Abstract
During bacterial infection, the bone marrow hematopoietic activity shifts toward granulocyte production, which is critical for host defenses. Along with this enhancement of granulopoiesis, the bone marrow also increases its release of hematopoietic precursors. At the present time, little is known about the commitment of hematopoietic precursor cells including hematopoietic stem cells and progenitors in this response. To investigate the hematopoietic precursor cell response to bacterial infection, bacteremia was established in Balb/c mice by intravenous injection of Escherichia coli. Bacteremia caused a 10-fold increase in the number of lineage (lin)-c-kit+Sca-1+ cells in the bone marrow. This dramatic expansion of the lin-c-kit+Sca-1+ cell pool resulted from both increased mitosis of these cells and inversion from lin-c-kit+Sca-1- cell phenotype. Lipopolysaccharide, tumor necrosis factor-α, and interleukin-6 were potent factors capable of mediating phenotypic inversion of lin-c-kit+Sca-1- cells. Cells in the expanded lin-c-kit+Sca-1+ cell pool contained an increased number of colony-forming unit-granulocyte/macrophage (CFU-GM). Mobilization of lin-c-kit+Sca-1+ cells into the circulation was significantly enhanced following bacteremia. These results demonstrate that the lin-c-kit+Sca-1+ cell population in the bone marrow constitutes a key component of the host defense response to bacteremia. Functional modifications of these primitive hematopoietic precursors are critical for enhancing granulocyte production following bacterial infection.
Keywords: Mouse, Stem/progenitor Cells, Granulopoiesis, Hematopoiesis, Bone Marrow, Immunity
Introduction
Hematopoietic stem cells are functionally defined by their unique capacity of self-renewal and differentiation into all types of mature blood cells [1]. Under normal conditions, the process of hematopoietic stem cell self-renewal, as well as their conversion into lineage-committed progenitors, is tightly controlled in order to maintain daily blood cell production [1, 2]. Accumulated evidence has shown that the equilibrium of bone marrow hematopoiesis is altered during bacterial infection whereby production of phagocytes, particularly granulocytes and monocytes, becomes predominant with inhibition of other lineage (lymphoid and erythroid) development [3-6]. Studies have shown that in response to bacterial infection, bone marrow generation of granulocytes or polymorphonuclear leukocytes (PMNs) from their precursors is accelerated [7]. The transit time of PMNs through the marrow mitotic (or proliferative) and postmitotic (maturation-storage) pools to blood is significantly shortened in both experimental animals and hospitalized patients with bacterial infections [8, 9]. Since PMNs constitute the first line of phagocytic defense in the systemic circulation, this enhancement of granulopoietic activity is critically important. Failure to develop an adequate granulopoietic response to infection results in increased morbidity and mortality [10, 11]. Despite the biological significance of changes in hematopoiesis during bacterial infection, relatively little is known about the commitment of the up-stream hematopoietic precursor cells including hematopoietic stem cells in this response.
During bacterial infection, the infected tissues produce large amounts of cytokines including granulocyte colony-stimulating factor (G-CSF) and CXC chemokines [interleukin-8 (IL-8) in humans and keratinocyte-derived chemokine (KC) in mice] [12-15]. These mediators are potent stimulants for mobilization of hematopoietic precursor cells from the bone marrow into the systemic circulation [16-18]. The mobilized hematopoietic precursor cells have been shown to play a significant role in the process of tissue repair [19-21]. At the present time, knowledge concerning the release of hematopoietic precursor cells from the bone marrow in relation to changes in the marrow pool of these cells as well as its effects on granulopoiesis during bacterial infection remains limited.
In this study, we investigated the hematopoietic precursor cell response to bacterial infection using a murine model of intravenous (i.v.) challenge with Escherichia coli (E. coli). Previous studies have shown that C57BL/6 mouse bone marrow lineage (lin)-c-kit+Sca-1+ cells are highly purified (or enriched) hematopoietic stem cells, while lin-c-kit+Sca-1- cells contain more committed progenitors [22, 23]. Similarly, in Balb/c and FVB/N mouse strains, CFU-spleen (CFU-S) cells express high levels of the Sca-1 antigen [24]. C57BL/6 mice express the Ly6b (Ly6A) isoform of Sca-1, whereas Balb/c mice possess the Ly6a (Ly6E) haplotype. The difference between the native Ly6A and Ly6E antigens consists of two amino acid differences [25]. In contrast to human hematopoietic stem cells that are highly enriched in CD34+ cell population in the bone marrow, mouse long-term repopulating hematopoietic stem cells are CD34 low/negative [26]. Our current observations show that the bone marrow lin-c-kit+Sca-1+ cell population is rapidly expanded following E. coli bacteremia in Balb/c mice. This increase in the number of marrow lin-c-kit+Sca-1+ cells results from both mitosis of these cells and phenotypic inversion from the lin-c-kit+Sca-1- cell population. Cells in the expanded marrow lin-c-kit+Sca-1+ cell pool are functionally activated for CFU-GM formation. Mobilization of lin-c-kit+Sca-1+ cells into the circulation was significantly enhanced from 12 to 48 h after initiation of bacteremia.
Materials and Methods
Animals
Male Balb/c mice (7 to 10 weeks old; Charles River, Wilmington, MA) with a body weight of 26.8±0.3 g were maintained on a standard laboratory diet and were housed in a controlled environment with a 12-h light/dark cycle. Intravenous challenge with live E. coli (E11775 from the American Type Culture Collection, Rockville, MD; ∼1 × 106 CFUs in 50 μl of pyrogen-free saline/mouse) or recombinant murine granulocyte colony-stimulating factor (G-CSF, 50 μg/Kg bodyweight in 50 μl of 5% dextrose, Amgen, Thousand Oaks, CA) was given to mice via penile vein injection under isoflurane anesthesia. Control mice were injected with an equal volume of vehicle. The animals were sacrificed at selected time points thereafter as indicated in the figure legend of each figure in the results. In a subgroup of animals, 5-bromo-2-deoxyuridine [BrdU, 1 mg in 100 μl of phosphate-buffered saline (PBS)/mouse, BD PharMingen, San Diego, CA] was i.v. administered along with the bacterial challenge. Upon sacrifice, a heparinized blood sample was obtained by cardiac puncture. Plasma was separated and stored at -80°C. Peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte-Mammal density separation medium (Cedarlane, Homby, Ontario, Canada) and protocols provided by the manufacturer. Femurs and tibias were collected and bone marrow cells were flushed out with a total volume of 2 ml PBS containing 2% bovine serum albumin (BSA, HyClone Laboratories, Logan, UT) through a 23-gauge needle. Bone marrow cells were filtered through a 70 micron nylon mesh (Sefar America Inc. Kansas City, MO). Erythrocytes in isolated PBMCs and bone marrow cell samples were lysed with Purescript® RBC lysis solution (Gentra Systems, Valencia, CA). After washing twice with PBS containing 2% BSA, the remaining nucleated cells were quantified under a light microscope with a hemacytometer. The experiments described here were performed in adherence to the National Institutes of Health guidelines on the use of experimental animals. Approval of the Animal Care and Use Committee of the Louisiana State University Health Sciences Center was obtained prior to initiating these experiments.
Flow cytometric analysis
Nucleated bone marrow cells or isolated PBMCs suspended in RPMI-1640 (Invitrogen, Grand Island, NY) containing 2% fetal calf serum (2 × 106 cells in 100 μl medium) were added with a mixed panel of biotinylated anti-mouse lineage markers [10 μg/mL of each antibody against CD3e (clone 145-2C11), CD45R/B220 (clone RA3-6B2), CD11b/CD18 (Mac-1, clone M1/70), Gr-1 (Ly-6G/Ly-6C, clone RB6-8C5), or TER 119 (clone TER-119)] or isotype control antibodies (clones A19-3, R35-95, A95-1) (BD PharMingen, San Diego, CA). Following incubation for 15 min at 4°C, PE-conjugated streptavidin (10 μg/mL) and 10 μg/mL of each fluorchrome conjugated anti-mouse c-kit (CD117, clone 2B8), anti-mouse Sca-1 (Ly-6A/E, clone D7), and anti-mouse CD34 (clone RAM34) (BD PharMingen, San Diego, CA), or the matched isotype control antibodies (clones A95-1 and R35-95) were added into the incubation system. The samples were further incubated in the dark for 15 min at 4°C. The cells were then washed with cold PBS. For measuring BrdU incorporation, the cells were further processed using a BD BrdU Flow Kit (BD PharMingen, San Diego, CA). At the end of the staining procedure, cells were suspended in 0.5 ml of PBS containing 1% paraformaldehyde. Analysis of cell phenotypes and BrdU incorporation was performed on a FACSAria™ or a SLR-II flow cytometer with FACSDiva software (Becton Dickinson, San Jose, CA). In each sample, 500,000 cells were acquired for analysis.
Sorting of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells
Pooled nucleated bone marrow cells or PBMCs were suspended in StemSpan serum-free medium (StemCell Technologies, Vancouver, BC, Canada). Staining procedure for cell surface markers was similar as described above except that the amount of antibodies used for each sample was increased proportionally. Sorting of bone marrow or circulating lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells was performed on the FACSAria™ flow cytometer with FACSDiva software. The purity of sorted cell population was 97-100%.
In vitro culture of bone marrow lin-c-kit+Sca-1- cells
Sorted marrow lin-c-kit+Sca1- cells from normal mice were plated into a 96-well tissue culture plate with 5 × 104 cells per well in a total volume of 100 μl StemSpan serum-free medium. The cells were stimulated with the following treatments: (a) 50% plasma from mice which received i.v. saline for 6 h; (b) 50% plasma from mice which received i.v. E. coli for 6 h; (c) 15 ng of murine G-CSF (Amgen, Thousand Oaks, CA); (d) a cocktail of growth factors [1000 ng of heparin, 1 ng of murine stem cell factor (SCF), 2 ng of murine thrombopoietin (TPO), 2 ng of murine insulin-like growth factor-II (IGF-II), 1 ng of human fibroblast growth factor-1 (FGF-1) (R&D Systems Inc., Minneapolis, MN), and 15 ng of murine G-CSF]; (e) 10 μg of lipopolysaccharide (LPS, E. coli 0111:B4, List Biological Laboratories, Inc., Campbell, CA); (f) 10 ng of murine tumor necrosis factor-α (TNF-α, Biosource International, Inc., Camarillo, CA); (g) 10 ng of murine interleukin-6 (IL-6, Biosource International, Inc., Camarillo, CA); (h) 25 ng (∼200 U) of murine interferon-γ (IFN-γ, Biosource International, Inc., Camarillo, CA); and (i) combination of 10 ng murine TNF-α, 10 ng murine IL-6, and 25 ng murine IFN-γ. Cells in control wells were not exposed to any stimulants. The cells were incubated at 37°C in an atmosphere of 5% CO2 for 24 h. At the end of culture, cells are stained with Fluorchrome-conjugated anti-mouse c-kit and anti-mouse Sca-1 antibodies. Flow cytometric analysis of live (propidium iodide negative) cells was conducted on a LSR-II™ flow cytometer with FACSDiva software (Becton Dickinson, San Jose, CA).
Colony forming unit (CFU) assay
CFU assays of freshly sorted bone marrow or circulating lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells as well as lin-c-kit+Sca-1+ cells derived from inversion of marrow lin-c-kit+Sca-1- cells following in vitro culture for 24 h with TNF-α (100 ng/ml) or IFN-γ (250 ng/ml) were performed by culturing the cells on Methocult GF M3434 and Methocult GF M3534 media (StemCell Technologies, Vancouver, BC, Canada), respectively. One milliliter of Methocult GF M3434 or Methocult GF M3534 media containing 100 of bone marrow lin-c-kit+Sca-1+ or lin-c-kit+Sca-1- cells or 30 circulating lin-c-kit+Sca-1+ or lin-c-kit+Sca-1- cells was plated to a 35 mm Nunclon™ dish (Nunc, Rodkilde, Denmark). The cultures were conducted for seven days at 37°C in an atmosphere of 5% CO2. Colonies containing 50 or more cells were then enumerated.
Luminex assay and ELISA measurement of plasma cytokines
Plasma concentrations of cytokines including TNF-α, IFN-γ, IL-1α, IL-1β, IL-3, IL-6, G-CSF, granulocyte macrophage colony-stimulating factor (GM-CSF), KC, FGF, and vascular endothelial growth factor (VEGF) were measured using a Mouse Cytokine Twenty-Plex kit (Biosource International, Camarillo, CA). All luminex assays were performed on the Bio-Plex™ Protein Array System (Bio-Rad Laboratories, Hercules, CA). Plasma concentrations of SCF, TPO, and IGF-II were measured using the Quantikine Mouse SCF and TPO ELISA Kits and the DuoSet Mouse IGF-II ELISA Kit (R&D Systems Inc., Minneapolis, MN).
Statistical analysis
Data are presented as mean ± SEM. The sample size is indicated in the legend of each figure. Statistical analyses of data were conducted using unpaired Student t test (for comparison between two groups) or one-way analysis of variance followed by Student-Newman-Keuls test (for comparisons among multiple groups). Differences were considered statistically significant at P < 0.05.
Results
Alteration of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cell pools
In order to examine changes in the bone marrow hematopoietic precursor cell populations following systemic infection with E. coli, bone marrow cells were analyzed by flow cytometry based on their phenotypic surface markers. As shown in Figure 1, the lin-c-kit+Sca-1+ cell population in the bone marrow of control mice was very small. Similar observations have been reported in C57BL/6 mice previously [22]. However, the number of lin-c-kit+Sca-1+ cells in the bone marrow was markedly increased following E. coli infection. At 12 h post i.v. E. coli, the lin-c-kit+Sca-1+ cell population was increased 7 fold in the bone marrow. Between 24 to 48 h after the infection, the bone marrow lin-c-kit+Sca-1+ cell population was further expanded to approximately 10 times of the control values. The changes in the number of lin-c-kit+Sca-1+CD34- cells were in parallel with the alterations of lin-c-kit+Sca-1+ cell population in the bone marrow following E. coli challenge. In contrast to this increase in the lin-c-kit+Sca-1+ population, the number of lin-c-kit+Sca-1- cells was reduced significantly following E. coli infection. This cell population decreased to approximately 40% of control value at 12 h post i.v. E. coli. Between 24 to 48 h after E. coli infection, the bone marrow lin-c-kit+Sca-1- cell population gradually recovered but remained significantly lower compared to the control values. Similarly, the number of lin-c-kit+ cell (lin-c-kit+Sca-1+ plus lin-c-kit+Sca-1- cells) in the bone marrow was reduced at 12 h after the E. coli infection. The decrease in these cells was no longer evident by 24 h. By 48 h post i.v. E. coli challenge, the bone marrow lin-ckit+ cell population was moderately increased when compared to the controls.
Figure 1.
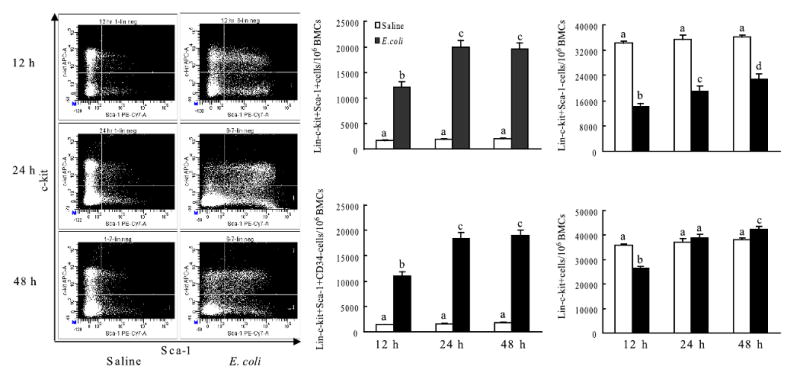
Changes in cell populations in bone marrow following bacteremia and representative dot plots of c-kit-APC vs. Sca-1-PE-Cy7 of lineage negative bone marrow cells. N=5∼9. BMCs: bone marrow cells; Bars with different letters in each panel are statistically different (p<0.05).
Alteration of cell BrdU incorporation
In order to understand the mechanisms underlying this expansion of marrow lin-c-kit+Sca-1+ cell pool following bacteremia, in vivo BrdU incorporation technique was utilized to determine the activities of hematopoietic precursor cell proliferation. At both 12 h and 24 h after E. coli infection, the percentage of BrdU+ cells in the marrow lin-c-kit+Sca-1+ cell population was significantly reduced compared to those of control mice (Figure 2) although the absolute number of lin-c-kit+Sca-1+BrdU+ cells in bone marrow was increased in mice with E. coli infection in comparison to the controls. In contrast, the percentage of BrdU+ cells in the marrow lin-c-kit+Sca-1- cell population was moderately increased in mice at 12 h and 24 h post E. coli infection. However, the absolute number of lin-c-kit+Sca-1-BrdU+ cells in the bone marrow was reduced in mice with E. coli infection at 24 h in comparison to controls.
Figure 2.
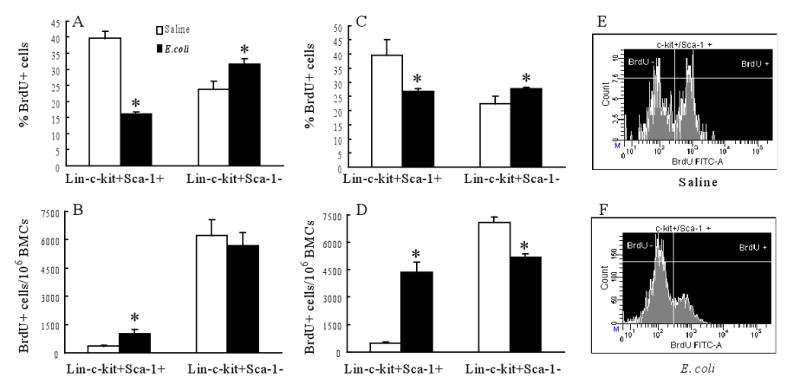
Changes in 5-bromo-2-deoxyuridine (BrdU) positive marrow lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells 12 h (A, B) and 24 h (C, D) following bacteremia. N= 3∼4. BMCs: bone marrow cells; *: p<0.05 vs. Saline group. Panels E and F are representative histograms of BrdU incorporation into marrow lin-c-kit+Sca-1+ cells 24 h after intravenous saline and E. coli, respectively.
The plasma cytokine response
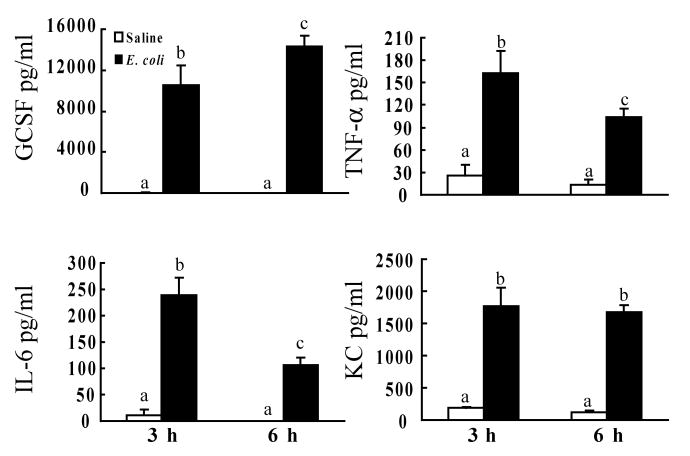
Cytokines are essential in mediating the alteration of hematopoietic activity in response to infection and inflammation. Several cytokines have also been shown to modulate Sca-1 expression by various cell types [27-29]. Therefore, we measured a panel of related plasma cytokines and growth factors during the early stage of E. coli infection. At 3 h and 6 h post i.v. challenge, the plasma concentrations of FGF, GM-CSF, IFN-γ, IGF-II, IL-1α, IL-1β, IL-3, SCF, TPO, and VEGF were very low. No differences of these cytokine levels were observed between control and E. coli groups (data not shown). At 3 h following E. coli bacteremia, the plasma level of G-CSF was markedly increased when compared to the control value (Figure 3). The G-CSF concentration in the plasma was further increased at 6 h after E. coli infection. IL-6 and TNF-α levels in the plasma were also increased at 3 h post i.v. E. coli challenge. This increase in plasma IL-6 and TNF-α concentrations was attenuated at 6 h following E. coli bacteremia. At both 3 h and 6 h after i.v. E. coli challenge, KC concentrations in the plasma were significantly increased compared to controls.
Figure 3.
Changes in plasma cytokine levels following bacteremia. N=4∼5. Bars with different letters in each panel are statistically different (p<0.05).
Inversion of phenotype following in vitro culture of lin-c-kit+Sca-1- cells
The in vivo BrdU data suggest that the observed increase in bone marrow lin-c-kit+Sca-1+ cells during E. coli bacteremia may not be primarily due to accelerated proliferation of existing lin-c-kit+Sca-1+ cells. Other mechanisms may be involved. Since the expansion of the lin-c-kit+Sca-1+ cell population was associated with a significant reduction in the number of lin-c-kit+Sca-1- cells in the bone marrow following E. coli infection, we hypothesized that lin-c-kit+Sca-1- cells might invert to lin-c-kit+Sca-1+ cells during E. coli bacteremia. Figure 4 shows the phenotypic shift of isolated lin-c-kit+Sca-1- cells following 24 h culture with conditioned plasma or different cytokines and growth factors. Incubation of lin-c-kit+Sca-1- cells with 50% plasma of control mice did not cause an increase in c-kit+Sca-1+ cells as compared to the background level detected using isotype control antibodies. However, exposure of lin-c-kit+Sca-1- cells to 50% plasma of E. coli-infected mice resulted in a significant increase in the number of c-kit+Sca-1+ cells in the culture system. Culture of lin-c-kit+Sca-1- cells with culture medium alone for 24 h showed a slight increase in c-kit+Sca-1+ cells in the culture system. Addition of G-CSF or a cocktail of growth factors into the culture system did not cause additional increase in the number of c-kit+Sca-1+ cells during the 24 h culture period. Interestingly, culture of lin-c-kit+Sca-1- cells with LPS caused a remarkable increase in Sca-1 expression by these cells. Similarly, TNF-α, IL-6, and IFN-γ each caused a marked increase in the number of c-kit+Sca-1+ cells in the culture system. However, IL-6-induced increase in Sca-1 expression in the cultured lin-c-kit+Sca-1- cells was relatively weak in comparison to those did by LPS, TNF-α, or IFN-γ. Lin-c-kit+Sca-1- cells cultured with a combination of TNF-α, IL-6, and IFN-γ showed the strongest increase in Sca-1 expression by these cells.
Figure 4.
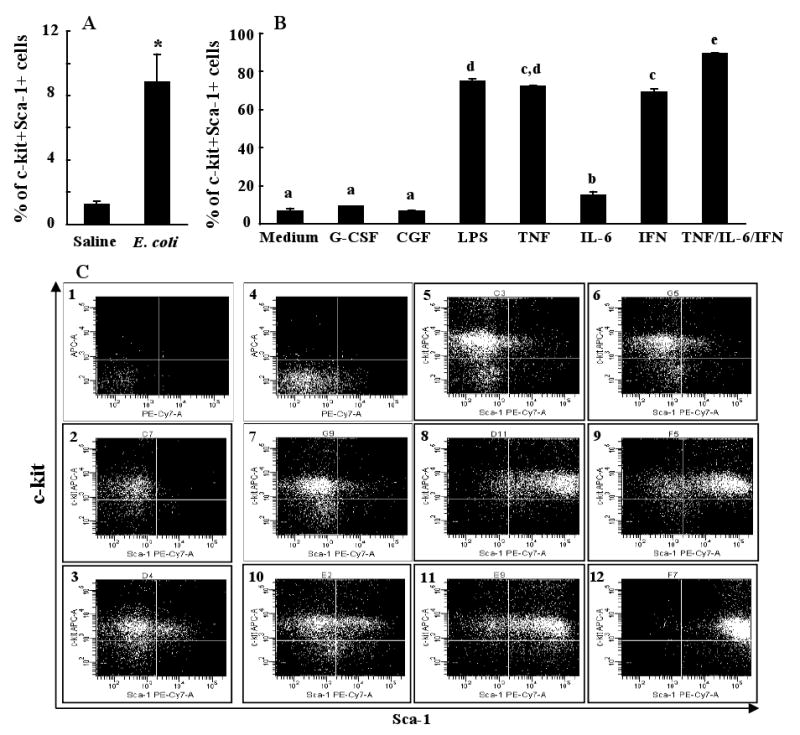
Phenotypic changes of marrow lin-c-kit+Sca-1- cells following 24 h culture. N = 5 in each group. Panel A: Culture with 50% plasma of saline or E. coli challenged mice; *: p < 0.05 vs. Saline group. Panel B: Culture with different cytokines and growth factors; CGF: cocktail of growth factors; Bars with different letters are statistically different (p < 0.05). Panel C: Representative flow cytometric dot plots of isotype control antibody stained cells cultured with No. 1: plasma from saline treated mice; No. 4: medium only; and representative flow cytometric dot plots of anti-c-kit and anti-Sca-1 stained cells cultured with: No. 2: plasma from saline treated mice; No. 3: plasma from mice with E. coli bacteremia; No. 5: medium only; No. 6: granulocyte colony-stimulating factor (G-CSF); No. 7: cocktail of growth factors (CGF); No. 8: lipopolysaccharide (LPS); No. 9: tumor necrosis factor-α (TNF-α); No. 10: interleukin-6 (IL-6); No. 11: interferon-γ (IFN-γ); No. 12: TNF-α/IL-6/IFN-γ.
Alteration of CFU activity in bone marrow lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells
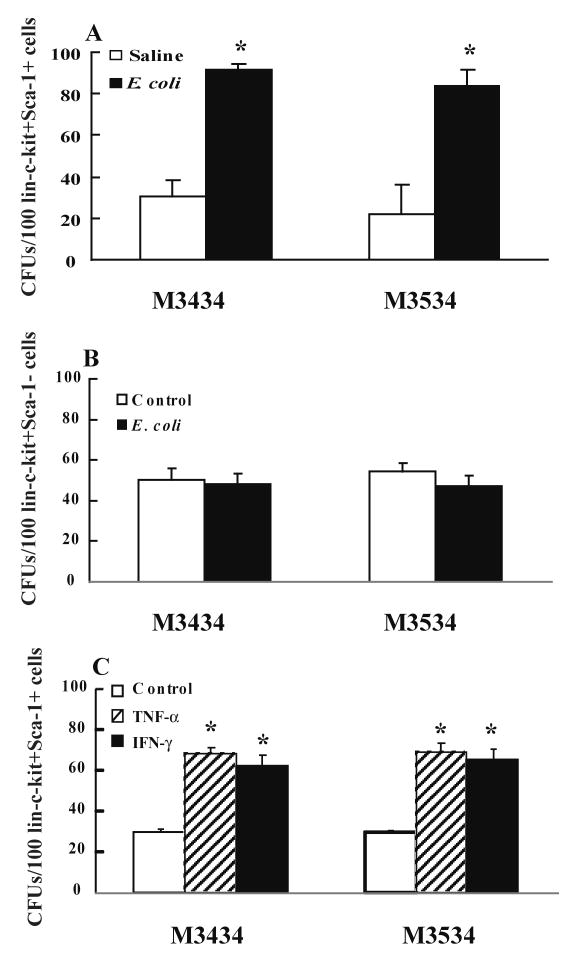
To test changes in functional activity of marrow lin-c-kit+Sca-1+ and lin-c-kit+Sca-1-cells following E. coli bacteremia, lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells sorted from mice at 24 h post i.v. saline or E. coli were cultured with Methocult GF M3434 and M3534 media. The Methocult GF M3434 medium supports the growth of multiple lineages including CFU-granulocyte/erythrocyte/macrophage/megakaryocyte (CFU-GEMM), CFU-granulocyte/macrophage (CFU-GM), CFU-granulocyte (CFU-G), CFU-macrophage (CFU-M), and brust-forming unit-erythroid (BFU-E), while Methocult GF M3534 only supports the growth of granulocyte and monocyte lineages including CFU-GM, CFU-G, and CFU-M. As shown in Figure 5A, lin-c-kit+Sca-1+ cells isolated from mice at 24 h post i.v. E. coli exhibited a significant increase in CFU activity when cultured on Methocult GF M3434. This increase in CFU formation resulted essentially from an increase in CFU-GM (including CFU-G and CFU-M) activity of these cells. CFU counts of isolated lin-c-kit+Sca-1- cells cultured on either Methocult GF M3434 or Methocult GF M3534 media did not show any statistically significant differences between control and E. coli bacteremic groups (Figure 5B).
Figure 5.
Colony-forming unit (CFU) activity of isolated bone marrow lin-c-kit+Sca-1+ (Panel A, N=3) and lin-c-kit+Sca-1- cells (Panel B, N=5) from mice at 24 h following i.v. challenge with saline or E. coli and CFU activity of isolated bone marrow lin-c-kit+Sca-1+ cells from control mice versus lin-c-kit+Sca-1+ derived from conversion of lin-c-kit+Sca-1- following culture for 24 h with TNF-α and IFN-γ (Panel C, N=5). Cells were cultured on Methocult GF M3434 and Methocult GF M3534 media for 7 days. *: p<0.05 vs. Saline group (Panel A), or p<0.05 vs. Control group (Panel C).
We subsequently tested CFU activity of lin-ckit+Sca-1+ cells derived from inversion of lin-c-kit+Sca-1- cells following in vitro culture for 24 h with TNF-a and IFN-g. As shown in Figure 5C, lin-c-kit+Sca-1+ cells inverted from lin-c-kit+Sca-1- cells showed a significant increase in CFU activity when cultured on Methocult GF M3434 media compared to freshly isolated lin-c-kit+Sca-1+ cells from control mice. This increase in CFU formation resulted essentially from an increase in CFU-GM activity of these cells, which is similar to the results of lin-c-kit+Sca-1+ cells freshly isolated from E. coli infected mice.
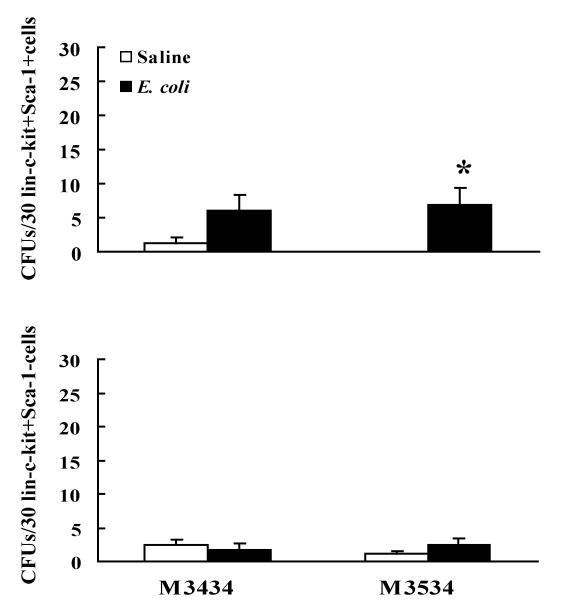
Circulating lin-c-kit+Sca-1+ cells sorted from mice 24 h following E. coli bacteremia also showed a higher CFU-GM activity compared to the control value (Figure 6). Circulating lin-c-kit+Sca-1+ cells of mice with E. coli bacteremia showed a tendency of increase in CFU activity when cultured on the Methocult GF M3434 media, but this change did not reach statistical significance as compared to the control value. Sorted circulating lin-c-kit+Sca-1- cells exhibited minimum CFU activities when cultured on both the Methocult GF M3434 and the Methocult GF M3534 media. No difference of CFU activity in circulating lin-c-kit+Sca-1- cells was observed between control and E. coli-infected groups.
Figure 6.
Colony-forming unit (CFU) activity of isolated circulating lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells from mice at 24 h following i.v. challenge with saline or E. coli. Cells were cultured on Methocult GF M3434 and Methocult GF M3534 media for 7 days. N=4∼5, *: p<0.05 vs. Saline group.
Alteration of bone marrow release of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells
During bacterial infection, the increased production of G-CSF and KC may stimulate the bone marrow to release hematopoietic precursor cells. Since the marrow pools of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells are dramatically altered after infection, this alteration may affect release of these cells from the bone marrow into the systemic circulation. As shown in Figure 7, the mobilization of lin-c-kit+Sca-1+ cells into the circulation was significantly increased at 12 h after E. coli infection. This mobilization of lin-c-kit+Sca-1+ cells was further enhanced at 24 h and 48 h. In contrast to the enhanced mobilization of lin-c-kit+Sca-1+ cells, the release of lin-c-kit+Sca-1- cells from the bone marrow into the systemic circulation was reduced during E. coli bacteremia.
Figure 7.
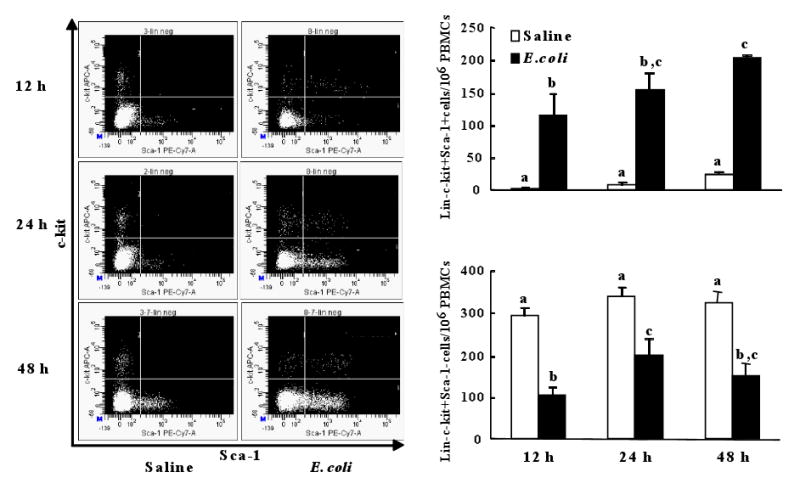
Changes in the number of lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cells in peripheral blood mononuclear cells (PBMCs) following bacteremia and representative dot plots of c-kit-APC vs. Sca-1-PE-Cy7 of lineage negative PBMCs. N=4∼6. Bars with different letters in each panel are statistically different (p<0.05).
Discussion
In response to bacterial infection, hematopoietic activity in the bone marrow is directed toward granulocyte production which is critical for enhancing host defenses against invading pathogens. At the present time, relatively little is known about how hematopoietic precursors are modified by this response. Early studies reported by Quesenberry and colleagues show that intraperitoneal administration of Salmonella typhosa endotoxin causes a rapid reduction of marrow granulocytic progenitor cells as assayed by in vitro colony forming cell technique (growing granulocyte and macrophage colonies in soft agar) [30]. This reduction of granulocytic progenitor cells occurs within 20 min after endotoxin, reaches a nadir at 6 h, and then returns to baseline values by 48 h. In contrast, Barthlen and colleagues observed an increase in CFU-GM in the bone marrow of mice at 12 h following the onset of abdominal sepsis [5]. In order to further clarify changes in hematopoietic precursor cells, we analyzed the bone marrow cells of mice with E. coli bacteremia using flow cytometry based on the phenotypic cell surface markers. The results of our current investigation showed that E. coli infection caused a dramatic reduction in the number of marrow lin-c-kit+Sca-1- cells. These results are in agreement with previous observations of endotoxin-treated mice reported by Quesenberry and colleagues [30]. Interestingly, the lin-c-kit+Sca-1+ or lin-c-kit+Sca-1+CD34- cell population in the bone marrow was markedly increased following E. coli infection in our current model despite the decrease in the number of lin-c-kit+Sca-1- cells. At 12 h post i.v. E. coli challenge, lin-c-kit+Sca-1+ cell population was increased 7 fold in the bone marrow. During 24 to 48 h after the infection, the number of bone marrow lin-c-kit+Sca-1+ cells was further increased to approximately 10 fold. In our other paralleled studies, we have observed that C57BL/6 mice intravenously challenged with live or heat-inactivated E. coli and Balb/c mice intratracheally challenged with Streptococcus pneumoniae also show a rapid increase in the lin-c-kit+Sca-1+ or lin-c-kit+Sca-1+CD34- cell population in the bone marrow (unpublished data). These data suggest that the expansion of the marrow lin-c-kit+Sca-1+ cell pool is likely a fundamental component of the host defense response to severe bacterial infection.
In order to understand the mechanisms underlying the expansion of the marrow lin-c-kit+Sca-1+ cell pool, we conducted studies utilizing in vivo BrdU incorporation. The results showed that the absolute number of BrdU positive lin-c-kit+Sca-1+ cells in the bone marrow was increased at both 12 h and 24 h following E. coli infection. This early increase in BrdU incorporation into marrow lin-c-kit+Sca-1+ cells reflects an increase in mitosis among these cells, which may contribute to the observed expansion of lin-c-kit+Sca-1+ cell pool in the bone marrow. However, this increase in mitosis of lin-c-kit+Sca-1+ cells appears not to be the sole event that causes the increase in the marrow lin-c-kit+Sca-1+ cell pool. The percentage of BrdU positive cells in the marrow lin-c-kit+Sca-1+ cell population was actually reduced at both 12 h and 24 h post E. coli infection when compared to the controls. A significant population of the increased lin-c-kit+Sca-1+ cells in the bone marrow was BrdU negative, which suggests that mechanism(s) other than proliferation of these cells may be involved.
Recent studies have shown that engraftable stem cells and progenitors exist in a reversible continuum, so called progenitor/stem cell inversions [31-33]. In an in vitro culture system, it has been observed that while traveling through cell cycle, stem cells acquire a progenitor phenotype and lose their stem cell phenotype during the late S and early G2 phases. This phenomenon is reversible by the next G1 phase. Colvin and colleagues have postulated that the progenitors and the stem cells may, in fact, be the same cell in different reversible functional states [33]. In our studies, E. coli bacteremia-induced expansion of the marrow lin-c-kit+Sca-1+ cell pool occurred in conjunction with a rapid reduction of the lin-c-kit+Sca-1- cell population in the bone marrow. A significant number of cells in the expanded marrow lin-c-kit+Sca-1+ cell pool appeared not to be derived from the proliferation of pre-existing lin-c-kit+Sca-1+ cells. We speculate that the majority (if not all) of this increased number of BrdU negative lin-c-kit+Sca-1+ cells in the bone marrow may be inverted from lin-c-kit+Sca-1- cells. To verify this, purified marrow lin-c-kit+Sca-1- cells were cultured with plasma of bacteremic mice. Our data showed that exposure to plasma of E. coli-infected mice resulted in a significant increase in conversion of these cells to c-kit+Sca-1+ cells, which strongly supports our hypothesis that the inversion of lin-c-kit+Sca-1- cells to lin-c-kit+Sca-1+ cells may contribute to the rapid expansion of the marrow lin-c-kit+Sca-1+ cell pool following E. coli bacteremia.
In order to further define factors capable of mediating phenotypic conversion of lin-c-kit+Sca-1- cells during bacteremia, a battery of related plasma cytokines and growth factors were determined during the early stage of bacteremia. The results showed that plasma levels of G-CSF were markedly increased in comparison to controls. However, intravenous administration of recombinant murine G-CSF at a dose of 50 μg/kg did not induce any increase in the number of marrow lin-c-kit+Sca-1+ cells following 48 h of G-CSF treatment (data not shown). In vitro culture of purified lin-c-kit+Sca-1- cells with recombinant murine G-CSF or G-CSF plus other growth factors (SCF, TPO, IGF-II, FGF-I, and heparin) also did not induce any increase in the conversion of these cell to c-kit+Sca-1+ cells. In our in vivo experiments, the plasma levels of TNF-α and IL-6 were increased in the early stage of E. coli bacteremia. Previously, it has been reported that LPS, IL-6, and IFN-γ stimulate Sca-1 expression by lymphocytes and TNF-α enhances Sca-1 expression by endothelial cells [27-29]. Therefore, we tested the effects of these factors in our in vitro culture system. The results showed that LPS, TNF-α, and IFN-γ each caused a remarkable increase in the number of c-kit+Sca-1+ cells in the culture system of marrow lin-c-kit+Sca-1- cells. IL-6 also induced a significant increase in Sca-1 expression in these cultured cells. In a paralleled study, mice were intravenously challenged with recombinant murine TNF-α (45 μg/kg or 105U/mouse), IL-6 (45 μg/kg or 104U/mouse), or IFN-g (540 μg/kg or 105U/mouse) in the absence of E. coli infection. The results showed that 24 h after challenge, TNF-α and IL-6 at the doses administered each caused a 2-fold expansion of marrow lin-c-kit+Sca-1+ cell population. IFN-g at the dose administered caused a greater than 6-fold increase in the lin-c-kit+Sca-1+ population in the bone marrow (unpublished data). Since IFN-γ concentration was not increased in the plasma during the early stage of bacteremia, it appears that TNF-α, IL-6, and LPS are major mediators potentially responsible for inducing the rapid phenotypic inversion of lin-c-kit+Sca-1+ cells in our current model of E. coli infection.
Previous studies indicate that accelerated differentiation of marrow hematopoietic progenitors into granulocytes and migration of these precursors to the spleen may account for the reduction of the progenitor cell population in the bone marrow during the early stage of endotoxin challenge [30, 34]. Our results suggest that the enhancement of phenotypic inversion to lin-c-kit+Sca-1+ cells may also be responsible for the rapid reduction of marrow lin-c-kit+Sca-1- cell pool following E. coli bacteremia. It appears that the lin-c-kit+Sca-1- cell population in the bone marrow serves as a functional reserve supporting the marrow responses to bacterial infection at both directions. These cells may function as the direct precursors for differentiation and production of granulocytes. In addition, they support the expansion of marrow lin-c-kit+Sca-1+ cell pool via expression of Sca-1 antigen. The decrease in the number of marrow lin-c-Kit+Sca-1- cells following bacteremia may result from both the accelerated differentiation of these cells into the down-stream granulocytic cells and phenotypic conversion of these cells to the lin-c-kit+Sca-1+ cells.
During bacterial infection, acceleration of myeloid lineage differentiation with enhancement of granulocyte production becomes a predominant feature of hematopoiesis [3-5]. In order to understand the expansion of the lin-c-kit+Sca-1+ cell pool in relation to the granulopoietic activity in the bone marrow following E. coli infection, isolated bone marrow lin-c-kit+Sca-1+ cells were cultured with Methocult GF M3434 and M3534 media. Our results showed that marrow lin-c-kit+Sca-1+ cells of mice with E. coli bacteremia displayed a significant increase in CFU-GM activity as compared to those of control animals. These data suggest that cells in the rapidly expanded lin-c-kit+Sca-1+ pool in the bone marrow following E. coli bacteremia are functionally activated for granulocyte lineage commitment. We also tested CFU forming activity in lin-c-kit+Sca-1+ cells inverted from lin-c-kit+Sca-1- cells following 24 h culture in vitro with TNF-α or IFN-γ. These inverted cells showed a marked increase in CFU-GM activity compared to lin-c-kit+Sca-1+ cells freshly isolated from control mice, which support our in vivo observation. Similarly, sorted circulating lin-c-kit+Sca-1+ cells from E. coli-infected mice showed an increase in CFU-GM activity in comparison to the value of saline-treated control mice.
E. coli bacteremia caused a marked increase in KC level in the systemic circulation. Both G-CSF and KC are potent factors mediating the bone marrow release of hematopoietic stem cells and progenitors in mice [16-18, 35]. In the present study, we observed that the mobilization of lin-c-kit+Sca-1+ cells into the circulation was significantly increased during bacteremia. In contrast, the number of lin-c-kit+Sca-1- cells in the circulation was reduced in the infected animals. These data indicate that the altered lin-c-kit+Sca-1+ and lin-c-kit+Sca-1- cell pools in the bone marrow affect the release of cells from each pool into the blood stream. The expanded marrow pool of lin-c-kit+Sca-1+ cells effectively endorses mobilization of lin-c-kit+Sca-1+ cells into the systemic circulation.
Our current observation of the hematopoietic precursor cell response to bacteremia is mainly based on changes of cells identified by their phenotypic markers. Further investigations on the alteration of other functions associated with these phenotypic changes, including competitive repopulating capacity of cells in the expanded marrow lin-c-kit+Sca-1+ cell pool and homing of the mobilized lin-c-kit+Sca-1+ cells in the systemic circulation following bacteremia will provide additional information for understanding the significance of the hematopoietic precursor cell response to bacterial infection. These investigations are currently under way in our group.
In summary, the lin-c-kit+Sca-1+ cell population in the bone marrow is rapidly expanded in response to bacteremia. Both enhanced mitosis of these cells and inversion from the lin-c-kit+Sca-1- cell phenotype contribute to the observed increase in the number of lin-c-kit+Sca-1+ cells in the bone marrow. Cells in the expanded marrow lin-c-kit+Sca-1+ cell pool exhibit increase in CFU-GM activity. In addition, mobilization of lin-c-kit+Sca-1+ cells into the systemic circulation is significantly enhanced along with the expansion of the marrow lin-c-kit+Sca-1+ cell pool. These data suggest that the marrow lin-c-kit+Sca-1+ cell population functions as an important component of the host immune defense response to severe bacterial infection.
Acknowledgments
We thank Amy B. Weinberg, Rhonda R. Martinez, Jane A. Schexnayder, and Joseph S. Soblosky for their expert technical assistance. The authors also thank Connie P. Porretta for her expert assistance with flow cytometric analyses and cell sorting.
This work was supported by Public Health Service Grants AA09803, HL075161, HL073770, and HL76100.
References
- 1.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Akala OO, Clarke MF. Hematopoietic stem cell self-renewal. Curr Opin Genet Dev. 2006;16:496–501. doi: 10.1016/j.gde.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann DW, Entringer MA, Robinson WA, et al. Regulation of granulopoiesis and distribution of granulocytes in early phase of bacterial infection. J Cell Physiol. 1981;109:17–24. doi: 10.1002/jcp.1041090103. [DOI] [PubMed] [Google Scholar]
- 5.Barthlen W, Zanti N, Pfeffer K, et al. Impact of experimental peritonitis on bone marrow cell function. Surgery. 1999;126:41–47. doi: 10.1067/msy.1999.99060. [DOI] [PubMed] [Google Scholar]
- 6.Santangelo S, Gamelli RL, Shankar R. Myeloid commitment shifts toward monocytopoiesis after thermal injury and sepsis. Ann Surg. 2001;233:97–106. doi: 10.1097/00000658-200101000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh JC, Boggs DR, Gartwright GE, et al. Neutrophil kinetics in acute infection. J Clin Invest. 1967;12:1943–1953. doi: 10.1172/JCI105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terashima T, Wiggs B, English D, et al. Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. Am J Physiol. 1996;271:L587–L592. doi: 10.1152/ajplung.1996.271.4.L587. [DOI] [PubMed] [Google Scholar]
- 9.Cronkite EP. Analytical review of structure and regulation of hemopoiesis. Blood Cell. 1988;14:313–328. [PubMed] [Google Scholar]
- 10.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. J A M A. 1996;275:134–141. [PubMed] [Google Scholar]
- 11.Perlino CA, Rimland D. Alcoholism, leucopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757–760. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- 12.Kagsbjerg P, Jones I, Viderfors T, et al. Diagnostic value of blood cytokine concentrations in acute pneumonia. Thorax. 1995;50:1253–1257. doi: 10.1136/thx.50.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauksen K, Elfman L, Ulfgren AK, et al. Serum levels of granulocyte-colony stimulating factor (G-CSF) in bacterial and viral infections, and in atypical pneumonia. Br J Haematol. 1994;88:256–260. doi: 10.1111/j.1365-2141.1994.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujishima S, Sasaki J, Shinozawa Y, et al. Serum MIP-1 alpha and IL-8 in septic patients. Intensive Care Med. 1996;22:1169–1175. doi: 10.1007/BF01709331. [DOI] [PubMed] [Google Scholar]
- 15.Shahbazian LM, Quinton LJ, Bagby GJ, et al. Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit Care Med. 2004;32:1740–1746. doi: 10.1097/01.ccm.0000132900.84627.90. [DOI] [PubMed] [Google Scholar]
- 16.Gazitt Y. Recent developments in the regulation of peripheral blood stem cell mobilization and engraftment by cytokines, chemokines and adhesion molecules. J Hematother Stem Cell Res. 2001;10:229–236. doi: 10.1089/15258160151134908. [DOI] [PubMed] [Google Scholar]
- 17.Starckx S, Van den Steen PE, Wuyts A, et al. Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization. Leuk Lymphoma. 2002;43:233–241. doi: 10.1080/10428190290005982. [DOI] [PubMed] [Google Scholar]
- 18.Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010–1020. doi: 10.1016/j.exphem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majka SM, Jackson KA, Kienstra KA, et al. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 23.Osawa M, Nakamura K, Nishi N, et al. In vivo self-renewal of c-Kit+ Sca-1+ Lin(low/-) hemapoietic stem cells. J Immunol. 1996;156:3027–3214. [PubMed] [Google Scholar]
- 24.Jurecic R, Van NT, Belmont JW. Enrichment and functional characterization of Sca-1+WGA+, lin-WGA+, lin-Sca-1+, and lin-Sca-1+WGA+ bone marrow cells from mice with an Ly-6a haplotype. Blood. 1993;82:2673–2683. [PubMed] [Google Scholar]
- 25.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 26.Osawa M, Hanada K, Hamada H, et al. Long-term lymphohematopoietic reconsititution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 27.Chen HC, Frissora F, Durbin JE, et al. Activation induced differential regulation of stem cell antigen-1 (Ly-6A/E) expression in murine B cells. Cell Immunol. 2003;225:42–52. doi: 10.1016/j.cellimm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Demoulin JB, Maisin D, Renauld JC. Ly-6A/E induction by interleukin-6 and interleukin-9 in T cells. Eur Cytokine Netw. 1999;10:49–56. [PubMed] [Google Scholar]
- 29.Luna G, Paez J, Cardier JE. Expression of the hematopoietic stem cell antigen Sca-1 (Ly-6A/E) in liver sinusoidal endothelial cells: Possible function of Sca-1 in endothelial cells. Stem Cell Dev. 2004;13:528–535. doi: 10.1089/scd.2004.13.528. [DOI] [PubMed] [Google Scholar]
- 30.Quesenberry PJ, Morley A, Miller M, et al. Effect of endotoxin on granulopoiesis and the in vitro colony-forming cell. Blood. 1973;41:391–398. [PubMed] [Google Scholar]
- 31.Quesenberry PJ, Colvin GA, Abedi M, et al. The marrow stem cell: the continuum. Bone Marrow Transplant. 2003;32:S19–S22. doi: 10.1038/sj.bmt.1703938. [DOI] [PubMed] [Google Scholar]
- 32.Colvin GA, Lambert JF, Moore BE, et al. Intrinsic hematopoietic stem cell/progenitor plasticity: inversions. J Cell Physiol. 2004;199:20–31. doi: 10.1002/jcp.10436. [DOI] [PubMed] [Google Scholar]
- 33.Siggins RW, II, Zhang P, Welsh D, et al. Stem cells, phenotypic inversion, and differentiation. Int J Clin Exp Med. 2008;1:2–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Quesenberry PJ, Morley A, Tyan M, et al. The effect of endotoxin on murine stem cell. J Cell Physiol. 1973;82:239–244. doi: 10.1002/jcp.1040820212. [DOI] [PubMed] [Google Scholar]
- 35.Nardini E, Morelli D, Aiello P, et al. CpG-oligodeoxynucleotides induce mobilization of hematopoietic progenitor cells into peripheral blood in association with mouse KC (IL-8) production. J Cell Physiol. 2005;204:889–895. doi: 10.1002/jcp.20360. [DOI] [PubMed] [Google Scholar]