Abstract
To develop effective vaccination strategies againstEhrlichia, we have previously reported developing an animal model of cross-protection in which C57BL/6 mice primed withE. muris were resistant to lethal infection withIxodes ovatus ehrlichia (IOE). Polyclonal antibody produced in mice after priming withE. muris and later injected with IOE-detected antigenic proteins inE. muris and IOE cell lysates. Cross-reaction of antigenic proteins was observed when we probed both theE. muris and IOE cell lysates with IOE andE. muris-specific polyclonal antibody. Analysis of the total proteins ofE. muris and IOE by two dimensional electrophoresis showed that bothE. muris and IOE have the same antigenic proteins. Finally, studies on post-translational protein modifications using a novel technique, Eastern blotting, showed thatE. muris proteins are more lipoylated and glycosylated than those of IOE.
Keywords: antigen, Ehrlichia, ehrlichiosis, IOE, Eastern blotting
INTRODUCTION
The obligate intracellular bacterium Ehrlichia chaffeensis that resides in mononuclear phagocytes is the aetiologic agent of human monocytotropic ehrlichiosis (HME). HME is an emerging, highly prevalent, and often life-threatening tick-transmitted infectious disease in the United States (1,2). Development of murine models of persistent ehrlichiosis has greatly facilitated our understanding of the pathogenesis and mechanisms of host defences against primary ehrlichial infections.
Mildly virulent E. muris infection in immunocompetent C57BL/6 mice results in persistent infection and mimics E. chaffeensis infection in its natural host, white-tailed deer (3), whereas infection of immunocompetent C57BL/6 mice with high dose of a highly virulent Ehrlichia species isolated from Ixodes ovatus ticks (I. ovatus ehrlichia-IOE), mimics severe E. chaffeensis infection in humans (4). Ehrlichia muris and IOE are antigenically and genetically closely related to each other and to E. chaffeensis (5,6). We had previously reported an effective vaccination strategy in a mouse model against fatal ehrlichiosis in which C57BL/6 mice primed with E. muris are cross-protected against lethal infection with IOE (4). Furthermore, we showed that prior infection with E. muris, but not with IOE, provided protection against lethal secondary IOE challenge. Cross-protection against lethal ehrlichial challenge was associated with substantial generation of IFN-γ-producing memory type 1 CD4+ and CD8+ T cells, type 1 cytokine production, the development of a strong anamnestic Ehrlichia-specific antibody response, and persistent E. muris infection. Lack of protection in IOE-primed mice was associated with low frequency of memory type 1 T cells (7).
This study was undertaken to determine which antigens of E. muris and IOE are reactive with polyclonal antibody produced in mice after priming with E. muris and later superinfected with IOE. Subsequently we observed that E. muris- and IOE-specific antibody cross-reacted with IOE and E. muris proteins, respectively. Furthermore, we analysed the total proteins of E. muris and IOE by two dimensional (2D) gel electrophoresis and found that both E. muris and IOE have the same antigenic proteins, but the level of protein modifications was more extensive in E. muris than in IOE.
MATERIALS AND METHODS
Bacterial culture
Two monocytotropic ehrlichial strains were used in this study, highly virulent Ehrlichiaspp. (designated IOE) isolated from I. ovatus ticks (a gift from Dr M. Kawahara, Nagoya City Public Health Research Institute, Nagoya, Japan) and mildly virulent E. muris (provided by Dr Y. Rikihisa, Ohio State University, Columbus, OH). Ehrlichia muris was cultivated in DH82 cells at 37°C in DMEM supplemented with 5% heat inactivated bovine calf serum. Ehrlichiae were harvested when approximately 90–100% of the cells were infected. To produce infectious stocks for reproducible studies, C57BL/6 mice were inoculated i.p. with 1 mL of a 10−1 dilution (5 × 108 E. muris) of the frozen stock. On day 7 after inoculation, the mice were sacrificed, the spleens were harvested, and splenic homogenate was prepared and suspended in DMEM medium. After centrifugation at 11 000 g the cells were suspended in PBS. The total protein concentrations of the resulting bacterial preparations were determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). DH82 cells or uninfected mouse spleen was used as the negative control.
Antibodies
For polyclonal antibody production E. muris (from infected mouse spleen) was inoculated intraperitoneally into mice and the blood collected on day 45 after the first injection. To generate IOE-specific antibodies we inoculated sublethal doses of IOE at 2 week intervals, and serum was collected after 30 days. For E. muris/IOE antibody, mice primed with E. muris were infected with IOE on day 30 and the blood collected on day 75 after primary infection.
Western immunoblots
Total cell lysate from uninfected spleen, spleen infected with E. muris and IOE were loaded on to 4–12% Bis–Tris gel (Invitrogen) and the proteins transferred to a nitrocellulose membrane. The membranes were probed with polyclonal sera against E. muris, IOE and E. muris/IOE (1 : 100 dilution), followed by incubation with goat antimouse alkaline phosphatase (AP). The bands were detected by a chemiluminescence substrate (KPL, MD).
2D electrophoresis
For 2D gel electrophoresis, we used an IEF strip (pH 4–7) (NuPage, Invitrogen, CA), followed by separation in a 4–12% Bis-Tris gel. The gels were run according to the protocol of the manufacturers.
Probes for detecting antigenic protein modification
For detecting lipids, blots were probed with Cholera Toxin B subunit (10 µg/mL) (Sigma, MO), followed by probing with rabbit anti CTB (1 : 5000) and goat antirabbit AP (1 : 1000).
Glucosyl residues were detected using wheat germ agglutinin (WGA) and Con-A. WGA binds Gal NAcα1-ser/thr glycoproteins, whereas Con-A binds glucosyl and mannosyl residues. Biotinylated WGA (0·05 µg/mL) or Con-A (0·005 µg/mL) (Vector Laboratories, CA) was diluted in PBS-BSA3%-Tween 20 and the membrane probed for one hour at room temperature, followed by incubating with Streptavidin-HRP (1 : 15 000). Positive spots were detected by a chemiluminescence substrate (Millipore, MA).
Phosphorylated proteins were detected using the gelcode phosphoprotein detection kit (Pierce-Thermofisher, IL) following manufacturers instruction.
RESULTS
Cross-reaction of Ehrlichia antibodies
Western blot of one dimensional gel electrophoresis showed that the polyclonal E. muris/IOE antibody detected antigenic proteins in both E. muris and IOE cell lysates. The predominant antigens were the 60 and 28 kDa proteins. We then explored if the E. muris antibody cross-reacted with the IOE proteins. The E. muris polyclonal antibody cross-reacted with IOE proteins; similarly, the E. muris antigens cross-reacted with the IOE specific antibody (Figure 1). Since the sensitivity of the IOE antibody was less compared to E. muris or E. muris/IOE polyclonal antibody, we excluded it from further studies. All the three antibodies also detected the antigenic proteins in E. muris-infected DH82 cells (Figure 2).
Figure 1.
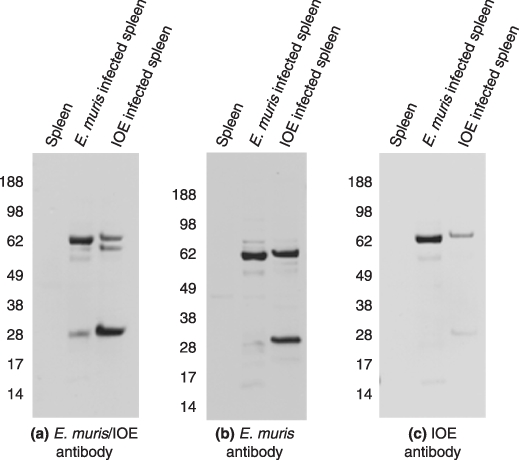
Western blot of one dimensional gel electrophoresis probed with polyclonal antibodies against (a) E. muris/IOE (b) E. muris and (c) IOE (1 : 100). Five micrograms of cell lysate from mouse spleen, spleen infected with E. muris or IOE was used in the study. Representative images based on three independent experiments.
Figure 2.
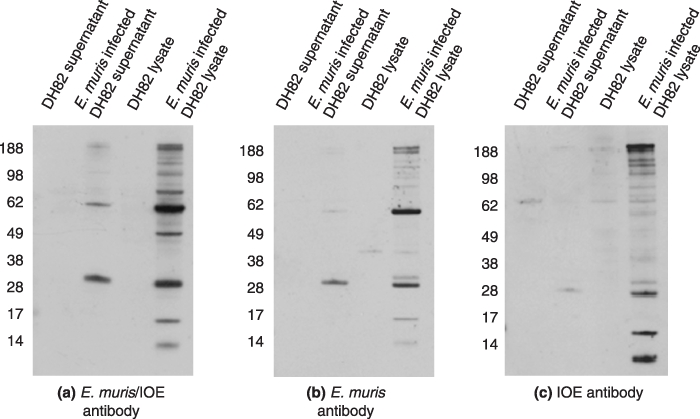
Western blot of one dimensional gel electrophoresis probed with polyclonal antibodies against (a) E. muris/IOE (b) E. muris and (c) IOE (1 : 100). Five micrograms of cell lysate from supernatant of DH82 cell line, supernatant of DH82 cell line infected with E. muris, DH82 cell lysate and E. muris infected DH82 cell lysate were used in the study. Representative images based on three independent experiments.
Coomassie staining of the 2D PAGE gel showed that E. muris has more proteins detected than IOE or the uninfected spleen (Figure 3). Both the E. muris/IOE and E. muris polyclonal antibody detected the E. muris and IOE antigenic proteins (Figure 4). The polyclonal antibodies did not detect any antigen in uninfected spleen (data not shown). There was an increase in detection of p28 protein expression in IOE compared to E. muris when probed with the E. muris/IOE polyclonal antibody; whereas the 60 kDa protein was highly expressed in E. muris.
Figure 3.
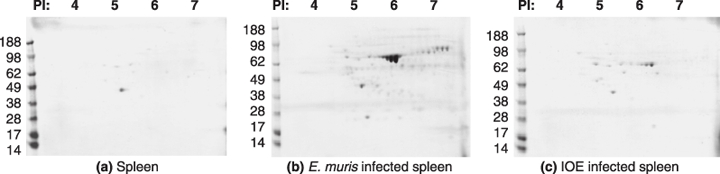
Two dimensional gel of (a) uninfected spleen (b) spleen infected with E. muris, and (c) spleen infected with IOE stained with Coomassie blue. Fifty micrograms of protein was used to run the first dimensional gel. Representative images based on three independent experiments.
Figure 4.
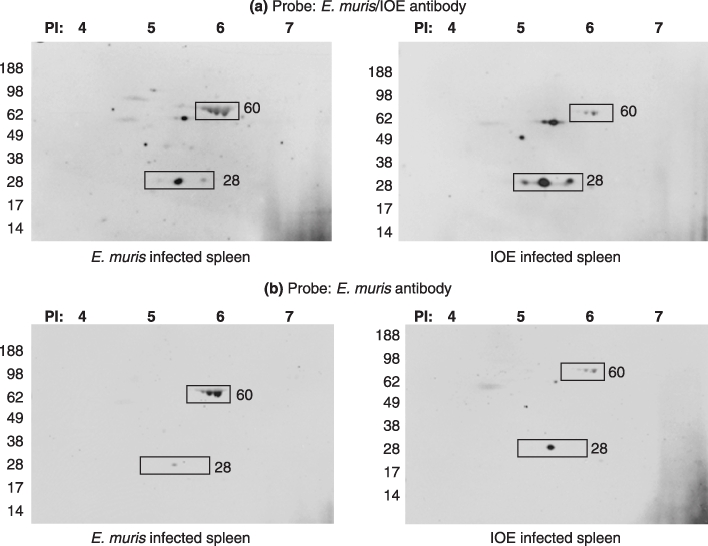
Western blot of two dimensional gels (E. muris infected spleen and IOE infected spleen) probed with polyclonal antibodies against (a) E. muris/IOE and (b) E. muris. Fifty micrograms of protein was used to run the first dimensional gel. The proteins of interest are marked in the image (rectangle).
Detection of protein modifications (Eastern blotting) in antigenic proteins of Ehrlichia muris and IOE
It is estimated that 50–90% of proteins are post-translationally modified. These modifications are necessary for the biological functions of a vast array of proteins (8). Studies have suggested important roles for post-translational modifications in a variety of effector-cell functions, including antigen processing, signal transduction and the expression of cytokines and chemokines (9,10). Since the immune recognition of and activation by E. muris and IOE are different, we probed for post-translational modifications in the proteins of E. muris and IOE.
Cholera toxin is used for the detection of lipids and cholesterol (11,12). We probed the E. muris and IOE from infected spleen with cholera toxin B subunit (CTB). The CTB bound to the 60 kDa protein in both E. muris and IOE (Figure 5). No spots were observed in the uninfected spleen (data not shown). The 60 kDa protein of E. muris was more lipoylated than that of IOE. Lipoproteins are known to induce innate and humoral responses (13), and removal of the lipid moiety from bacterial lipoproteins renders them nonfunctional (14,15).
Figure 5.
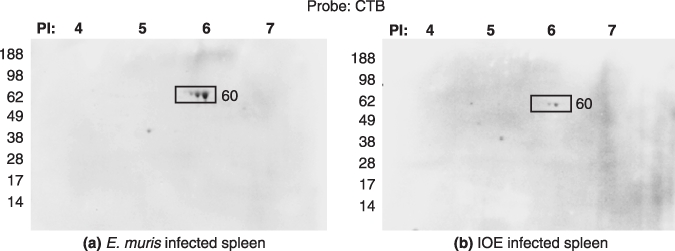
Two dimensional gels of (a) E. muris infected spleen and (b) IOE infected spleen probed with Cholera Toxin B subunit for the detection of lipids. Fifty micrograms of protein was used to run the first dimensional gel. Representative images based on three independent experiments. The proteins of interest are shown in boxes.
WGA a lectin, primarily reacts with N-acetylglucosamine (23 O-glycans), whereas Con-A binds to mannosyl and glucosyl residues and both are useful for the detection of glycoproteins. When E. muris and IOE were probed with WGA, the probe detected glycoproteins at PI 7·0; whereas, on probing for glycoproteins using Con-A we found that it detected the 60 kDa protein in E. muris but not in IOE or the uninfected spleen (Figure 6). Thus in our experiments Con-A was found to be more sensitive for detection of the antigenic glycoprotein. Glycoproteins are known to modulate the immune system and many workers have utilized that property in the production of vaccines (16,17).
Figure 6.

Two dimensional gels of (I) uninfected spleen (II) E. muris-infected spleen and (III) IOE infected spleen probed with (a) wheat germ agglutinin and (b) Con-A for the detection of glucose moieties. For the first dimension 50 µg protein was used to run gel. The protein of interest is marked in the image.
Knowledge about covalent modifications and their regulation is essential for the understanding of protein function. Regulation of protein activity is often modulated by reversible phosphorylation, and information about specific sites of phosphorylation is vital for understanding cellular signalling pathways. Two-dimensional gel electrophoresis is still the most common method used for detecting large-scale changes in phosphorylation (18). We probed E. muris and IOE in 2D gels for phosphorylated proteins and found that both the 60 kDa and 28 kDa proteins of both the species are phosphorylated. Overall, the E. muris antigenic proteins were more phosphorylated than those of IOE (Figure 7).
Figure 7.
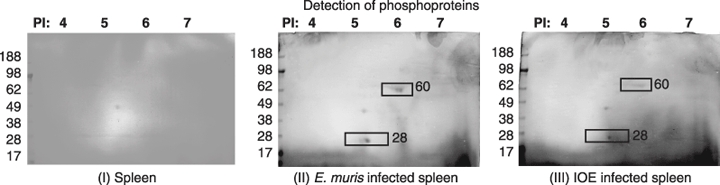
Two dimensional gels of (I) uninfected spleen (II) E. muris infected spleen and (III) IOE-infected spleen probed for phosphoproteins. Fifty micrograms of protein was used to run the first dimensional gel. The images are the representative of five independent experiments. The proteins of interest are marked in the images (rectangle).
The above results show that the 60 kDa antigenic protein of E. muris is more post-translationally modified than the IOE counterpart. Collectively, our data show that the 60 kDa E. muris antigenic protein is a phospho-lipo-glycoprotein, whereas in IOE this antigenic protein is a phospho-lipoprotein.
DISCUSSION
HME manifests as a flu-like illness that can progress in severity to resemble toxic shock-like syndrome, with meningoencephalitis or adult respiratory distress syndrome, and half of patients require hospitalization (1). Currently there are no vaccines against ehrlichial infections. Previous studies in our laboratory in a mouse model have shown that IOE infected mice die within 7–12 days, whereas E. muris infected mice are resistant to lethal infection with IOE. In this study we showed that antibodies to both the avirulent E. muris and virulent IOE cross-reacted reciprocally with the other ehrlichiae and E. muris antigenic proteins had greater post-translation modifications viz., lipoylation, glycosylation and phosphorylation compared to the IOE proteins.
16S rRNA sequences and partial GroEL amino acid sequence of IOE showed the extremely close genetic relatedness of the Anan strain, E. chaffeensis, and E. muris (19). The 60 kDa protein detected by the E. muris and IOE antibody corresponds to GroEL (heat shock protein) (20) and the 28 kDa protein corresponds to the p28 family (21,22). The groEL genes, which encode the 60-kDa heat shock protein GroEL, are ubiquitous in both prokaryotes and eukaryotes and encode highly conserved housekeeping proteins which are essential for the survival of these cells (23). In our studies E. muris and IOE polyclonal antibodies detected IOE and E. muris antigens; hence we presume that both the 60 kDa heat shock protein and p28 are homologous proteins in both the species of Ehrlichia.
The technique of gel blotting is useful for visualizing a particular subset of macromolecules – proteins (Western blot), fragments of DNA (Southern blot) or RNA (Northern blot). Since protein modifications are important for signal transduction and it is known that post-translational protein modifications (modified by lipid, glucose or phosphate groups) are immunomodulators and have practical applications for vaccine development we name the technique of detecting protein modifications as Eastern blotting. For our studies we used cholera toxin, Con-A and nitrophospho–molybdate complex for the detection of lipid, glucose and phosphate residues, respectively, on the native proteins of Ehrlichia. In this article we showed that the Eastern blotting technique could detect protein modifications in the 60 and 28 kDa proteins of E. muris and IOE. The technique shows that among the Ehrlichia species E. muris proteins have more post-translational modifications than IOE.
The technique of far-Eastern blotting was developed by Ishikawa and Taki (24) as a method for transferring lipids from an HPTLC plate to a PVDF membrane and later probed with antibodies. The technique was also used by Fukuda et al. (25) to separate ginsenosides by TLC and blotted to a PVDF membrane treated with NaIO4 solution followed by bovine serum albumin (BSA) which resulted in a ginsenoside–BSA conjugate on the PVDF membrane. The blotted spots were finally stained by antiginsenoside monoclonal antibodies. Though the technique used is the same far-eastern technique described by Ishikawa and Taki (24) the authors used the term Eastern blot for their studies. Our technique of Eastern blotting is a simpler technique that proteins blotted from the SDS-PAGE gel to a PVDF or nitrocellulose membrane are analysed for post-translational protein modifications using probes specifically designed to detect lipids, carbohydrate or phospho moieties.
Bacterial lipopolysaccharides (LPS), peptidoglycan, and lipoproteins contribute to pathogenesis by inducing proinflammatory cytokines leading to inflammation and stimulating innate immunity to confer initial host resistance to pathogens (26). The innate immune responses influence the nature of subsequent acquired immune responses, thereby, in combination with inflammation, ultimately affecting host morbidity and mortality. Ehrlichia lacks genes for cholesterol biosynthesis (27). A recent study by Huang et al. (28) demonstrated that multiple lipoproteins were expressed by E. chaffeensis in cell culture. The inhibition of E. chaffeensis infection by globomycin (28) or methyl-β-cyclodextrin (27) treatment suggests that lipoproteins are required for E. chaffeensis infection of host cells. Lipoproteins are the first components of E. chaffeensis shown to induce delayed type hypersensitivity reactions. Ehrlichia chaffeensis lipoproteins also induce delayed type hypersensitivity reactions in dogs. Thus, lipoproteins may be considered as potential candidates for a vaccine against HME.
Many bacterial heat shock proteins (GroEL) are immunogenic and are used as vaccine (29,30). GroEL is the predominant antigenic protein in both E. muris and IOE and our studies have shown that the protein is phospho-lipo-glycosylated, hence we hypothesize that GroEL is a potential vaccine candidate.
We had reported in previous published articles about the presence of gp140 (5) and gp153 in E. canis (31) and gp120 in E. chaffeensis (5). We did not detect glycoproteins corresponding to these proteins probably due to species specificity. In addition to the glycosylated 60 kDa we detected glycoproteins of 70 kDa in both E. muris and IOE at a PI of 7·0.
Singu et al. (20) demonstrated that the p28 outer membrane protein of E. chaffeensis is both phosphorylated and glycosylated, whereas in our studies the 28 kDa protein was found to be only phosphorylated in both E. muris and IOE.
As recombinant proteins are post-translationally modified by the host bacterium (32) we presume that the technique of Eastern blotting will be able to distinguish protein modifications in recombinant and native proteins. Previous studies have shown that post-translational modifications have the ability to mask epitopes, potentially representing a strategy that pathogens have evolved for their survival in the host (8). As protein post-translational modifications are involved in immune modulation we presume that post-translational modifications observed in both E. muris and IOE explain the pathogenecity in mouse model of ehrlichiosis.
Acknowledgments
This research was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI031431).
REFERENCES
- 1.Walker DH. Ehrlichia under our noses and no one notices. Arch Virol Suppl. 2005;19:147–156. doi: 10.1007/3-211-29981-5_12. [DOI] [PubMed] [Google Scholar]
- 2.Walker DH, Ismail N, Olano JP, McBride JW, Yu XJ, Feng HM. 2004 Ehrlichia chaffeensis: a prevalent, life-threatening, emerging pathogen. Trans Am Clin Climatol Assoc. 2004;115:375–384. [PMC free article] [PubMed] [Google Scholar]
- 3.Olano JP, Wen G, Feng HM, McBride JW, Walker DH. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am J Pathol. 2004;165:997–1006. doi: 10.1016/S0002-9440(10)63361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ismail N, Soong L, McBride JW, et al. Overproduction of TNF-α by CD8+ type 1 cells and down-regulation of IFN-γ production by CD4+ Th1 cells contribute to toxic shock–like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J Immunol. 2004;172:1786–1800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- 5.McBride JW, Yu XJ, Walker DH. Glycosylation of homologous immunodominant proteins of Ehrlichia chaffeensis and Ehrlichia canis. Infect Immun. 2000;68:13–18. doi: 10.1128/iai.68.1.13-18.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu XJ, Zhang XF, McBride JW, Zhang Y, Walker DH. Phylogenetic relationships of Anaplasma marginale and ‘Ehrlichia platys’ to other Ehrlichia species determined by GroEL amino acid sequences. Int J Syst Evol Microbiol. 2001;51:1143–1146. doi: 10.1099/00207713-51-3-1143. [DOI] [PubMed] [Google Scholar]
- 7.Thirumalapura NR, Stevenson HL, Walker DH, Ismail N. Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge. Infect Immun. 2008;76:1920–1930. doi: 10.1128/IAI.01293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–449. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 9.Campbell KS. Signal transduction from the B-cell antigen receptor. Curr Opin Immunol. 1999;11:256–264. doi: 10.1016/s0952-7915(99)80042-9. [DOI] [PubMed] [Google Scholar]
- 10.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 11.Thomas S, Kumar R, Casares S, Brumeanu TD. Sensitive detection of GM1–lipid rafts and TCR partitioning in the T cell membrane. J Immunol Meth. 2003;275:161–168. doi: 10.1016/s0022-1759(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 12.Thomas S, Preda-Pais A, Casares S, Brumeanu TD. Analysis of lipid rafts in T cells. Mol Immunol. 2004;41:399–409. doi: 10.1016/j.molimm.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Schröder NW, Eckert J, Stübs G, Schumann RR. Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology. 2008;213:329–340. doi: 10.1016/j.imbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Bessler W, Resch K, Hancock E, Hantke K. Induction of lymphocyte proliferation and membrane changes by lipopeptide derivatives of the lipoprotein from the outer membrane of Escherichia coli. Z. Immunitatsforsch Immunobiol. 1977;153:11–22. [PubMed] [Google Scholar]
- 15.Erdile LF, Brandt MA, Warakomski DJ, et al. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993;61:81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna RV, Riding GA, Jarmey JM, Pearson RD, Willadsen P. Vaccination of cattle against the Boophilus microplus using a mucin-like membrane glycoprotein. Parasite Immunol. 1998;20:325–336. doi: 10.1046/j.1365-3024.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 17.Warfield KL, Bosio CM, Welcher BC, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci USA. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sickmann A, Meyer HE. Phosphoamino acid analysis. Proteomics. 2001;1:200–206. doi: 10.1002/1615-9861(200102)1:2<200::AID-PROT200>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S, Kawahara M, Rikihisa Y, et al. New Ehrlichia species closely related to Ehrlichia chaffeensisisolated from Ixodes ovatus ticks in Japan. J Clin Microbiol. 2000;38:1331–1338. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singu V, Liu H, Cheng C, Ganta RR. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect Immun. 2005;73:79–87. doi: 10.1128/IAI.73.1.79-87.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crocquet-Valdes PA, McBride JW, Feng HM, et al. Analysis of ehrlichial p28 gene expression in a murine model of persistent infection. Ann NY Acad Sci. 2005;1063:420–424. doi: 10.1196/annals.1355.076. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JZ, Popov VL, Gao S, Walker DH, Yu XJ. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell Microbiol. 2007;9:610–618. doi: 10.1111/j.1462-5822.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Park HS, Jang WJ, et al. Differentiation of rickettsiae by groEL gene analysis. J Clin Microbiol. 2003;41:2952–2960. doi: 10.1128/JCM.41.7.2952-2960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa D, Taki T. Micro-scale analysis of lipids by far-eastern blot (TLC blot) Nihon Yukagaku Kaishi. 1998;47:963–970. [Google Scholar]
- 25.Fukuda N, Shan S, Tanaka H, Shoyama Y. New staining methodology: Eastern blotting for glycosides in the field of Kampo medicines. J Nat Med. 2006;60:21–27. [Google Scholar]
- 26.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 27.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Lin M, Wang X, et al. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect Immun. 2008;76:3405–3414. doi: 10.1128/IAI.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BY, Horwitz MA, Clemens DL. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect Immun. 2006;74:4002–4013. doi: 10.1128/IAI.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paliwal PK, Bansal A, Sagi SS, Mustoori S, Govindaswamy I. Cloning, expression and characterization of heat shock protein 60 (groEL) of Salmonella enterica serovar Typhi and its role in protective immunity against lethal Salmonella infection in mice. Clin Immunol. 2008;126:89–96. doi: 10.1016/j.clim.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 31.McBride JW, Comer JE, Walker DH. Novel immunoreactive glycoprotein orthologs of Ehrlichia spp. Ann NY Acad Sci. 2003;990:678–684. doi: 10.1111/j.1749-6632.2003.tb07443.x. [DOI] [PubMed] [Google Scholar]
- 32.Giersing B, Miura K, Shimp R, et al. Posttranslational modification of recombinant Plasmodium falciparum apical membrane antigen 1: impact on functional immune responses to a malaria vaccine candidate. Infect Immun. 2005;73:3963–3970. doi: 10.1128/IAI.73.7.3963-3970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


