Experience tells us that the social and physical environments in which people live and work have a huge effect upon psychological states. The nature of these environments also affects physical and mental health and risk for disease. Yet the scientific study of this important topic has been frustrated and fragmented by disciplinary boundaries between such fields as environmental toxicology, social psychology, sociology, health psychology, economics, epidemiology, psychiatry and medicine. As a result, only some of the considerable knowledge has penetrated, albeit inconsistently, into the mainline of medical teaching and practice, and neuroscience has been largely out of the picture until recently. As a result, a coherent conceptual framework has been missing, because the brain has not been fully recognized as playing a central role in physiological adaptation and the effects of stress, as well as being a target of stress and related behaviors (McEwen, 2007).
Experiences involving social interactions and events in the physical environment are processed by the brain and are usually referred to under the rubric of “stress”. We now know, from animal models, that the brain changes in structure and function with experiences, including those of chronic stress, and that these changes in brain represent “adaptive plasticity”, in that they are largely reversible and appropriate for the conditions that cause them (McEwen, 2007). With the exciting advances in neuroimaging, the living human brain can now be studied in some detail as it responds to stressful experiences during the life course, as well as how its structure and functions are related to physiologic states in the body. This Special Issue of NeuroImage is focused on interactions between the brain, peripheral pathways and body organs since the bi-directional brain-body interactions can be thought of as mediating the relationship between psychological and social factors and physical and mental health.
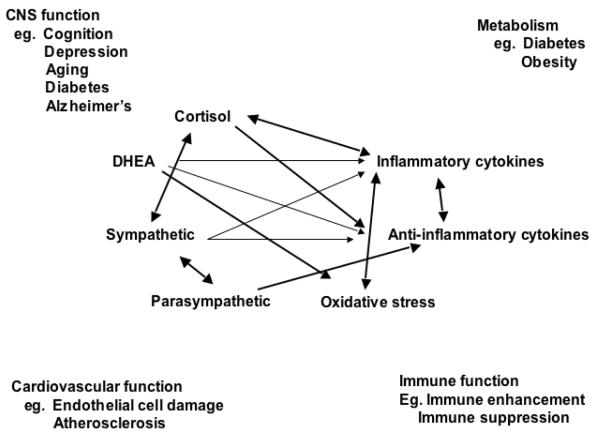
Indeed, the brain is the key organ of the adaptive and maladaptive responses to stress because it determines what is threatening and, therefore, potentially stressful, as well as initiating the behavioral, as well as many of the physiological responses to the stressors, which can be either adaptive or damaging (McEwen, 1998;McEwen, 2007). Stress involves two-way communication between the brain and the cardiovascular, immune and other systems via the autonomic nervous system and via endocrine mechanisms. The mediators of the actions operate in a non-linear manner (Figure 1) (McEwen, 2006). Beyond the “flight or fight” response to acute stress, there are events in daily life, including the individual life style, that produce a type of chronic stress and lead over time to wear and tear on the body (“allostatic overload”). Yet, the hormones and other mediators associated with stress and adaptation protect the body in the short-run and promote adaptation (“allostasis”).
Figure 1.
Non-linear network of mediators of allostasis involved in the stress response. Arrows indicate that each system regulates the others in a reciprocal manner, creating a non-linear network. Moreover, there are multiple pathways for regulation – e.g. inflammatory cytokine production is negatively regulated via anti-inflammatory cytokines, as well as via parasympathetic and glucocorticoid pathways, whereas sympathetic activity increases inflammatory cytokine production. Parasympathetic activity, in turn, contains sympathetic activity. Reprinted from McEwen 2006 by permission.
Animal models have provided insights into how the brain responds to stress (McEwen, 2007). The brain is a target of stress and the hippocampus was the first brain region, besides the hypothalamus, to be recognized as a target of glucocorticoids. Stress and stress hormones produce both adaptive and maladaptive effects on this brain region throughout the lifecourse. Early life events influence lifelong patterns of emotionality and stress responsiveness and alter the rate of brain and body aging. The amygdala and prefrontal cortex, as well as the hippocampus, undergo stress-induced structural remodeling, which alters behavioral and physiological responses, including anxiety, aggression, mental flexibility, memory and other cognitive processes (McEwen, 2007).
Human brain structural imaging has begun to reveal how the human hippocampus changes with experience. Recent evidence includes the relationship of 20 years of elevated perceived stress to reduced hippocampal volume, and how the hippocampus shrinks in disease states such as Cushing's disease, major depression, diabetes and PTSD and pre-disease conditions, such as resulting from chronic jet-lag (Cho, 2001) and elevated circulating inflammatory cytokines. Hippocampal volume is also smaller in both young and older people with low self esteem, accompanied by elevated HPA activity and lack of habituation to repeated stress. On the other hand, physical activity and fitness in elderly subjects is associated with greater hippocampal volume and better memory function, just as is greater activation of prefrontal cortical activity is associated with fitness and regular exercise and leads to better executive function.
The prefrontal cortex, which is reversibly, functionally impaired by increased levels of perceived stress in medical students studying for the board exam, is smaller in major depression and is smaller in people who self-report lower socioeconomic status. Functional activation of the prefrontal cortex is related to blood pressure responses (Gianaros – this issue;) whereas functional activation in the amygdala is related to the negative response to fearful faces which is exaggerated in people with early life adversity. Elevated amygdala functional activity is also related to the development of atherosclerosis.
Animal models teach us that experiences, including stress-induced changes in brain structure are largely reversible and that resilience in both brain structure and behavior is the name of the game in adapting to changing environments (McEwen, 2007). A corollary of this is that failure to show resilience is a feature of maladaptation and pathophysiology, including anxiety and depressive disorders and the downstream effects that these have on the rest of the body via the autonomic, neuroendocrine and immune systems. But how plastic is the human brain in response to interventions that effectively treat disorders that affect the brain as well as the rest of the body? A few studies have shown changes, for example, in functional activity and PFC structure in patients who successfully responded to behavioral therapy treatment for OCD and chronic fatigue, respectively. Another, cross-sectional study reports thicker cortical volume in right anterior insula and prefrontal cortex of subjects who had meditated for many years compared to matched controls. It is well known that, as an adjunct to pharmaceutical therapy, social and behavioral interventions, including regular physical activity and social support, are able to reduce the chronic stress burden and benefit brain and body health and resilience (McEwen, 2007). Therefore, studies of how the brain is changed by behavioral, as well as by pharmaceutical therapies, are important future applications of brain imaging.
Finally, it should be noted that the social and physical environments in which we live are, at least in part, the products of practices and policies of private enterprise and government and these can be changed by changing those policies. Indeed, virtually all of the policies of government and business have powerful effects on health. Indeed, they have a top down effect via the brain on all the physiological systems involved in stress and adaptation (McEwen, 2007). Therefore, monitoring how the brain is affected by such policies is another important future direction of neuroimaging research because animal models can only give clues, but the study of the adaptability of the human brain is the ultimate goal!
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nature Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness. J. Mol. Neurosci. 2004;24:9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JWM, Toni I. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009 doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol. Psychiat. 2008 doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. SCAN1-13. 2007a doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure. Hypertension. 2007b;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007c;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. NeuroReport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol. Psychiat. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and Damaging Effects of Stress Mediators. New England J.Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dial. in Clin. Neurosci.: Stress. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. & Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nature Neurosci. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick RMNK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Jr, Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch. Gen. Psychiat. 1996;53:109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiat. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]