Abstract
Bidirectional signaling has emerged as an important signature by which Ephs and ephrins control biological functions. Eph/ephrin signaling participates in a wide spectrum of developmental processes, and cross-regulation with other communication pathways lies at the heart of the complexity underlying their function in vivo. Here, we review in vitro and in vivo data describing molecular, functional, and genetic interactions between Eph/ephrin and other cell surface signaling pathways. The complexity of Eph/ephrin function is discussed in terms of the pathways that regulate Eph/ephrin signaling and also the pathways that are regulated by Eph/ephrin signaling.
[Keywords: Cell surface receptors, development, proteases, synaptic plasticity, cell adhesion]
Since the cloning of the first eph gene 20 years ago (Hirai et al. 1987) and the identification of ligands for Eph receptors (ephrins) a few years later (Bartley et al. 1994; Beckmann et al. 1994; Cheng and Flanagan 1994), much work has been done in trying to understand the function of this receptor/ligand pair. Due to a great number of studies related to Eph/ephrin signaling, we are beginning to grasp the full range of action of this signaling cascade. Eph receptors form the largest subfamily of receptor tyrosine kinases (RTKs). They interact with cell surface-bound ligands that are also part of a family of related proteins. Structural differences distinguish two classes of ephrins: Ephrins-A (A1–A6) are tethered to the plasma membrane via a glycosyl phosphatidyl inositol moiety, while ephrins-B (B1–B3) span the plasma membrane and possess a short cytoplasmic tail (Fig. 1). Eph receptors and ephrins are also grouped into class A and class B based on their degree of sequence similarity. One of the unique features of Eph/ephrin signaling is the fact that both receptors and ligands are competent to transduce a signaling cascade upon interaction. Eph-activated signaling is termed forward, and ephrin-activated signaling is termed reverse. Another level of complexity stems from the fact that interactions between Eph receptors and ephrins can happen in trans (between two opposing cells) or in cis (within the same cell). It is commonly assumed that trans interactions are activating while cis interactions are inhibiting (Fig. 1).
Figure 1.
Main features of Eph/ephrin signaling. (A) Both classes of Eph receptors and ephrins activate bidirectional signaling. Interaction between Eph receptors and ephrins leads to activation of forward and reverse signaling in neighboring cells. (B) Eph receptors and ephrins expressed in opposing cells interact in trans and activate bidirectional signaling. Eph receptors and ephrins coexpressed in the same cell interact in cis. Cis interaction has been shown to inhibit trans interaction and/or signaling.
Eph receptors and ephrins are expressed in virtually all tissues of a developing embryo, and they are involved in a wide array of developmental processes such as cardiovascular and skeletal development, axon guidance, and tissue patterning (Palmer and Klein 2003). In many developmental processes, the biological function of Eph/ephrin signaling boils down to the modulation of cell adhesion: growth cone retraction in axon guidance, cell sorting in embryo patterning, cell migration and fusion in craniofacial development, and platelet aggregation, among others. Although Eph/ephrins have been studied classically in a developmental context, their physiological functions in the adult are coming to light. They have been implicated recently in learning and memory (Gerlai 2002), in bone homeostasis (Zhao et al. 2006), and in insulin secretion (Konstantinova et al. 2007). Alterations of Eph/ephrin signaling in humans leads to congenital diseases and cancer (Pasquale 2005).
As our understanding of Eph/ephrin signaling improves, so does our reckoning that Eph/ephrins do not act in isolation, but are part of a complex network of regulatory pathways that must act in concert to control appropriate biological responses (Table 1). This review presents genetic, biochemical, and functional evidence for cross-talks between Eph/ephrin and other signaling pathways.
Table 1.
Cross-talk between Eph/ephrin and other signaling pathways
(ND) Not determined; (VSMC) vascular smooth muscle cells; (Y phosphorylation) tyrosine phosphorylation.
Cell surface receptors
Fibroblast growth factor receptor (FGFR)
The notion of cross-talks between Eph/ephrins and other RTKs was proposed in one of the original papers describing bidirectional signaling. It was shown in this study that ephrin-Bs could be phosphorylated on tyrosine in response to Platelet-Derived Growth Factor receptor activation (Bruckner et al. 1997). Since then, a number of studies have reported interactions between Eph/ephrin and another family of RTK, FGFR. Jones et al. (1998) reported that injection of ephrin-B1 in both blastomeres of a two-cell stage Xenopus embryo resulted in blastomere dissociation at the mid-blastula stage, and that the phenotype could be rescued by culturing the injected embryos in the presence of basic FGF. In addition, this activity was associated with the cytoplasmic and transmembrane portions of the ephrin-B1 protein, and the extracellular domain was not required for the induced deadhesion. In a follow-up paper (Chong et al. 2000), the same laboratory elucidated the mechanisms by which FGF signaling could rescue the ephrin-induced cell dissociation. Activated FGFR bound directly to ephrin-B1 in cis and induced its phosphorylation on tyrosine, which in turn inhibited the ability of ephrin-B1 to induce blastomere dissociation (Chong et al. 2000).
The relevance of this interaction was subsequently illustrated in the context of eye field formation in Xenopus. Retinal specification is a multistep process in which cellular movements during gastrulation and neurulation are critical to allow retinal progenitors to populate the eye field. Moore et al. (2004) showed that activated FGFR2 repressed eye field formation by restricting movements of retinal progenitors, therefore limiting their access to the eye field. Ectopic expression of ephrin-B1 rescued that phenotype, and ephrin-B1 knockdown phenocopied the repression associated with activated FGFR2. Moody (2004) concluded from their studies that these two signaling pathways coordinately regulate access to the eye field by modulating cell movement. Altogether, these studies point to antagonistic interaction between FGF and ephrin signaling pathways.
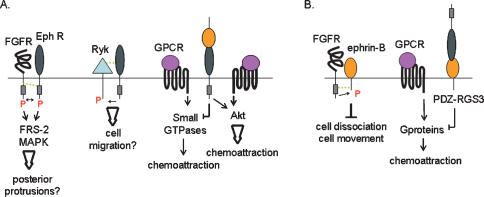
Other studies have reported a direct agonistic interaction between FGFR and Eph/ephrin signaling pathways. Indeed, in mammalian cells, FGFR and Eph-A4 could trans-phosphorylate each other and activate common downstream signaling pathways. Moreover, costimulation of both receptors resulted in the potentiation of mitogen-activated protein kinase (MAPK) stimulation (Yokote et al. 2005). No functional significance for the Eph-A4/FGFR potentiation was reported in that study (Yokote et al. 2005); however, an earlier study (Park et al. 2004) in Xenopus had also revealed an agonistic interaction between Eph-A4 and FGF signaling. Overexpression of Eph-A4 (like ephrin-B1) induced cell dissociation in early Xenopus embryos. As development proceeded, embryos recovered from the loss of cell adhesion; however, overexpression of Eph-A4 induced ectopic formation of posterior protrusions (Park et al. 2004) These tail-like structures are multicellular protrusions that express a number of posterior markers. They are not a direct consequence of the early loss of cell adhesion, but rather reflect the role of Eph/ephrin signaling in directing morphogenetic movements during development. Similar ectopic structures were induced following activation of FGFR1 in Xenopus embryos, and FGF8 knockdown rescued the Eph-A4-induced protrusions, indicating that FGF signaling could be involved in the Eph-A4-induced phenotype. Moreover, coinjection of both Eph-A4 and FGFR1 receptors increased the incidence of these structures (Park et al. 2004), which is consistent with the potentiating effect described in vitro (Yokote et al. 2005). While the sites of interaction between FGFR3 and Eph-A4 were mapped to the juxtamembrane domain in FGFR3 and the N-terminal portion of the tyrosine kinase domain in Eph-A4 (Yokote et al. 2005), the binding site for ephrin-B1 on FGFRs has not been reported (Fig. 2).
Figure 2.
Interactions with cell surface receptors. (A) Eph receptors interact with FGFR, Ryk, and chemokine receptors. Direct interactions are indicated by dashed green lines. Arrows represent agonistic interaction, while blunted lines indicate antagonistic regulation of downstream effectors or biological processes. Tyrosine phosphorylation events are shown in red. (B) Ephrins interact with FGFR and chemokine receptors.
More recently, the interplay between FGF and Eph/ephrin signaling has been involved in regulating asymmetric cell division and cell fate determination in Ciona embryos (Picco et al. 2007). In these chordate embryos, notochord and neural precursors share a common mother cell, and the binary choice between both fates is determined by differential extracellular signal-regulated kinase (ERK) activation. Picco et al. (2007) have shown that FGF signaling and Eph/ephrin signaling act in coordination to differentially regulate ERK and have proposed that local inhibition of ERK by an activated Eph receptor polarizes the mother cell and initiates asymmetric cell division leading to the acquisition of distinct cell fates. Again, this study illustrates how the antagonistic relationship between FGF signaling and Eph/ephrin signaling controls key developmental processes. Picco et al. (2007) inferred that the cross-talk between both pathways was downstream from the receptors, at the level of ERK activation; however, it does not necessarily preclude direct binding of FGFR and Eph receptor. Because MAPK is a cytoplasmic effector common to a number of receptor/ligand pairs, modulation of MAPK activity could be a mechanism by which Eph/ephrin impinge on a number of signaling cascades.
Ryk
RYK is an atypical RTK that contains a catalytically inactive tyrosine kinase domain. Genetic studies in Drosophila and the mouse have implicated RYK in regulating axon guidance and craniofacial development, two developmental processes that involve cell migration (Halford and Stacker 2001). Because these biological functions overlap with those attributed to Eph/ephrin signaling, a potential interaction between these pathways has been sought. In the mouse, homozygous deletion of Ryk resulted in craniofacial defects similar to defects observed in Eph-B2/Eph-B3-deficient embryos. In addition, RYK directly interacted with Eph receptors, and activated Eph receptors could phosphorylate RYK on tyrosines (Halford et al. 2000). The molecular mechanisms by which RYK influences Eph/ephrin signaling are still unclear; however, the authors (Halford et al. 2000; Halford and Stacker 2001) have proposed that RYK could facilitate the recruitment of AF-6, a cell junction-associated protein, to Eph receptors, therefore facilitating activation of downstream signaling events such as cell migration. Surprisingly, human RYK also interacted with Eph-B2 and Eph-B3, but this interaction did not lead to tyrosine phosphorylation of RYK, and no interaction could be detected between human RYK and AF-6 (Trivier and Ganessan 2002). Direct interaction between RYK and Eph receptors has also been observed in a rat model. The site of interaction between rat RYK and Eph-B3 was mapped to the extracellular domain of RYK (Kamitori et al. 2005; Fig. 2).
Interaction between RYK and Eph receptors is also involved in regulating the migration of neuroprogenitors. Indeed, overexpression of full-length RYK, but not a mutant form that does not bind to Eph-B3, inhibited radial migration of cortical neuroprogenitors in the rat cortex. Conversely, a mutant form of RYK lacking the kinase domain (including six out of nine tyrosines) had no effect on radial cell migration and still bound Eph-B3, suggesting that the function of RYK in cortical cell migration is independent of tyrosine phosphorylation but correlates with Eph-B3 binding (Kamitori et al. 2005). Altogether, these results suggest that RYK and Eph receptors act as agonists in regulating cortical cell migration during development (Fig. 2).
The latter study, however, must be interpreted in light of recent findings showing that RYK is an alternate receptor for Wnt proteins (Inoue et al. 2004; Lu et al. 2004) and that the Wnt/β-catenin pathway is also involved in cortical cell migration (Machon et al. 2003). To complicate matters further, a recent study uncovered an antagonistic relationship between Wnt/RYK and Eph/ephrin pathways in controlling retinotectal topographic mapping (Schmitt et al. 2006). Topographic mapping is a process ensuring that axons project to their appropriate target in the brain. Eph/ephrin signaling has been involved in positioning retinotectal projections along the medial–lateral axis; however, it cannot alone account for correct mapping in the tectum. In their very elegant study, Schmitt et al. (2006) demonstrate that the Wnt3/RYK pathway acts as a lateral mapping force to counterbalance the Eph-B/ephrin-B medial mapping force in the tectum. Although Schmitt et al. (2006) favor the idea of two independent pathways, their results do not rule out the possibility that both pathways directly modulate each other.
Chemokine receptors
Chemokines and their cell surface G-protein-coupled receptors are major regulators of cell trafficking. Among the wide number of chemokines and chemokine receptors (Zlotnik and Yoshie 2000), the CXCR4/SDF-1 pair stands out since the function of the CXCR4/SDF-1 signaling pathway is not limited to cell trafficking but also encompasses important roles in organogenesis and embryonic development (Kucia et al. 2004). The CXCR4/SDF-1 pathway has been shown to regulate multiple cellular processes such as locomotion, adhesion, secretion, and potentially survival and proliferation (Kucia et al. 2004). In 2001, Lu et al. (2001) demonstrated that activation of ephrin-B reverse signaling inhibited SDF-1-induced chemotaxis of cerebellar granule cells. The mechanistic basis for this inhibition was partly elucidated with the identification of PDZ-RGS3, a protein that binds the cytoplasmic domain of ephrin-Bs and is able to inactivate G-protein signaling via its GAP activity (Fig. 2; Lu et al. 2001). Similar results were obtained in T cells, as it was shown that activation of Eph-A receptors inhibited SDF-1-induced chemotaxis by altering the balance of small GTPases activity in these cells (Fig. 2; Sharfe et al. 2002). Down-regulation of chemokine-induced migration by Eph/ephrin signaling was also shown to be important to regulate trophoblast movement involved in arteriole remodeling during human placentation (Red-Horse et al. 2005). Unlike the studies above, which demonstrated an antagonistic relationship between Eph/ephrin and CXCR4/SDF-1 signaling, Salvucci et al. (2006) reported that an agonistic interaction between these pathways regulates endothelial movement and morphogenesis of blood vessels (Fig. 2). Stimulation of the Eph/ephrin signaling cascade enhanced SDF-1-induced chemotaxis in endothelial cells, and both pathways synergized to activate/phosphorylate AKT (Salvucci et al. 2006). Therefore, these studies clearly demonstrate that Eph/ephrin signaling and CXCR4/SDF-1 signaling cooperate to regulate a number of biological processes involving cell chemotaxis. Although the data so far point to a cross-talk at the level of downstream effectors, it would be interesting to test for direct interactions between these proteins, especially in light of the fact that both pathways localize in lipid rafts (Gauthier and Robbins 2003; Wysoczynski et al. 2005; Giri et al. 2007).
Together, these studies show that Eph/ephrin signaling interacts with a number of cell surface receptors and that these interactions can be agonistic or antagonistic (Table 1).
Adhesion molecules
Integrins
Due to the prominent role of Eph/ephrin signaling in cell migration, a relationship with integrin signaling was postulated early on. Despite numerous studies showing cross-talks between Eph/ephrin signaling and integrin signaling, there is still a surprising degree of uncertainty with respect to the outcome of the interaction between both pathways: While a number of reports show that Eph/ephrin signaling increased integrin-mediated cell adhesion (Huynh-Do et al. 1999, 2002; Davy and Robbins 2000; Gu and Park 2001; Huai and Drescher 2001; de Saint-Vis et al. 2003; Prévost et al. 2004, 2005), others demonstrate a counter-effect of Eph/ephrin on integrin-mediated cell adhesion (Zou et al. 1999; Miao et al. 2000, 2005; Deroanne et al. 2003; Bourgin et al. 2007). These outcomes are not linked to a specific class of Eph/ephrin pair, nor does it seem linked to either forward or reverse signaling. It is important to note, however, that all the studies focusing on reverse signaling have reported an increased integrin-mediated adhesion following activation of class A and class B ephrins (Davy and Robbins 2000; Huai and Drescher 2001; Huynh-Do et al. 2002; Prévost et al. 2004). Common sense now attributes the opposite effects of Eph/ephrin signaling on integrin function to distinct cellular contexts, since all the above-mentioned studies use different cell types (primary, transformed, or nontransformed cell lines) and different modes of expression of the proteins of interest (endogenous vs. ectopic) (Table 1). Nevertheless, the conclusion from these studies is that Eph/ephrin signaling impinges on integrin signaling (Fig. 3). In fact, cooperation between Eph/ephrin and integrin signaling is supported by genetic data: itga5 (integrin α5) and fn (fibronectin) are required for somite development in zebrafish, and reducing Eph/ephrin signaling in the context of a mutant fn or itga5 background worsened the somite phenotype (Koshida et al. 2005). The point of convergence between both pathways appears to be at the level of cytoplasmic kinases (FAK, PI3K, MAPK) and/or small GTPases (Rac, Rho, Ras, Rap1). Only one study reports a direct interaction between an Eph receptor and an integrin (Prévost et al. 2005).
Figure 3.
Regulation of adhesion proteins. Eph/ephrin signaling regulates cell–cell adhesion and cell–matrix adhesion by impinging on formation/stability of tight, adherens, and gap junctions, as well as on integrin function. In Eph-expressing cells (blue), activation of forward signaling induces the redistribution of E-cadherin to the cell surface while destabilizing claudins. In ephrin-expressing cells (orange), activation of reverse signaling leads to inhibition of GJC, while interaction with claudins destabilizes tight junctions. Both forward and reverse signaling act on integrin-mediated adhesion. Together, these cascades participate in Eph/ephrin-induced cell sorting.
Immunoglobulin superfamily (IgSF) proteins
Like Eph/ephrin, IgSF proteins are implicated in many aspects of the nervous system development, including axon pathfinding, target recognition, and synapse formation (Rougon and Hobert 2003). It was reported recently that the Caenorhabditis elegans IgSF protein, WRK-1, interacts with Eph/ephrin signaling to provide a midline guidepost function (Boulin et al. 2006). Midline guideposts are necessary for developing axons to decide whether to cross the midline or not, a decision that underlies the ability of the nervous system to coordinate events occurring on each side of the body. WRK-1 is expressed in embryonic midline motoneurons (eMNs), and wrk-1 loss-of-function mutations caused nonautonomous midline axon guidance defects in the worm similar to those observed in mutants for Eph/ephrin signaling. Epistasis experiments indicated that these genes act in a single pathway. Interestingly, the cell autonomy of vab-1 (coding for an Eph receptor) and wrk-1was investigated, and although these genes act in the same pathway, they are required in different cells. The proposed model is that vab-1 and wrk-1 are expressed in opposing and presumably interacting neurons (Boulin et al. 2006). The authors further demonstrate a direct interaction between WRK-1, VAB-1, and VAB-2 (an ephrin), leading to a proposed model in which WRK-1 and VAB-2 are coexpressed in eMNs, where they interact with each other and provide a repulsive signal to VAB-1-expressing axons in trans (Fig. 5, below; Table 1; Boulin et al. 2006).
Figure 5.
Interactions at growth cones. Binding of WRK-1 and Eph/VAB-1 in trans prevents midline crossing. VAB-1 also interacts with SAX-3 in cis. Growth cone retraction requires termination of contact between Eph- and ephrin-expressing cells. This is achieved by cleavage of ephrin ectodomain by ADAM10 in trans and/or endocytosis of Eph/ephrin complexes, followed by processing of the ectodomain in the endosomal/lyzosomal pathway. Subsequent to ectodomain shedding, processed ephrins are targets for PS cleavage that releases the ICD in the cytosol.
Robo proteins are members of the IgSF family also involved in midline axon guidance (Dickson and Gilestro 2006). Ghenea et al. (2005) identified sax-3 as a candidate gene functioning with vab-1 during embryogenesis. Using genetic and molecular approaches, Ghenea et al. (2005) demonstrated that SAX-3/Robo functions with VAB-1 to regulate embryonic morphogenesis and axon guidance in C. elegans. sax-3 mutants displayed defects that are similar to vab-1 mutants, and analysis of a combination of double mutants revealed a gene-dosage sensitivity between these genes. In addition, direct interaction between VAB-1 and SAX-3 was demonstrated using two-hybrid and GST-pull-down assays (Ghenea et al. 2005). Since the domain of interaction was mapped to the juxtamembrane domain of SAX-3, and both proteins were coexpressed in a subset of neuroblasts, Ghenea et al. (2005) proposed that these two receptors form a complex in cis and act together during embryogenesis (Fig. 5, below). Given the well-known role of Robo proteins as midline guideposts and the data described above (Boulin et al. 2006), it would be interesting to assess midline crossing of ventral axons in double sax-3/vab-1 mutants. In vertebrates, evidence for interplay between Eph/ephrin and IgSF proteins is limited to one member of the family. Zisch et al. (1997) showed that L1 is a substrate for Eph-B2 tyrosine kinase, while Suh et al. demonstrated that growth cones stimulated with L1 lost their responsiveness to Eph-B (Suh et al. 2004). Together, these studies show the importance of the interplay between Eph/ephrin and IgSF proteins and highlight the complexity of interactions between cell surface proteins, which can happen either in cis or in trans.
Cadherins
In addition to the regulation of cell migration and axon navigation, one of the prominent biological outcomes of Eph/ephrin signaling is the regulation of cell sorting, a process by which populations of cells physically segregate from each other to generate distinct tissues or compartments (Xu et al. 1999; Poliakov et al. 2004). The cellular and molecular mechanisms by which Eph/ephrin signaling control cell sorting behaviors are still not well characterized; however, because homotypic interactions via cadherins play an important role in cell sorting (Tepass et al. 2002), it was postulated that these two pathways might cooperate to regulate cell adhesion and segregation. Surprisingly, there is little evidence supporting this hypothesis. In Xenopus embryos, ectopic expression of an Eph receptor or an ephrin leads to cell dissociation, a phenotype that could be rescued by overexpressing C-cadherin (Winning et al. 1996; Jones et al. 1998). However, the fact that no alteration of the cadherin/β-catenin interaction could be detected following ectopic expression of Eph or ephrin suggests that overexpression of C-cadherin might not specifically rescue Eph/ephrin-induced dissociation but might serve as indiscriminate SuperGlue. In mammalian epithelial cells, it was shown that Eph-A2 localizes to sites of cell–cell contact and that this subcellular localization was dependent on E-cadherin. In addition, ectopic expression of E-cadherin expression in breast cancer cells that lack endogenous E-cadherin increased tyrosine phosphorylation of Eph-A2 and led to a decreased cell adhesion to the extracellular matrix (Zantek et al. 1999). Zantek et al. (1999) concluded that Eph-A2 function is dependent on E-cadherin; however, they also report that neither coprecipitation nor coclustering between the two proteins could be detected using their system. This led them to argue that E-cadherin could primarily serve to stabilize cell–cell contacts and thereby promote interactions between Eph-A2 and its ligands (Zantek et al. 1999).
The most convincing data showing a direct role of E-cadherin in Eph/ephrin-induced cell sorting was reported by the Batlle group (Cortina et al. 2007). Expression of Eph-B3 and ephrin-B1 in colorectal cancer cells (CRC) induced cell sorting and stimulation of Eph-expressing CRC resulted in cell clustering and redistribution of E-cadherin to the plasma membrane. Importantly, down-regulation of E-cadherin expression prevented Eph-induced clustering and sorting between Eph- and ephrin-expressing cells (Cortina et al. 2007).
Claudins
In contrast to cadherins, claudins have clearly been shown to directly interact with Eph/ephrin proteins in epithelial cells. Claudins are components of tight junctions located in the subapical region of the lateral membranes. Tight junctions serve as paracellular barriers restricting movements of molecules across epithelial barriers (Hartsock and Nelson 2007). Direct interaction between Eph-A2 and claudin-4 was reported and mapped to their extracellular domains. This interaction led to the phosphorylation on tyrosine of claudin-4, which then reduced its integration in tight junctions, thus increasing paracellular permeability (Fig. 3; Tanaka et al. 2005a). Interestingly, claudin-4 also binds to ephrin-B1, and the interaction between these proteins, which was mapped to their extracellular domain, led to tyrosine phosphorylation of ephrin-B1 that in turn affected intercellular adhesion (Fig. 3; Tanaka et al. 2005b). Tyrosine phosphorylation of claudin-4 in this context was not discussed. An interesting bit of data was that interaction between claudin-4 and ephrin-B1 happened in trans, lending support to the notion that ephrins could have Eph-independent functions (Tanaka et al. 2005b).
These studies reveal the interplay between Eph/ephrins and integrins, cadherins, and claudins, which are involved in the regulation of intercellular permeability and cell adhesion and probably participate in cell sorting (Fig. 3; Table 1).
Channels and pores
Connexins/innexins
As mentioned above, one of the outcomes of Eph/ephrin signaling is cell sorting. Cell sorting during embryo development permits the formation of distinct compartments with distinct developmental fates. This process is necessary from early embryo patterning to organ formation. An important characteristic of developmental compartments is the fact that all cells in the compartment exchange information by direct coupling of their cytoplasms. On the contrary, cells of a given compartment are not directly coupled to cells of an adjacent compartment, and this break in coupling forms a developmental boundary. The structures that allow for direct coupling of cytoplasms and transfer of small molecules are called gap junctions. Connexins are the main structural subunits of gap junctions in vertebrates (Laird 1996).
Wilkinson and colleagues (Mellitzer et al. 1999) have shown that in addition to their role in cell sorting, Eph/ephrins are also involved in negatively regulating gap junction communication (GJC). Eph/ephrin bidirectional signaling was required to restrict cell intermingling, but unidirectional signaling via Eph receptors or ephrins was sufficient to inhibit GJC between Eph- and ephrin-expressing cells. These results clearly demonstrated that inhibition of GJC is not a mere consequence of an Eph/ephrin-induced deadhesion, since inhibition was observed even in conditions in which cells intermingled (Mellitzer et al. 1999). We showed more recently that inhibition of GJC by Eph/ephrin signaling has adverse consequences on skeletal development in the mouse embryo (Davy et al. 2006). Gap junctions play a critical role in nearly all aspects of skeletal development, from limb bud patterning to differentiation of osteoblasts; however, the mechanisms underlying this function remain unclear (Stains and Civitelli 2005). As a result of the linkage of the Efnb1 gene (coding for ephrin-B1) to the X-chromosome, heterozygous females that carry one copy of a loss-of-function Efnb1 allele are mosaic with respect to ephrin-B1 expression. This mosaicism leads to the formation of ectopic Eph/ephrin boundaries in ephrin-B1 heterozygous females and correlates with the appearance of skeletal phenotypes that are never seen in ephrin-B1-null animals (Compagni et al. 2003; Davy et al. 2004). This is similar to what has been observed in patients carrying a mutation in the Efnb1 gene (Twigg et al. 2004; Wieland et al. 2004). Importantly, the skeletal defects observed in ephrin-B1 heterozygotes could be partially rescued by overexpression of connexin 43 (Cx43) (Davy et al. 2006), suggesting that inhibition of GJC at ectopic Eph/ephrin boundaries is the underlying cause of the skeletal defects in ephrin-B1 heterozygotes. More recently, interaction between GJC and Eph/ephrin signaling has also been shown to be involved in insulin secretion (Konstantinova et al. 2007).
The mechanism by which Eph/ephrin signaling inhibits GJC is still unclear; however, we reported a biochemical interaction between ephrin-B1 and Cx43, raising the possibility that Eph/ephrin directly regulates GJC. One possible mechanism could be via internalization of gap junctions. Our results also suggested that interaction between ephrin-B1 and Cx43 could be involved in the process of cell sorting itself (Fig. 3). A recent publication on the role of gap junction in regulating radial migration in the developing cortex lends support to this idea since it demonstrates that gap junction proteins are involved in providing adhesive contacts necessary for radial migration, independently of cell–cell communication (Elias et al. 2007).
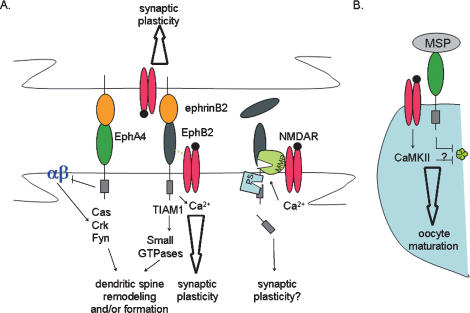
An overlap between Eph/ephrin signaling and GJC has been uncovered in C. elegans. In the adult hermaphrodite gonad, oocytes are arrested in meiotic prophase and resume maturation in the presence of a sperm signal, MSP. Eph/ephrins are doubly involved in the process of oocyte maturation: First, Eph/ephrin signaling is required to block maturation of oocytes in the absence of sperm; second, VAB-1/Eph is one of the MSP receptors responsible for lifting the maturation block in the presence of sperm (Miller et al. 2003). In addition to Eph/ephrin signaling, oocyte maturation is also inhibited by a parallel pathway involving CEH-18 in sheath somatic cells. Binding of MSP to an unknown receptor on sheath cells lifts this inhibition (Miller et al. 2003). Interestingly, breakdown of gap junctions was observed between oocytes and sheath cells in ceh-18 mutants, correlating with an increased rate of oocyte maturation. These observations indicated that gap junctions could be involved downstream from CEH-18 to provide an inhibitory signal to the oocyte. Two recent studies have confirmed that gap junctions are indeed involved in regulating oocyte maturation (Govindan et al. 2006; Whitten and Miller 2007). Mutants in innexins (the proteins forming gap junction pores in invertebrates) exhibited higher than normal rates of oocyte maturation, which is similar to the Eph/ephrin mutants. Based on these results, the current model is that gap junction pores between sheath cells and oocytes allow for the transfer of a signal inhibiting oocyte maturation. In presence of sperm, the MSP-induced cascades in the oocyte (which involve VAB-1/Eph) and in the sheath cells lead to destabilization of gap junctions, blocking the transfer of information to the oocyte. In this model based on genetic data, Eph/ephrin signaling runs parallel to GJC, while MSP/VAB-1 antagonizes GJC. Interestingly, this switch from negative to positive regulation of oocyte maturation by VAB-1 involves NMR1, another type of cell surface receptor (ligand-gated ion channels, further discussed below) (Fig. 4; Corrigan et al. 2005). Although there is currently no evidence for a direct interaction between Eph/ephrin and GJC in this system, these studies clearly highlight the delicate intricacies between Eph/ephrin signaling and GJC.
Figure 4.
Interactions with synaptic proteins. (A) The figure shows a holistic view of Eph/ephrin interactions at sites of synapse formation/regulation. Eph-A4-induced forward signaling inhibits β1-integrin, which induces spine remodeling. Eph-B receptors and ephrins-B interact with NMDAR and potentiates its activity. Recruitment of Tiam1 to the Eph-B/NMDAR complex and activation of small GTPases facilitates spine formation. Activation of NMDAR induces processing of Eph receptors by MMPs and PS. (B) Interaction between Eph/VAB-1 and NMDAR participates in oocyte maturation, which also involves down-regulation of GJC.
NMDA receptor (NMDAR)
A number of molecular signals control aspects of synapse development, including secreted factors that affect the competence of neurons to generate synapses, cell–cell adhesion proteins that locally drive the organization and maturation of synaptic specializations, and ligand- or voltage-gated ion channels that respond to neuronal activity (Li and Sheng 2003; Scheiffele 2003; Craig et al. 2006). There is a growing body of evidence for a tight cooperation between Eph/ephrins and ion channels in regulating excitatory neurotransmission and synaptic plasticity.
Initial studies showed a direct association between the N-terminal domains of Eph-B2 and the NMDA receptor 1 (NR1) subunit of the NMDARs at the post-synaptic membrane (Dalva et al. 2000). This interaction was enhanced by the presence of ephrin-B acting in trans (Fig. 4). In parallel, activation of Eph-B by soluble ephrin-B, in vitro, induced Eph-B kinase-dependent formation of dendritic spines, suggesting that the interaction between Eph-B/ephrin signaling and NMDAR could contribute to local changes influencing spinogenesis (Penzes et al. 2003). In these early studies, Eph-B signaling was implicated in post-synaptic differentiation, where ephrin-B treatment increased the density of synaptic release sites (Dalva et al. 2000) and of synaptic markers apposed to spines (Penzes et al. 2003). Studies using cultured cortical neurons further revealed that the activation of Eph-B by exogenous addition of ephrin-B2 could potentiate NMDAR clustering and enhanced NMDAR-dependent Ca2+ flux, suggesting a mechanism whereby activity-dependent and -independent signals converge in the regulation of synaptic plasticity (Takasu et al. 2002).
In agreement with these findings, animal models lacking Eph-B2 display abnormal NMDAR-dependent synaptic plasticity and a reduction in synapse-associated NMDARs (Grunwald et al. 2001; Henderson et al. 2001). Analysis of Eph B1, B2, and B3 triple-knockout mice show few dendritic NMDAR clusters and, interestingly, contained a reduced AMPA receptor (AMPAR) density (Henkemeyer et al. 2003), thereby indicating that Eph-B is more broadly involved in post-synaptic development. Further examination of the triple-knockout model revealed that Eph-B preferentially regulates the development and maturation of functional excitatory synaptic contacts between neurons, at pre- and post-synaptic membranes in vitro (Kayser et al. 2006). Using cultured neurons, Kayser et al. (2006) demonstrate that Eph-B2 colocalizes with AMPARs in cortical neurons, and that the PDZ-binding domains but not the kinase domain of Eph-B2 are required for this colocalization. They further demonstrate that the disruption of the Eph-B2–AMPAR association, by mutating the Eph-B2 PDZ-binding domain, did not result in a decrease in dendritic spines but could still regulate dendritic spine development in vitro. Therefore, Kayser et al. (2006) put forward two potential scenarios for the function of Eph-B in synaptogenesis: first, that Eph-B might initiate de novo formation of excitatory synapses with the ephrin-B/Eph-B/NMDAR complex serving to converge protein–protein interaction, or, alternatively, that Eph-B may traffic to pre-existing NMDAR-containing synaptic sites organized by other synaptogenic molecules and act to recruit AMPAR, induce spine formation, and modulate presynaptic functions via trans-synaptic signaling.
Recent studies conducted by Tolias et al. (2007) have further unraveled how Eph-B receptors may complex with NMDAR and positively regulate their function. Here the authors show that Tiam1, a large multidomain protein that is necessary for proper spine and synapse development (Tolias et al. 2005), specifically interacts with Eph-B2 (Tolias et al. 2007). The activation of Eph-B by ephrin-B induced the phosphorylation and recruitment of Tiam1 to Eph-B complexes containing NMDAR, which in turn leads to Rac-dependent actin remodeling required for spine formation (Fig. 4). It is worth noting that besides Tiam1, ephexin and intersectin, which are also guanine nucleotide exchange factors (GEFs), have been involved in regulating dendritic spine formation downstream from Eph signaling (Nishimura et al. 2006; Fu et al. 2007).
While most studies to date focused on the actions of Eph-B forward signaling at the pre- and/or post-synaptic membranes, there is emerging evidence describing how ephrin-B reverse signaling functions in synaptogenesis and spine formation. Post-synaptic Eph-B trans-activates ephrin-reverse signaling to regulate presynaptic differentiation (Grunwald et al. 2004; Segura et al. 2007). In CA1 hippocampal neurons, ephrin-Bs are localized to the post-synaptic membrane where they regulate NMDAR-dependent long-term plasticity (Grunwald et al. 2004; Fig. 4). An interaction between ephrin-B2 and metabotropic Glutamate receptors group 1 (mGlu1) was shown (Calo et al. 2005). mGlu1 receptors interact with NMDARs and are involved in the regulation of synaptic plasticity during development and in adulthood (Spooren et al. 2003). Although the functional significance of this interaction between ephrin-B2 and mGlu1 is unknown, Calo et al. (2005) speculate that it may facilitate NMDAR activation, or directly amplify mGlu1 responses. Together, these studies demonstrate that Eph-B/ephrin-B signaling positively regulates the activity of a number of ligand-gated ion channels, which underlies their role in regulating dendritic spine formation and synaptic plasticity (Fig. 4).
Eph-A4, which belongs to the other class of Eph receptors, has been shown to regulate synaptic plasticity (Grunwald et al. 2004) and spine remodeling (Murai et al. 2003; Bourgin et al. 2007) by distinct mechanisms. Eph-A4 indirectly regulates synaptic plasticity by activating reverse signaling post-synaptically (Grunwald et al. 2004). In addition, Eph-A4-induced forward signaling activates a number of cytosolic effectors that then inhibit β1-integrin function and induce dendritic spine remodeling (Fig. 4; Murai et al. 2003; Bourgin et al. 2007).
Cell surface proteases
ADAM
Cognate Eph and ephrin typically bind at nanomolar affinity (Zimmer et al. 2003), where after the initial binding event, the ligand–receptor pairs oligomerize to form large signaling aggregates. How this tight binding of a membrane-bound receptor to a ligand tethered to an opposing cell is reconciled with the typically repulsive activity of the Eph/Ephrin binding began to emerge in the last few years. The initial studies demonstrated that ephrin-A2 forms a stable complex with the metalloproteinase Kuzbanian (KUZ), the Drosophila homolog of ADAM 10 (Hattori et al. 2000). These studies also demonstrated that upon formation of the Eph-A3/ephrin-A2 signaling complex, KUZ catalyzed the proteolytic shedding of ephrin-A2 from its membrane tether. ADAMs, which can process or remove the extracellular domains of cell surface proteins, are particularly intriguing in that they contain both cell adhesion and proteolytic domains (Kaushal and Shah 2000; Primakoff and Myles 2000). By finding that ephrin-A2 is a metalloprotease (MMP) substrate, Hattori et al. (2000) showed that cell surface MMPs not only modulate the strength of axon guidance signals, but are also intimately involved in defining their outcomes.
More recently, Janes et al. (2005) shed new light on the mechanism by which ADAM10 interacts with an ephrin signaling complex. This work revealed that the acidic pocket within the ADAM10 cysteine-rich domain mediates cleavage of both ephrin-A5 and ephrin-A2 (Janes et al. 2005). A key finding was that ADAM10 cleaves Eph-A-bound ephrin-As from their membrane tether in trans (Fig. 5). Thus, consistent with findings that ADAM10 constitutively associates with the Eph-A3 receptor in cis, the studies showed that ADAM10 must be presented by the juxtaposed cell for cleavage of ephrin-A2 or ephrin-A5. This is in contrast to other characterized ADAM-mediated proteolytic events that so far have been shown to occur only in cis (Blobel 1997). Nonetheless, ADAM10 appears to be the first example of a protease that cleaves its substrate in a manner that is cell-nonautonomous. The phenomenon for cleavage of Ephs and ephrins at the cell surface has quickly begun to represent a general strategy underlying Eph/ephrin repulsive signals in many cell types.
Presenilins (PS)
More recently, two independent studies showed that ephrin-Bs are substrates for γ-secretase (Georgakopoulos et al. 2006; Tomita et al. 2006). PS are highly conserved polytopic transmembrane proteins that represent the active component of γ-secretase, a multiprotein complex of Nicastrin, APH1, and PEN-2 (Takasugi et al. 2003). γ-Secretase is an unusual aspartate protease that cleaves single-span transmembrane proteins within the transmembrane domain. Although it is unable to cleave the full-length form of its substrates, γ-secretase cleaves subsequent to ectodomain shedding to liberate N-terminal small fragments and C-terminal intracellular domains (ICD) into the luminal and cytoplasmic sides, repectively (Kopan and Ilagan 2004; Tomita and Iwatsubo 2004). Georgakopoulos et al. (2006) revealed a novel signaling cascade whereby Eph-B/ephrin-B2 binding stimulates the sequential processing of ephrin-B2 by MMPs and γ-secretase to produce ephrin-B2 ICD (Fig. 5). The ephrin-B2 ICD binds Src and promotes its dissociation from a negative Src regulator called C-terminal Src Kinase (Csk), thus allowing for the autophosphorylation and activation of Src. Likewise, degradation of the ephrin-B2 ICD results in Src dephosphorylation and deactivation. Georgakopoulos et al. (2006) extrapolate their findings to previously established consequences of Eph-B/ephrin-B signaling that lead to the recruitment of cellular factors to cytoplasmic ephrin-B and to the rearrangement of the actin cytoskeleton (for a review on ephrin-induced reverse signaling, see Palmer and Klein 2003).
Parallel studies reported that Eph-B/ephrin-B1 binding mediates ephrin-B1 ectodomain shedding, and the membrane-tethered fragment is sequentially cleaved by γ-secretase to release the ICD (Tomita et al. 2006). These studies further showed that the overexpression of the membrane-tethered ephrin-B1 led to the formation of cellular protrusions consisting of F-actin, which was negatively regulated by γ-secretase activity. Moreover, overexpression of the ephrin-B1 ICD and inhibition of the proteasome resulted in the nuclear localization of the ephrin-B1 ICD. To date, the functions of γ-secretase-generated ICDs as transcriptional activators (i.e., Notch, APP, CD44) or repressors (Jagged, N-Cadherin) within the nucleus have been reported (for review, see Kopan and Ilagan 2004); however, functional analysis of the nuclear ephrin-B1 ICD awaits further study.
While ephrin-Bs are shown to constitutively undergo ectomembrane shedding and sequential cleavage by γ-secretase to release the ephrin-B ICDs, new findings support an analogous process for the Eph-B2 receptor (Litterst et al. 2007). To date, reports have indicated that the mechanism by which adhesive and signaling interactions between Eph receptors and ephrin ligands are terminated include endocytosis of the cell surface Eph/ephrin complexes and cleavage of the ectodomain of ephrin ligands (Hattori et al. 2000; Marston et al. 2003; Zimmer et al. 2003). Recent work shows that two distinct pathways regulate the proteolytic processing of Eph-B2 receptor and its complexes: one stimulated by calcium influx and the other by ephrin-B2 ligand binding (Litterst et al. 2007). Eph-B2 processing was stimulated by N-methyl-D-aspartic acid (NMDA) treatment and calcium influx and was sensitive to a broad spectrum of MMP inhibitors, particularly the inhibition of ADAM10. Therefore, these data show an additional physiological consequence of the interaction between Eph-B2 and NMDAR (Fig. 4), and identified ADAM10 as the protease involved in calcium-induced processing of the Eph-B2 receptor. ADAM10 processing of Eph-B2 did not require endocytosis but rather resulted in the rapid shedding of the extracellular domain of Eph-B2, suggesting that the ectodomain shedding and γ-secretase cleavage involved in this pathway occur at or near the plasma membrane. In addition to this first pathway, a ligand-induced processing of Eph-B2 was demonstrated in the endosomal/lysosomal pathway. Eph-B2/ephrin-B2 binding resulted in increased ectodomain shedding followed by γ-secretase processing and rapid degradation of Eph-B2 ectodomain following ubiquitination (Fig. 5).
Taken together, the findings demonstrate that both Eph receptors and Ephrin ligands are processed by MMPs and/or γ-secretase, a catalytic event necessary for some of their functions (Table 1; Ethell and Ethell 2007).
Conclusions and perspectives
Our understanding of the Eph/ephrin pathway has improved tremendously over the last 10 years—we have discovered that it is involved in many developmental processes, we know it affects a number of cellular functions, and we partly characterized its mechanistic mode of action. The challenge we face now is to consider Eph/ephrin signaling not in isolation but as part of a network of information. Every single cell constantly receives numerous signals that have to be instantaneously integrated and translated into coherent cellular responses. Direct or indirect regulatory interactions between pathways serve as a mechanism to simplify the interpretation of the many environmental factors confronting cells at every decisional key point, since upstream cross-regulation eliminates the requirement of having a specific signaling cascade for each extracellular cue.
One of the obvious difficulties in assembling a model from the studies presented above is the fact that Eph/ephrin signaling clearly impinges on multiple pathways simultaneously to achieve its biological function. A second difficulty lies in the fact that the outcome (agonistic or antagonistic) of a given cross-talk seems to be highly dependent on cellular context. Resolution of these apparent discrepancies awaits the characterization of all players in the various signaling cascades. Indeed, one could hypothesize that the presence or absence of (as yet) unknown partners could switch outcomes from agonistic to antagonistic. Another possibility that has been little explored is that different Eph/ephrin pairs could regulate different signaling cascades, therefore leading to opposite outcomes.
For many years, Eph/ephrin signaling has been studied in a developmental context; however, recent publications clearly highlighted its involvement in organ function and in disease in the adult. Although regulation of cell adhesion, migration, and morphology underlies most of the developmental roles of Eph/ephrin signaling, these proteins seem to regulate a different set of biological outcomes in the adult. In the future, it will be interesting to compare signaling cascades and signal integration in the context of the roles of Eph/ephrins in the adult.
The discovery that Eph/ephrins are substrates for cell surface proteases was satisfying, as it resolved one of the paradoxes of this signaling pair: how the initial Eph/ephrin adhesion turned into cell repulsion. However, cleavage of Eph receptors and ephrins and release of their ectodomain in the extracellular milieu raises the mind-boggling possibility that the action of these proteins might not be limited to short-range cell–cell interactions, but might also encompass long-range paracrine interactions.
Acknowledgments
We apologize to the authors whose work we were not able to cite due to space constraints. We thank Phil Soriano, David Wilkinson, and our laboratory colleagues for critical reading of the manuscript, and Michael Miller for his insightful comments. D.A. is supported by the Centre National de la Recherche Scientifique. A.D. is supported by the Centre National de la Recherche Scientifique. Work from our laboratory is supported by grants from the Centre National de la Recherche Scientifique, from the Association Française contre les Myopathies, and from the Human Frontier Science Program Organization.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1630408
References
- Bartley T.D., Hunt R.W., Welcher A.A., Boyle W.J., Parker V.P., Lindberg R.A., Lu H.S., Colombero A.M., Elliott R.L., Guthrie B.A., et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- Beckmann M.P., Cerretti D.P., Baum P., Vanden Bos T., James L., Farrah T., Kozlosky C., Hollingsworth T., Shilling H., Maraskovsky E., et al. Molecular characterization of a family of ligands for eph-related tyrosine kinase receptors. EMBO J. 1994;13:3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel C.P. Metalloprotease-disintegrins: Links to cell adhesion and cleavage of TNFα and Notch. Cell. 1997;90:589–592. doi: 10.1016/s0092-8674(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Boulin T., Pocock R., Hobert O. A novel Eph receptor-interacting IgSF protein provides C. elegans motoneurons with midline guidepost function. Curr. Biol. 2006;16:1871–1883. doi: 10.1016/j.cub.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Bourgin C., Murai K.K., Richter M., Pasquale E.B. The EphA4 receptor regulates dendritic spine remodeling by affecting β1-integrin signaling pathways. J. Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner K., Pasquale E.B., Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Calo L., Bruno V., Spinsanti P., Molinari G., Korkhov V., Esposito Z., Patane M., Melchiorri D., Freissmuth M., Nicoletti F. Interactions between ephrin-B and metabotropic glutamate 1 receptors in brain tissue and cultured neurons. J. Neurosci. 2005;25:2245–2254. doi: 10.1523/JNEUROSCI.4956-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.J., Flanagan J.G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Chong L.D., Park E.K., Latimer E., Friesel R., Daar I.O. Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol. Cell. Biol. 2000;20:724–734. doi: 10.1128/mcb.20.2.724-734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagni A., Logan M., Klein R., Adams R.H. Control of skeletal patterning by ephrinB1–EphB interactions. Dev. Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Corrigan C., Subramanian R., Miller M.A. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 2005;132:5225–5237. doi: 10.1242/dev.02083. [DOI] [PubMed] [Google Scholar]
- Cortina C., Palomo-Ponce S., Iglesias M., Fernández-Masip J.L., Vivancos A., Whissell G., Humà M., Peiro N., Gallego L., Jonkheer S., et al. EphB–ephrinB interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- Craig A.M., Graf E.R., Linhoff M.W. How to build a central synapse: Clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva M.B., Takasu M.A., Lin M.Z., Shamah S.M., Hu L., Gale N.W., Greenberg M.E. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Davy A., Robbins S.M. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A., Aubin J., Soriano P. EphrinB1 forward and reverse signaling are required during mouse development. Genes & Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A., Bush J.O., Soriano P. Inhibition of gap junction communication at ectopic ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:1763–1776. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint-Vis B., Bouchet C., Gautier G., Valladeau J., Caux C., Garrone P. Human dendritic cells express neuronal Eph receptor tyrosine kinases: Role of EphA2 in regulating adhesion to fibronectin. Blood. 2003;102:4431–4440. doi: 10.1182/blood-2003-02-0500. [DOI] [PubMed] [Google Scholar]
- Deroanne C., Vouret-Craviari V., Wang B., Pouysségur J. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 2003;116:1367–1376. doi: 10.1242/jcs.00308. [DOI] [PubMed] [Google Scholar]
- Dickson B.J., Gilestro G.F. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- Elias L.A., Wang D.D., Kriegstein A.R. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Ethell I.M., Ethell D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Fu W.Y., Chen Y., Sahin M., Zhao X.S., Shi L., Bikoff J.B., Lai K.O., Yung W.H., Fu A.K., Greenberg M.E., et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Gauthier L.R., Robbins S. Ephrin signaling: One raft to rule them all? One raft to sort them? One raft to spread their call and in signaling bind them? Life Sci. 2003;74:207–216. doi: 10.1016/j.lfs.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A., Litterst C., Ghersi E., Baki L., Xu C., Serban G., Robakis N.K. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. EphB and NMDA receptors: Components of synaptic plasticity coming together. Trends Neurosci. 2002;25:180–181. doi: 10.1016/s0166-2236(02)02165-3. [DOI] [PubMed] [Google Scholar]
- Ghenea S., Boudreau J.R., Lague N.P., Chin-Sang I.D. The VAB-1 Eph receptor tyrosine kinase and SAX-3/Robo neuronal receptors function together during C. elegans embryonic morphogenesis. Development. 2005;132:3679–3690. doi: 10.1242/dev.01947. [DOI] [PubMed] [Google Scholar]
- Giri B., Dixit V.D., Ghosh M.C., Collins G.D., Khan I.U., Madara K., Weeraratna A.T., Taub D.D. CXCL12-induced partitioning of flotillin-1 with lipid rafts plays a role in CXCR4 function. Eur. J. Immunol. 2007;37:2104–2116. doi: 10.1002/eji.200636680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan J.A., Cheng H., Harris J.E., Greenstein D. Gαo/i and Gαs signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr. Biol. 2006;16:1257–1268. doi: 10.1016/j.cub.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Grunwald I.C., Korte M., Wolfer D., Wilkinson G.A., Unsicker K., Lipp H.P., Bonhoeffer T., Klein R. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–1040. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- Grunwald I.C., Korte M., Adelmann G., Plueck A., Kullander K., Adams R.H., Frotscher M., Bonhoeffer T., Klein R. Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Gu C., Park S. The EphA8 receptor regulates integrin activity through p110γ phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol. Cell. Biol. 2001;21:4579–4597. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford M.M., Stacker S.A. Revelations of the RYK receptor. Bioessays. 2001;23:34–45. doi: 10.1002/1521-1878(200101)23:1<34::AID-BIES1005>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Halford M.M., Armes J., Buchert M., Meskenaite V., Grail D., Hibbs M.L., Wilks A.F., Farlie P.G., Newgreen D.F., Hovens C.M., et al. Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat. Genet. 2000;25:414–418. doi: 10.1038/78099. [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2007 doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Osterfield M., Flanagan J.G. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Henderson J.T., Georgiou J., Jia Z., Robertson J., Elowe S., Roder J.C., Pawson T. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., Itkis O.S., Ngo M., Hickmott P.W., Ethell I.M. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J. Cell Biol. 2003;163:1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Maru Y., Hagiwara K., Nishida J., Takaku F. A novel putative tyrosine kinase receptor encoded by the Eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Huai J., Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J. Biol. Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- Huynh-Do U., Stein E., Lane A.A., Liu H., Cerreti D.P., Daniel T.O. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through αvβ3 and αvβ1 integrins. EMBO J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh-Do U., Vindis C., Liu H., Cerretti D.P., McGrew J.T., Enriquez M., Chen J., Daniel T.O. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J. Cell Sci. 2002;115:3073–3081. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- Inoue T., Oz H.S., Wiland D., Gharib S., Deshpande R., Hill R.J., Katz W.S., Sternberg P.W. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Janes P.W., Saha N., Barton W.A., Kolev M.V., Wimmer-Kleikamp S.H., Nievergall E., Blobel C.P., Himanen J.P., Lackmann M., Nikolov D.B. Adam meets Eph: An ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jones T.L., Chong L.D., Kim J., Xu R.-H., Kung H.-F., Daar I.O. Loss of cell adhesion in Xenopus laevis embryo mediated by the cytoplasmic domain of XLerk, an Eph ligand. Proc. Natl. Acad. Sci. 1998;95:576–581. doi: 10.1073/pnas.95.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitori K., Tanaka M., Okuno-Hirasawa T., Kohsaka S. Receptor related to tyrosine kinase RYK regulates cell migration during cortical development. Biochem. Biophys. Res. Commun. 2005;330:446–453. doi: 10.1016/j.bbrc.2005.02.177. [DOI] [PubMed] [Google Scholar]
- Kaushal G.P., Shah S.V. The new kids on the block: ADAMTSs, potentially multifunctional metalloproteinases of the ADAM family. J. Clin. Invest. 2000;105:1335–1337. doi: 10.1172/JCI10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser M.S., McClelland A.C., Hughes E.G., Dalva M.B. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J. Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova I., Nikolova G., Ohara-Imaizumi M., Meda P., Kucera T., Zarbalis K., Wurst W., Nagamatsu S., Lammert E. EphA–Ephrin-A-mediated β cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359–370. doi: 10.1016/j.cell.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kopan R., Ilagan M.X. γ-Secretase: Proteasome of the membrane? Nat. Rev. Mol. Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Koshida S., Kishimoto Y., Ustumi H., Shimizu T., Furutani-Seiki M., Kondoh H., Takada S. Integrinα5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev. Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kucia M., Jankowski K., Reca R., Wysoczynski M., Bandura L., Allendorf D.J., Zhang J., Ratajczak J., Ratajczak M.Z. CXCR4–SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Laird D.W. The life cycle of a connexin: Gap junction formation, removal, and degradation. J. Bioenerg. Biomembr. 1996;28:311–318. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- Li Z., Sheng M. Some assembly required: The development of neuronal synapses. Nat. Rev. Mol. Cell Biol. 2003;4:833–841. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- Litterst C., Georgakopoulos A., Shioi J., Ghersi E., Wisniewski T., Wang R., Ludwig A., Robakis N.K. Ligand binding and calcium influx induce distinct ectodomain/γ-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Sun E., Klein R., Flanagan J. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G-protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Lu W., Yamamoto V., Ortega B., Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Machon O., van den Bout C.J., Backman M., Kemler R., Krauss S. Role of β-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- Marston D.J., Dickinson S., Nobes C.D. Rac-dependent trans-endocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nat. Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- Mellitzer G., Xu Q., Wilkinson D.G. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- Miao H., Burnett E., Kinch M., Simon E., Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Miao H., Strebhardt K., Pasquale E.B., Shen T.L., Guan J.L., Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J. Biol. Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Ruest P.J., Kosinski M., Hanks S.K., Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes & Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody S.A. To differentiate or not to differentiate: Regulation of cell fate decision by being in the right place at the right time. Cell Cycle. 2004;3:564–566. [PubMed] [Google Scholar]
- Moore K.B., Mood K., Daar I.O., Moody S.A. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev. Cell. 2004;6:55–67. doi: 10.1016/s1534-5807(03)00395-2. [DOI] [PubMed] [Google Scholar]
- Murai K.K., Nguyen L.N., Irie F., Yamaguchi Y., Pasquale E.B. Control of hippocampal dendritic spine morphology through ephrin-A3/Eph-A4 signaling. Nat. Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Yamaguchi T., Tokunaga A., Hara A., Hamaguchi T., Kato K., Iwamatsu A., Okano H., Kaibuchi K. Role of numb in dendritic spine development with a Cdc42 GEF intersectin and EphB2. Mol. Biol. Cell. 2006;17:1273–1285. doi: 10.1091/mbc.E05-07-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A., Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes & Dev. 2003;17:1429–1450. doi: 10.1101/gad.1093703. [DOI] [PubMed] [Google Scholar]
- Park E.K., Warner N., Bong Y.S., Stapleton D., Maeda R., Pawson T., Daar I.O. Ectopic EphA4 receptor induces posterior protrusions via FGF signaling in Xenopus embryos. Mol. Biol. Cell. 2004;15:1647–1655. doi: 10.1091/mbc.E03-09-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Penzes P., Beeser A., Chernoff J., Schiller M.R., Eipper B.A., Mains R.E., Huganir R.L. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB–EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Picco V., Hudson C., Yasuo H. Ephrin–Eph signalling drives the asymmetric division of notochord/neural precursors in Ciona embryos. Development. 2007;134:1491–1497. doi: 10.1242/dev.003939. [DOI] [PubMed] [Google Scholar]
- Poliakov A., Cotrina M., Wilkinson D.G. Diverse roles of Eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Prévost N., Woulfe D.S., Tognolini M., Tanaka T., Jian W., Fortna R.R., Jiang H., Brass L.F. Signaling by ephrinB1 and Eph kinases in platelets promotes Rap1 activation, platelet adhesion, and aggregation via effector pathways that do not require phosphorylation of ephrinB1. Blood. 2004;103:1348–1355. doi: 10.1182/blood-2003-06-1781. [DOI] [PubMed] [Google Scholar]
- Prévost N., Woulfe D.S., Jiang H., Stalker T.J., Marchese P., Ruggeri Z.M., Brass L.F. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc. Natl. Acad. Sci. 2005;102:9820–9825. doi: 10.1073/pnas.0404065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P., Myles D.G. The ADAM gene family: Surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- Red-Horse K., Kapidzic M., Zhou Y., Feng K.T., Singh H., Fisher S.J. EPH-B4 regulates chemokine-evoked trophoblast responses: A mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132:4097–4106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- Rougon G., Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu. Rev. Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- Salvucci O., de la Luz Sierra M., Martina J.A., McCormick P.J., Tosato G. Eph-B2 and Eph-B4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling. Blood. 2006;108:2914–2922. doi: 10.1182/blood-2006-05-023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P. Cell–cell signaling during synapse formation in the CNS. Annu. Rev. Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Schmitt A.M., Shi J., Wolf A.M., Lu C.C., King L.A., Zou Y. Wnt–Ryk signalling mediates medial–lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Segura I., Essmann C.L., Weinges S., Acker-Palmer A. Grb4 and GIT1 transduce ephrin-B reverse signals modulating spine morphogenesis and synapse formation. Nat. Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Sharfe N., Freywald A., Toro A., Dadi H., Roifman C. Ephrin stimulation modulates T cell chemotaxis. Eur. J. Immunol. 2002;32:3745–3755. doi: 10.1002/1521-4141(200212)32:12<3745::AID-IMMU3745>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Spooren W., Ballard T., Gasparini F., Amalric M., Mutel V., Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: Behavioural characterization and implications for the treatment of CNS disorders. Behav. Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- Stains J.P., Civitelli R. Gap junctions in skeletal development and function. Biochim. Biophys. Acta. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Suh L.H., Oster S.F., Soehrman S.S., Grenningloh G., Sretavan D.W. L1/Laminin modulation of growth cone response to Eph-B triggers growth pauses and regulates the microtubule destabilizing protein SCG10. J. Neurosci. 2004;24:1976–1986. doi: 10.1523/JNEUROSCI.1670-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu M.A., Dalva M.B., Zigmond R.E., Greenberg M.E. Modulation of NMDA receptor-dependent calcium influx and gene expression through Eph-B receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., Thinakaran G., Iwatsubo T. The role of presenilin cofactors in the γ-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamata R., Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J. Biol. Chem. 2005a;280:42375–42382. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kamata R., Sakai R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell–cell contact formation. EMBO J. 2005b;24:3700–3711. doi: 10.1038/sj.emboj.7600831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Godt D., Winklbauer R. Cell sorting in animal development: Signalling and adhesive mechanisms in the formation of tissue boundaries. Curr. Opin. Genet. Dev. 2002;12:572–582. doi: 10.1016/s0959-437x(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Tolias K.F., Bikoff J.B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R.J., Greenberg M.E. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Tolias K.F., Bikoff J.B., Kane C.G., Tolias C.S., Hu L., Greenberg M.E. The Rac1 guanine nucleotide exchange factor Tiam1 mediates Eph-B receptor-dependent dendritic spine development. Proc. Natl. Acad. Sci. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Iwatsubo T. The inhibition of γ-secretase as a therapeutic approach to Alzheimer’s disease. Drug News Perspect. 2004;17:321–325. doi: 10.1358/dnp.2004.17.5.829036. [DOI] [PubMed] [Google Scholar]
- Tomita T., Tanaka S., Morohashi Y., Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol. Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivier E., Ganessan T.S. RYK, a catalytically inactive receptor tyrosine kinase, associates with Eph-B2 and Eph-B3 but does not interact with AF-6. J. Biol. Chem. 2002;277:23037–23043. doi: 10.1074/jbc.M202486200. [DOI] [PubMed] [Google Scholar]
- Twigg S.R.F., Kan R., Babbs C., Bochukova E.G., Robertson S.P., Wall S.A., Moriss-Kay G.M., Wilkie A.O.M. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary, cause craniofrontonasal syndrome. Proc. Natl. Acad. Sci. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten S.J., Miller M.A. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev. Biol. 2007;301:432–446. doi: 10.1016/j.ydbio.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Wieland I., Jacubiczka S., Muschke P., Cohen M., Thiele H., Gerlach K.L., Adams R.H., Wieacker P. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am. J. Hum. Genet. 2004;74:1209–1215. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winning R.S., Scales J.B., Sargent T.D. Disruption of cell adhesion in Xenopus embryos by Pagliaccio, an Eph-class receptor tyrosine kinase. Dev. Biol. 1996;179:309–319. doi: 10.1006/dbio.1996.0262. [DOI] [PubMed] [Google Scholar]
- Wysoczynski M., Reca R., Ratajczak J., Kucia M., Shirvaikar N., Honczarenko M., Mills M., Wanzeck J., Janowska-Wieczorek A., Ratajczak M.Z. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- Xu Q., Mellitzer G., Robinson V., Wilkinson D.G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Yokote H., Fujita K., Jing X., Sawada T., Liang S., Yao L., Yan X., Zhang Y., Schlessinger J., Sakaguchi K. Trans-activation of Eph-A4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc. Natl. Acad. Sci. 2005;102:18866–18871. doi: 10.1073/pnas.0509741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantek N.D., Azimi M., Fedor-Chaiken M., Wang B., Brackenbury R., Kinch M.S. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- Zhao C., Irie N., Takada Y., Shimoda K., Miyamoto T., Nishiwaki T., Suda T., Matsuo K. Bidirectional ephrinB2–EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zimmer M., Palmer A., Kohler J., Klein R. EphB–ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- Zisch A.H., Stallcup W.B., Chong L.D., Dahlin-Huppe K., Voshol J., Schachner M., Pasquale E.B. Tyrosine phosphorylation of L1 family adhesion molecules: Implication of the Eph kinase Cek5. J. Neurosci. Res. 1997;47:655–665. doi: 10.1002/(sici)1097-4547(19970315)47:6<655::aid-jnr12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokines: A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zou J.X., Wang B., Kalo M.S., Zisch A.H., Pasquale E.B., Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc. Natl. Acad. Sci. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]