Abstract
The blending of key structural features from the purine and pyrimidine nucleobase scaffolds gives rise to a new class of hybrid nucleosides. The purine-pyrimidine hybrid nucleosides can be viewed as either N-3 ribosylated purines or 5,6-disubstituted pyrimidines, thus recognition by both purine- and pyrimidine-metabolizing enzymes is possible. Given the increasing reports of the development of resistance in many enzymatic systems, a drug that could be recognized by more than one enzyme could prove highly advantageous in overcoming resistance mechanisms related to binding site mutations. In that regard, the design, synthesis and results of preliminary biological activity for a series of carbocyclic uracil derivatives with either a fused imidazole or thiazole ring are presented herein.
Introduction
Efforts in our laboratories have focused on the strategic manipulation of key aspects of the nucleoside scaffold in an effort to probe structure and function for biologically significant enzymes. Increasing reports of viral and antibacterial resistance has rendered many current chemotherapeutics less effective, however recent examples of the success of dual inhibitors have opened new avenues for investigation. The most common example of a dual inhibitor involves two known drugs covalently linked together. The drugs bind to two different sites in the same enzyme, but can be delivered at the same time and in the same levels, thereby ensuring more effective inhibition. Another type is a drug that can be recognized and inhibit two different enzymes in the same replication pathway, or in two different mechanistic pathways that are involved in the same disease.1-4 This should also increase the chances for effective inhibition, especially if mutations have developed, thus a nucleoside analogue that could be recognized by both purine- and pyrimidine-metabolizing enzymes might prove to be a highly effective approach for circumventing some resistance mechanisms.
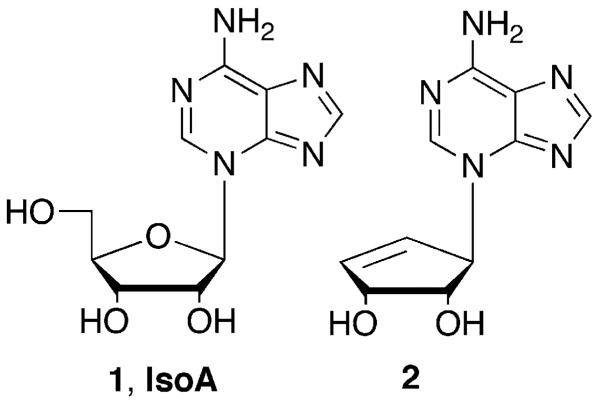
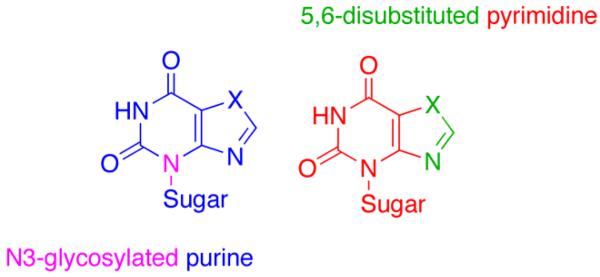
In that regard, exploitation of the nonstandard connectivity of the purine analogue isoadenosine (1, IsoA, Figure 1) was envisioned as a strategic starting point for constructing hybrid nucleosides that resemble both purines and pyrimidines. IsoA is a structural isomer of adenosine where the purine ring is connected to the sugar moiety at N-3 (purine numbering), rather than the traditional N-9.5, 6 As depicted in Figure 2, N-3 glycosolated purines can be viewed as 5,6-disubstituted pyrimidines, and conversely, 5,6-disubstituted pyrimidines are often considered as purine mimics, thus providing impetus for the design goals.7, 8
Figure 1.
Isoadenosine (IsoA) and DHCe-isoA.
Figure 2.
Purine-pyrimidine hybrid nucleosides.
Although IsoA was initially considered an interesting analogue since it was recognized by a number of biologically significant enzymes, the proclivity to undergo 1,3-migration in both basic and acidic conditions to result in adenosine, made synthesis tedious and low yielding.6 As an answer to this, employing the carbocyclic nucleoside scaffold was viewed as highly advantageous for several reasons; first, carbocyclic nucleosides are stable to glycosidic bond cleavage, since replacement of the furanose oxygen with a methylene group converts the bond between the sugar and the base from an unstable hemiaminal to a more robust tertiary amine. This structural modification would provide an answer to IsoA's instability towards acids and bases, as well as the propensity to undergo glycosidic migration, since it would require cleavage of a tertiary amine bond.9 Second, carbocyclic purine nucleosides as a class, are known potent inhibitors of S-adenosylhomocysteine hydrolase (SAHase), and indirectly, DNA methyltransferase (DNA MeTase).10-12 Both are critical enzymes in the replication pathway of many viruses, parasites and cancers, thus an analogue that could inhibit both steps in a single pathway would increase the likelihood of achieving complete inhibition.10, 13 Drawing upon these leads, it appeared that a hybrid of the pyrimidine and purine ring scaffolds merged with the carbocyclic “sugar” might offer forth a successful strategy for the design of more effective inhibitors.
Chemistry
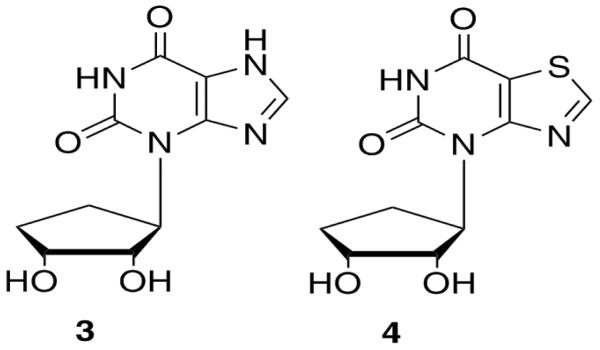
Although initial efforts for this project had focused on the synthesis of the parent purine isoA ring system (2, Figure 1)9, the ultimate goal was to construct a more versatile hybrid of the pyrimidine and purine ring systems. In considering the design of possible targets, it was necessary to retain two key structural features; the aromaticity and hydrogen bonding capabilities of the heterocyclic ring system. In addition to the parent carbocyclic isoxanthosine (3, Figure 3), the isoelectronic exchange of a sulfur atom for the nitrogen was also considered (4, Figure 3). This would allow for retention of the aromaticity desired for the heterocyclic base, as well as to retain uridine's acceptor-donor-acceptor pattern.
Figure 3.
Hybrid tragets.
Although Townsend et al had previously reported7, 8 several thiazolopyrimidine ribose-based nucleosides, there were no reports of any carbocyclic analogues. Since the routes to the carbocyclic analogues differ significantly from those used to obtain the ribose analogues, these were of interest synthetically. Additionally, because the 4'-deoxy derivatives of Aristeromycin and Neplanocin A exhibited less toxicity as compared to the parent compounds14-17, the truncated analogues were initially chosen for the design of the carbocyclic portion of the nucleoside. As a result, the first targets considered were the fused uridine-imidazole analogues shown in Figure 3.
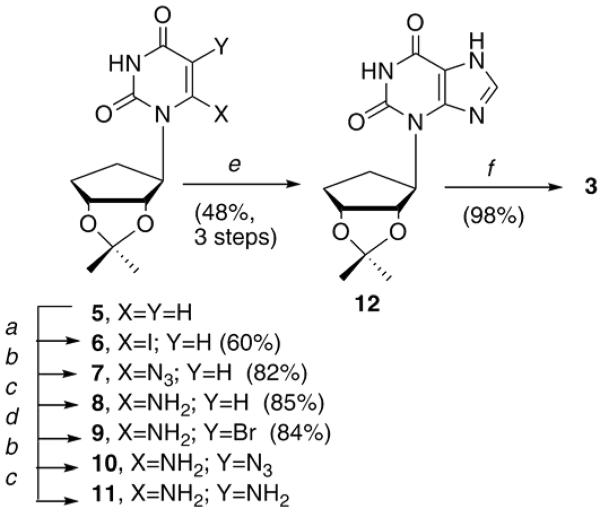
The synthesis of 3 was envisioned from functionalizing the C-5 and C-6 positions of a carbocyclic uridine analogue recently reported18 from our laboratories (Scheme 1). Depending upon the substituents, this intermediate could provide a facile starting point to realize both of the desired bicyclic analogues. Treatment of 5 with lithium diisopropyl amide (LDA) and iodine provided the 6-iodo intermediate 6.19 Subsequent displacement with sodium azide, followed by reduction afforded amine 8. Bromination of C-5 gave the difunctionalized 9, which was then subjected to the same set of reactions as was used to functionalize the C-6 position, resulting in diamino 11. It should be noted that these reactions were not particularly high-yielding, since several of the intermediates were unstable, however the reaction times were short, thus the entire series of reactions, including ring closure, could be accomplished in a matter of a few days. Finally, ring closure with triethylorthoformate and deprotection gave 3 in a 16.5% overall yield from 5.
Scheme 1.
Reagents and conditions: a, LDA, THF, I2, -78 °C, 5 h; b, NaN3, DMF, rt, 1h; c, 10% Pd/C, MeOH, H2, rt, 1h; d, NaHCO3, Br2, rt 1h; e, HC(OEt)3, reflux, 1.5h; f, TFA/H2O (2:1), rt, 2h.
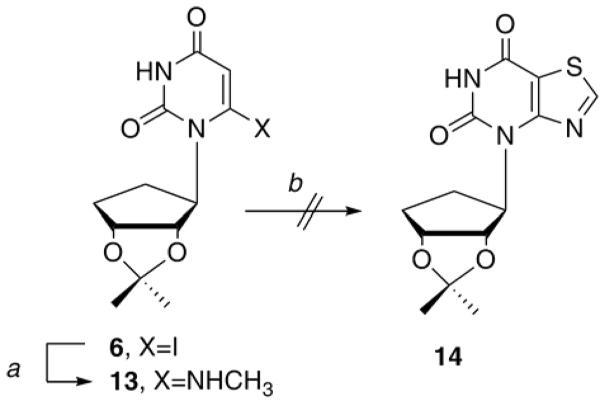
Attention then turned to the thiazole analogue 4. Using a two-step procedure reported in the literature20, 21, the synthesis of 4 initially appeared to be much more facile than the low-yielding and tedious route employed for 3. As shown below in Scheme 2, starting with 6, treatment with methylamine gave 13 in a 94% yield. Treatment with thionylchloride should have resulted in the bicyclic intermediate 14 similarly to the reports of Mizuno et al21, however no reaction was observed, despite repeated manipulation of the reaction times and temperatures.
Scheme 2.
Reagents and conditions: a, 33% NH2Me, EtOH, rt, 1.5h, 94%; b, SOCI2, pyridine, reflux, 3h
Since the original paper contained a thiazole substituted with methyl groups on N-3 and N-1, speculation that the electronics of the pyrimidine nitrogen might be hindering the ring closure reaction dictated investigating the possibility that a protecting group for N-3 might solve the problem. Several traditional protecting groups were considered, since a facile method for selective N-3 protection was available in the literature.22 Surprisingly, the outcome of these trials were not as straightforward as anticipated, and are summarized in Table 1.
Starting with the most obvious choice, benzoylation was tried first, however upon treatment of 5a with LDA and iodine as before, iodination occurred at multiple sites, including on the benzoyl group. Turning next to the Bn group, iodination proceeded with no problems, and the ring closure to give the desired fused pyrimidine-thiazole ring system was accomplished in good yield, removal of the Bn group proved difficult. The presence of the sulfur precluded the use of the traditional deblocking systems that employ hydrogenation catalysts such as Pd/C, thus other methods such as the use of ethanethiol, which had been successful in deblocking our thieno-expanded purine nucleosides23-25, were attempted. These proved to be very low-yielding, as did the Lewis acid deblocking methods. Additionally, the use of dissolving metal catalysis with sodium and ammonia led to the complete loss of the thiazole portion of the bicyclic base, reverting to the starting material, Bn-protected uridine 5b, which was similar to other reports. The benzyloxymethyl (BOM) group appeared to be a reasonable option, however again, all attempts to remove the BOM group resulted in either no reaction under mild conditions, or with more stringent conditions, conversion to a methyl group, which then proved to be completely recalcitrant to removal.
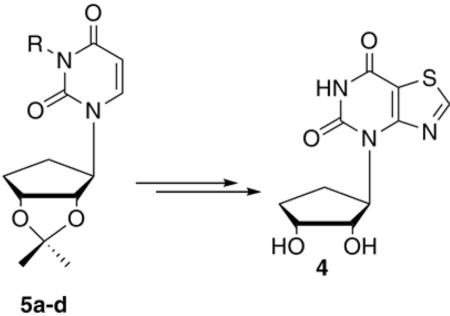
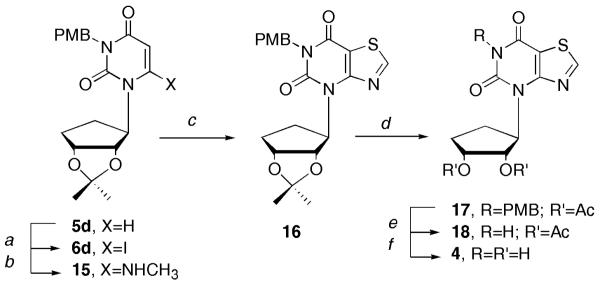
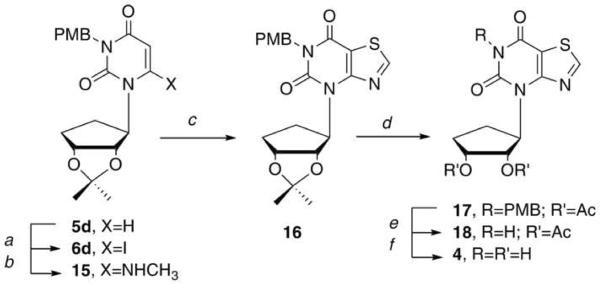
Finally, the p-methoxybenzyl (PMB) group was considered. As detailed in Scheme 3, the iodination, followed by displacement with methylamine, and subsequent ring closure gave PMB-protected 16 in very good yield. At this point, deblocking of the PMB group was undertaken, but initial attempts with the traditional method employing ceric ammonium nitrate (CAN), surprisingly resulted in loss of the isopropylidine group instead, leaving the PMB group untouched. To our knowledge this observation has not previously been noted in the literature. All attempts to remove both groups using excess CAN gave rise to an inseparable mixture of highly polar products. Finally, reprotection of the unmasked hydroxyls of 16 with the acetate group gave 17. Deprotection of the PMB group with CAN afforded 18, followed by removal of the acetates, provided 4 in a 65 % yield.
Scheme 3.
Reagents and conditions: a, LDA, THF, I2, -78 °C, 3h, 58%; b, 33% NH2Me, EtOH, rt, 1.5h, 94%; c, SOCI2, pyridine, reflux, 3h, 80%; d, i, TFA:H2O (2:1), rt, 18h; ii. Ac2O, pyridine, DMAP, CH2CI2, 12h, 82% for 2 steps; e, 10:1 CH3CN:H2O, CAN, 55 °C, 3h, 86%; f NH3, MeOH, rt, 15h, 65%.
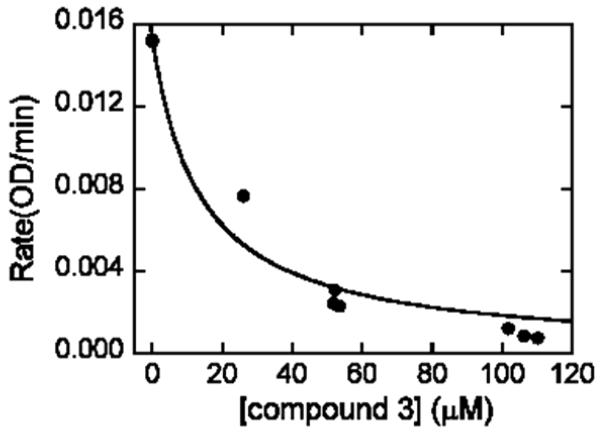
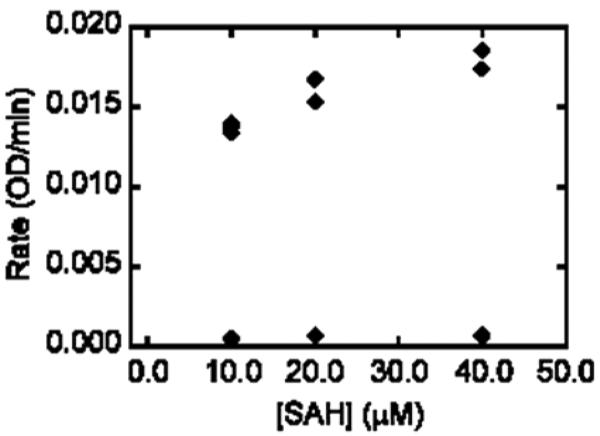
Once in hand, the targets were screened for their ability to inhibit SAHase and DNA MeTase. Weak to no activity was observed against DNA MeTase (data not shown) for either compound, however 3 exhibited good activity against SAHase while 4 showed no appreciable activity. For SAHase, in the first experiment the concentration of inhibitor was varied while the concentration of substrate (SAH) was held constant at 10 μM. Conversion of SAH to adenosine and then to inosine was monitored at 265 nm (Figure 4). In the second experiment the concentration of inhibitor was held constant at 45 μM while the concentration of substrate was varied. Initial rates in the presence and absence of inhibitors were measured (Figure 5). There was no change in absorbance at 265 nm in the absence of SAH. These experimental data best fit a competitive inhibition mechanism. The Ki value for compound 3 was found to be 5.0 ± 0.9 μM against SAHase in the hydrolysis direction, based on a Km value of 7.9 μM for the SAH substrate.26 Interestingly, in the presence of the inhibitor, biphasic enzyme kinetics were observed; albeit, the initial phase was much shorter than the later one. These data suggested that the mechanism may be more complicated than a simple competitive inhibition mechanism. Detailed mechanistic studies will be reported in the future.
Figure 4.
Inhibition of SAHase by 3. Assay solutions contained 50 mM potassium phosphate at pH 7.4, 0.39 units of adenosine deaminase, 132 nM of SAHase, 10 1M of SAH (substrate), and various concentrations of 10. Consumption of substrate was monitored at 265 nM.
Figure 5.
The concentration of inhibitor 3 was held constant at 45 °M while the concentration of substrate was varied. Initial rates in the presence (data points at the bottom of the plot) and absence (data points at the top of the plot) of inhibitors were measured. There was no change in absorbance at 265 nM in the absence of SAH.
Summary
In summary, given the unique electronic nature of many heterocyclic ring systems, it is clear that the choice of protecting groups is not always straightforward, since reactivity for these systems varies greatly. Additional analogues are currently being pursued, the results of which should provide potentially interesting information for further investigation of the hybrid purine-pyrimidine scaffold.
EXPERIMENTAL
General
All chemicals were obtained from commercial sources and used without further purification unless otherwise noted. Anhydrous DMF, MeOH, DMSO and toluene were purchased from Fisher Scientific. Anhydrous THF, acetone, CH2Cl2, CH3CN and ether were obtained using a solvent purification system (mBraun Labmaster 130). Melting points are uncorrected. NMR solvents were purchased from Cambridge Isotope Laboratories (Andover, MA). All 1H and 13C NMR spectra were obtained on a JEOL ECX 400 MHz NMR, operated at 400 and 100 MHz respectively, and referenced to internal tetramethylsilane (TMS) at 0.0 ppm. The spin multiplicities are indicated by the symbols s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet), and b (broad). Reactions were monitored by thin-layer chromatography (TLC) using 0.25 mm Whatman Diamond silica gel 60-F254 precoated plates. Column chromatography was performed using silica gel (63-200 μ) from Dynamic Adsorptions Inc. (Norcross, GA), and eluted with the indicated solvent system. Yields refer to chromatographically and spectroscopically (1H and 13C NMR) homogeneous materials. Mass spectra were recorded at the Johns Hopkins Mass Spectrometry Facility (Baltimore, MD). Elemental analyses were recorded at Atlantic Microlabs, Inc. (Norcross, GA).
Preparation of 6-Iodo-1-(2',3'-O-isopropylidenedioxy-cyclopent-1-yl)uracil (6)
To a flame dried flask under argon atmosphere was added anhydrous THF (5 mL) and freshly distilled diisopropylamine (0.23 g, 2.27 mmol). The temperature was lowered to -78 °C and n-BuLi was added dropwise (2.5 M in hexanes, 2.27 mmol, 0.91 mL). After stirring for 15 min, a solution of 5 (0.26 g, 1.03 mmol) in anhydrous THF (10 mL) was added. The mixture was stirred at -78 °C for an additional 30 min, at which point a solution of I2 (0.58 g, 2.27 mmol) in anhydrous THF (5 mL) was added and the mixture was allowed to continue stirring at this temperature for 2 h. Following quenching with acetic acid (10 drops) and NaHCO3 (1 mL), the solvent was removed by evaporation and the residue dissolved in EtOAc (50 mL), washed with sodium thiosulfate (25 mL) and brine (25 mL), dried (MgSO4), filtered, and concentrated under vacuum. The residue was purified by column chromatography eluting with n-hexanes:EtOAc (1:4) to afford 0.23 g of 6 as a yellow solid (60 %). mp 180-182 °C. 1H NMR (DMSO-d6): δ 1.38 (s, 3H), 1.41 (s, 3H), 1.51-1.61 (m, 2H), 1.76-1.86 (m, 2H), 3.88 (m, 1H), 3.93 (m, 1H), 4.50 (t, 1H), 6.57 (s, 1H), 11.00 (br s, 1H). 13C NMR (DMSO-d6): δ 19.3, 24.3, 25.9, 26.6, 56.1, 81.9, 88.4, 102.1, 107.3, 110.7, 150.9, 163.6. HRMS: calcd for C12H15IN2O4 1215IN2O4 (M+H), 379.0077; found, 379.0176.
Preparation of 6-Azido-1-(2',3'-O-isopropylidenedioxy-cyclopent-1-yl)uracil (7)
A solution of 6 (0.10 g, 0.26 mmol) and NaN3 (0.094 g, 1.45 mmol) in anhydrous DMF (3 mL) was stirred at rt for 30 min, at which point H2O (20 mL) was added and the mixture extracted with EtOAc (3 × 50 mL), washed with brine (50 mL), dried (MgSO4), filtered, and concentrated. The crude product was recrystallized from ethanol to afford 7 as a white solid (0.066 g, 0.23 mmol, 85 %), mp 159-160 °C. 1H NMR (DMSO-d6): δ 1.36 (s, 3H), 1.41 (s, 3H), 1.51-1.61 (m, 2H), 1.76-1.86 (m, 2H), 3.88 (m, 1H), 3.93 (m, 1H), 4.50 (t, 1H), 5.80 (s, 1H), 11.00 (br s, 1H). 13C NMR (DMSO-d6): δ 20.2, 24.3, 25.9, 26.6, 52.5, 81.9, 89.3, 100.0, 110.7, 142.0, 150.9, 163.6. Anal. Calcd. For C12H15N5O4 (0.25 EtOH): C, 49.28, H, 5.46, N, 23.00. Found: C, 49.41, H, 5.31, N, 22.88.
Preparation of 6-Amino-1-(2',3'-O-isopropylidenedioxy-cyclopent-1-yl)uracil (8)
To a solution of 7 (0.066 g, 0.23 mmol) in absolute EtOH (50 mL) was added Pd/C (10%, 10 mg), and the mixture subjected to hydrogentation at 25 psi for 20 min. The Pd/C was removed by filtration over a pad of celite, rinsed and the filtrate concentrated to afford 0.060 g of 8 as a white solid (quantitative), mp 225-227 °C. 1H NMR (DMSO-d6): δ 1.36 (s, 3H), 1.41 (s, 3H), 1.51-1.61 (m, 2H), 1.76-1.86 (m, 2H), 2.00 (br s, 2H), 3.88 (m, 1H), 3.93 (m, 1H), 4.50 (t, 1H), 4.70 (s, 1H), 11.00 (br s, 1H). 13C NMR (DMSO-d6): δ 20.2, 24.3, 25.9, 26.6, 52.5, 75.4, 81.9, 89.3, 110.7, 150.9, 153.1, 163.6. Anal. Calcd. for C12H17N3O4 (0.75 H2O): C, 51.36, H, 6.65, N, 14.98. Found: C, 51.37, H, 6.59, N, 15.06.
Preparation of 6-Amino-5-bromo-1-(2',3'-O-isopropylidenedioxy-cyclopent-1-yl) uracil (9)
To a solution of 8 (0.74 g, 2.77 mmol) and NaHCO3 (2.09 g, 24.93 mmol) in absolute EtOH (25 mL), was added dropwise, Br2 (5%, EtOH) until no starting material remained as observed by TLC. After removing the EtOH under vacuum, EtOAc (50 mL) was added, followed by washing with brine (3 × 50 mL). The organic layer was dried (MgSO4), filtered, concentrated, and purified via column chromatography eluting with nhexanes:EtOAc (1:4) to afford 0.77 g of 9 as a white solid (80 %). mp 214-216 °C. 1H NMR (DMSO-d6): δ 1.36 (s, 3H), 1.41 (s, 3H), 1.51-1.61 (m, 2H), 1.76-1.86 (m, 2H), 2.00 (br s, 2H), 3.88 (m, 1H), 3.93 (m, 1H), 4.50 (t, 1H), 11.00 (br s, 1H). 13C NMR (DMSO-d6): δ 20.2, 24.3, 25.9, 26.6, 51.8, 62.7, 81.9, 89.3, 110.7, 150.9, 159.9, 162.3. Anal. Calcd. For C12H16N3O4Br (0.20 EtOAc): C, 42.39, H, 4.89, N, 11.59. Found: C, 42.78, H, 4.91, N, 11.73.
Preparation of 3-(2',3'-O-isopropylidenedioxy-cyclopent-1-yl)xanthine (12)
A solution of 9 (0.77 g, 2.22 mmol) and NaN3 (0.72 g, 11.10 mmol) in anhydrous DMF (20 mL) was stirred at rt for 30 min, at which point H2O (20 mL) was added and the mixture extracted with EtOAc (3 × 50 mL), washed with brine (50 mL), dried (MgSO4), filtered, and concentrated to yield 0.54 g of 10, which was used directly without further purification.
To a solution of 10 (0.54 g, 1.75 mmol) in absolute EtOH (50 mL) was added Pd/C (10%, 0.054 g). This mixture was hydrogenated at a pressure of 25 psi for 20 min. The Pd/C was removed by filtration and the filtrate was concentrated to afford 0.49 g of 11 as a white solid, which was used directly in the next step without further purification.
Diamino 11 was dissolved in triethylorthoformate, heated at reflux for 1 h and the reaction monitored by TLC. Upon completion, the triethylorthoformate was removed by evaporation under reduced pressure and the resulting residue was purified by column chromatography eluting with EtOAc:MeOH (9:1) to afford 0.30 g of 12 as a white solid (59 %), mp 220-222 °C. 1H NMR (DMSO-d6): δ 1.38 (s, 3H), 1.41 (s, 3H), 1.51-1.61 (m, 2H), 1.76-1.86 (m, 2H), 3.88 (m, 1H), 3.93 (m, 1H), 4.50 (t, 1H), 8.30 (s, 1H), 10.00 (s, 1H), 13.4 (s, 1H). 13C NMR (DMSO-d6): δ 19.7, 24.2, 26.6, 27.1, 51.5, 81.8, 88.8, 110.7, 114.6, 144.6, 152.0, 154.9. HRMS: calcd for C13H16N4O4 (M+H)+, 2931172; found, 293.1247.
Preparation of 3-(2,3-Dihydroxy-cyclopent-1-yl)xanthine (3)
To a solution of 12 (0.30 g, 1.03 mmol) in TFA/H2O (2:1, 20 mL) was allowed to stir for 3 h at rt upon which time the TFA/H2O was removed by evaporation. The resulting residue was coevaporated with MeOH (3 × 10 mL) to remove trace amounts of TFA. The resulting residue was then purified first by column chromatography (EtOAc:acetone:EtOH:H2O, 4:1:1:0.5), and lastly by using C-18 HPLC eluting 90:10, H2O:MeOH → 50:50, H2O:MeOH → 10:90, H2O:MeOH to give 0.25 g of 3 as an off white solid (98 %), mp 254-256 °C. 1H NMR (CD3OD): δ 1.74-1.77 (m, 1H), 2.11-2.24 (m, 3H), 4.14-4.16 (m, 1H), 4.81-4.83 (m, 1H), 5.19-5.26 (q, 1H), 7.88 (s, 1H). 13C NMR (CD3OD): δ 19.4, 26.1, 53.4, 77.1, 85.4, 114.6, 144.6, 150.2, 152.0, 154.9. Anal. Calcd. For C10H12N4O4: C, 47.62, H, 4.80, N, 22.21. Found: C, 47.49, H, 4.89, N, 21.92.
Preparation of 1-[(2',3'-O-Isopropylidene)-cyclopent-1'-yl]-3-(4-methoxybenzyl)-6-iodouracil (6d)
Freshly distilled diisopropylamine (145 μL, 1.03 mmol) in THF (10 mL) was treated with n-butyllithium (414 μL, 2.5M in hexanes) at -78 °C and to this a stirred solution of 5d (192 mg, 0.517 mmol) in THF (10 mL) was added dropwise. The mixture was stirred for 30 min at which point a solution of I2 (525 mg, 2.07 mmol) in THF (10 mL) was added slowly and stirred for 3 h while maintaining the temperature at -78 °C. The reaction was quenched with a few drops glacial acetic acid, diluted with CHCl3 (100 mL), and the organic layer washed with saturated Na2S2O3 (2 × 25 mL), brine (3 × 50 mL), dried and the solvent removed under reduced pressure. The crude product was purified by column chromatography eluting with hexanes:EtOAc (1:1) to give 200 mg of 6d as a yellow foam (78%). 1H NMR (DMSO-d6): δ 1.18 (s, 3H), 1.40 (s, 3H), 1.82 (m, 2H), 2.19 (m, 2H) 3.68 (s, 3H), 4.74 (m, 1H, H-3'), 4.76 (t, 1H, H-1'), 4.79 (s, 2H, CH2), 4.86 (dd, 1H, H-2'), 6.46 (d, 1H, H-5), 6.83 (d, 2H), 7.18 (d, 2H). 13C NMR (CDCl3): δ 25.0, 27.6, 29.6, 29.8, 31.9, 44.1, 55.3, 76.3, 81.9, 84.5, 111.3, 113.8, 113.9, 116.0, 128.6, 130.6, 159.3.
Preparation of 1-[(2',3'-O-Isopropylidene)-cyclopent-1'-yl]-3-(4-methoxybenzyl)-6-methylaminouracil (15)
A stirred solution of 6d (1.12 g, 2.25 mmol) in NH2CH3 (30 mL, 33% EtOH) and stirred at rt for 1.5 h. The mixture was then condensed under reduced pressure and the residue co-evaporated from EtOH (3 × 50 mL). The crude product was purified using column chromatography eluting with hexanes:EtOAc (1:4) to give 15 as a sticky white solid (851 mg, 94%). 1H NMR (DMSO-d6): δ 1.16 (s, 3H), 1.37 (s, 3H), 1.76 (m, 2H), 2.19 (m, 2H), 2.64 (d, 3H), 3.67 (s, 3H), 4.59 (s, 2H), 4.75 (m, 1H, H-3'), 4.80 (t, 1H, H-1'), 4.86 (dd, 1H, H-2'), 6.46 (d, 1H, H-5), 6.81 (d, 2H), 7.15 (d, 2H). 13C NMR (CDCl3): δ 14.3, 24.7, 27.4, 28.6, 29.8, 30.2, 31.7, 43.2, 55.3, 60.5, 63.0, 81.2, 84.8, 112.0, 113.7, 130.2, 154.6, 158.9. HRMS calculated for C21H27N3O5 [M+H]+402.2029; found, 402.2020.
Preparation of 4-[(2',3'-O-Isopropylidene)-cyclopent-1'-yl]-6-(4-methoxybenzyl)-thiazolo[4,5-d]pyrimidine-5,7-dione (16)
To a stirred solution of 15 (62.5 mg, 0.156 mmol) in pyridine (1 mL) was added thionylchloride (5 mL) and refluxed for 3 h, then cooled and additional pyridine (5 mL) was added and the mixture evaporated under reduced pressure. The residue was co-evaporated with pyridine (2 × 5 mL) and the residue purified by preparative TLC eluting with hexane:EtOAc (1:1) to afford 29.4 mg of 16 appeared as an off-white sticky solid (80%). 1H NMR (CDCl3): δ 1.29 (s, 3H), 1.52 (s, 3H), 1.91-2.36 (m, 4H), 3.76 (s, 3H), 4.92 (m, 1H, H-3'), 5.10 (t, 1H, H-1'), 5.12 (s, 2H), 5.50 (td, 1H, H-2'), 6.82 (d, 2H), 7.45 (d, 2H), 8.92 (s, 1H, H- thiazole). 13C NMR (CDCl3): δ 14.3, 21.1, 25.0, 27.5, 29.4, 31.8, 44.6, 55.3, 60.5, 64.1, 81.4, 84.0, 111.6, 113.9, 128.9, 130.8, 151.2, 159.0, 159.3. HRMS calculated for C21H23N3O5S [M+H]+ 430.1437; found, 430.1424.
Preparation of 4-[(2',3'-O-Diacetoxy)-cyclopent-1'-yl]-6-(4-methoxybenzyl)-thiazolo[4,5-d]pyrimidine-5,7-dione (17)
A solution of 16 (391 mg, 0.910 mmol) in TFA:H2O (2:1, 15 mL) was stirred at rt for 18 h. The solvents were removed under reduced pressure and the resulting residue coevaporated with EtOH (3 × 10 mL). The crude product was dissolved in pyridine (120 μL, 2.29 mmol) and dry CH2Cl2 (10 mL), acetic anhydride (250 mg, 2.45 mmol) and DMAP (20 mg, 0.16 mmol) added. The reaction was stirred at rt for 12 h before cooling to 0 °C and quenched with saturated NaHCO3 solution. The organic layer was washed with sat. NaHCO3 (3 × 20 mL), 1N HCl (3 × 20 mL), brine (20 mL), dried and concentrated. The resulting yellow oil was purified using column chromatography eluting with hexanes:EtOAc (1:4) to afford 354 mg of 17 (82%) as a white hygroscopic powder. 1H NMR (CDCl3): δ 1.94 (s, 3H), 2.10 (s, 3H), 2.17-2.44 (m, 4H), 3.76 (s, 3H), 5.15 (d, 2H), 5.52 (q, 1H, H-3'), 5.69 (qd, 1H, H-1'), 5.87 (dd, 1H, H-2'), 6.82 (d, 2H), 7.47 (d, 2H), 8.95 (s, 1H, H-thiazole). 13C NMR (CDCl3): δ 20.8, 21.1, 24.6, 28.2, 29.8, 44.8, 55.3, 73.2, 75.1, 77.3, 113.8, 128.9, 130.8, 157.4, 159.1, 159.3, 170.3, 170.5. HRMS calculated for C22H23N3O7S [M]+ 473.1257; found, 473.1254.
Preparation of 4-[(2',3'-O-Diacetoxy)-cyclopent-1'-yl]-thiazolo[4,5-d]pyrimidine-5,7-dione (18)
A stirred solution of 17 (354 mg, 0.748 mmol) in CH3CN:H2O (10:1, 11 mL) was heated to 55 °C, ceric ammonium nitrate (450 mg, 0.820 mmol) added and the mixture stirred for 3 h. The reaction was cooled, quenched with saturated NH4Cl solution (1 mL) and evaporated to dryness. The crude residue was purified by column chromatography eluting with hexanes:EtOAc (1:4) to afford 228.2 mg of 18 as a white foam (86%). 1H NMR (CDCl3): δ 1.95 (s, 3H), 2.09 (s, 3H), 2.32 (m, 2H), 2.45 (m, 2H), 5.49 (q, 1H, H-3'), 5.64 (dt, 1H, H-1'), 5.86 (dd, 1H, H-2'), 9.01(s, 1H), 9.93 (s, 1H). 13C NMR (MeOH-d6): δ 20.8, 21.1, 24.7, 28.2, 29.8, 31.0, 73.2, 75.0, 107.6, 151.2, 157.4, 157.7, 160.0, 170.4, 170.7.
Preparation of 1'-(Thiazolo[4,5-d]-pyrimidine-5,7-dion-4-yl)-cyclopentane-2',3'-diol (4)
To a solution of 18 (107 mg, 0.304 mmol) in EtOH (0.6 mL) was added concentrated NH4OH (1.0 mL) and stirred for 18 h. The mixture was then concentrated under reduced pressure and the crude residue purified by reverse phase C-18 HPLC eluting 90:10, H2O:CH3CN → 70:30, H2O:CH3CN → 50:50, H2O:CH3CN to afford 61.4 mg of 4 as a white crispy foam (75 %). 1H NMR (CD3OD): δ 2.08 (m, 2H), 2.20 (m, 2H), 5.56 (q, 1H, H-3'), 5.61 (dt, 1H, H-1'), 5.88 (q, 1H, H-2'), 9.29 (s, 1H, H-thiazole). 13C NMR (CD3OD): δ 13.1, 19.5, 20.7, 29.4, 60.2, 74.5, 113.7, 127.5, 171.7, 175.1. HRMS calculated for C10H11N3O4S [M+H]+ 270.0470; found, 270.0545.
SAHase Inhibition Assay
The enzyme-coupled continuous assay in the hydrolysis direction was performed as previously reported. Briefly, SAH is hydrolyzed to homocysteine and adenosine, which is subsequently converted by adenosine deaminase into ammonia and inosine, a process associated with a decrease of absorbance at 265 nm.26 Each assay was conducted in thermostatted 1 cm quartz cuvettes at 37 °C maintained by a Peltier unit on a Cary 100 ultraviolet-visible photospectrometer. Enzyme assay solution typically contained 50 mM potassium phosphate at pH 7.4, 0.39 units of adenosine deaminase (Worthington Biochemical, catalog number LS009043), 132 nM of SAHase (provided by Dr. Lynne Howell), 10 μM of SAH and various concentrations of inhibitors in a total volume of 928 μL. The reactions were initiated by the addition of SAH. In all cases, we ascertained that SAH hydrolysis catalyzed by SAHase was rate limiting under testing conditions (data not shown). The kinetic data were analyzed using KaleidaGraph 4.0 (Synergy). Based on a competitive inhibition mechanism, the Ki value was determined using the equation, ν = kcat × [S] × [E] /{Km × (1 + [I]/Ki) + [S]}; and ν, kcat, [S], [E], Km, [I] and Ki stand for the initial reaction rates, rate constant, substrate concentration, enzyme concentration, Michaelis-Menten constant, inhibitor concentration and dissociation constant of the enzyme-inhibitor complex, respectively. A previously reported Km value of 7.9 μM for SAH in the hydrolysis direction was used for all calculation.26
Scheme 4.
Reagents and conditions: a, LDA, THF, I2, -78 °C, 3h, 58%; b, 33% NH2Me, EtOH, rt, 1.5h, 94%; c, SOCI2, pyridine, reflux, 3h, 80%; d, i. TFA:H2O (2:1), rt, 18h; ii. Ac2O, pyridine, DMAP, CH2CI2, 12h, 82% for 2 steps; e, 10:1 CH3CN:H2O, CAN, 55 °C, 3h, 86%; f, NH3, MeOH, rt, 15h, 65%.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) (RO1 CA 97634 to KSR and 1R01AI58146 to Z.S.Z) and the Herman Frasch Foundation (541-HF02 to Z.S.Z.). We are grateful of Prof. Lynne Howell of the Hospital of Sick Children of providing SAHase and Dr. Phil Mortimer of the Johns Hopkins Mass Spectrometry Facility for his invaluable assistance with HRMS analysis. We also thank Ms. Sarah Zimmermann for her editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Silverman RB. The Organic Chemistry of Drug Design and Drug Action. Elsevier; Evanston: 2004. [Google Scholar]
- 2.Kompis IM, Islam K, Then RL. Chem. Rev. 2005;105:583. doi: 10.1021/cr0301144. [DOI] [PubMed] [Google Scholar]
- 3.Lyon MA, Ducruet AP, Wipf P, Lazo JS. Nat Rev Drug Discov. 2002;1:961. doi: 10.1038/nrd963. [DOI] [PubMed] [Google Scholar]
- 4.Gangjee A, Li W, Yang J, Kisliuk RL. J Med Chem. 2008;52:68. doi: 10.1021/jm701052u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard NJ, Laursen RA. J. Am. Chem. Soc. 1963;85:2026. [Google Scholar]
- 6.Leonard NJ, Laursen RA. Biochemistry. 1965;4:354. [Google Scholar]
- 7.Schmidt CL, Rusho WJ, Townsend LB. J. Chem. Soc., Chem. Commun. 1971:1515. [Google Scholar]
- 8.Schmidt CL, Townsend LB. J. Org. Chem. 1975;40:2476. doi: 10.1021/jo00905a013. [DOI] [PubMed] [Google Scholar]
- 9.Seley KL, Mosley SL, Zeng F. Org Lett. 2003;5:4401. doi: 10.1021/ol035696q. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq E. Nucleosides Nucleotides. 1998;17:625. doi: 10.1080/07328319808005205. [DOI] [PubMed] [Google Scholar]
- 11.Marquez VE. In: Adv Antivir Drug Des. De Clercq E, editor. Vol. 2. JAI Press; Greenwich: 1996. p. 89. [Google Scholar]
- 12.Marquez VE, Lim M-I. Med. Res. Rev. 1986;6:1. doi: 10.1002/med.2610060102. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq E. Biochem. Pharmacol. 1987;36:2567. doi: 10.1016/0006-2952(87)90533-8. [DOI] [PubMed] [Google Scholar]
- 14.Borcherding DR, Scholtz SA, Borchardt RT. J. Org. Chem. 1987;52:5457. [Google Scholar]
- 15.Hasobe M, Liang H, Ault-Riche DB, Borcherding DR, Wolfe MS, Borchardt RT. Antivir. Chem. Chemother. 1993;4:245. [Google Scholar]
- 16.Hasobe M, McKee JG, Borcherding DR, Borchardt RT. Antimicrob. Agents Chemother. 1987;31:1849. doi: 10.1128/aac.31.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasobe M, McKee JG, Borcherding DR, Keller BT, Borchardt RT. Mol. Pharmacol. 1988;33:713. [PubMed] [Google Scholar]
- 18.Mosley SL, Bakke BA, Sadler JM, Sunkara NK, Dorgan KM, Zhou ZS, Seley-Radtke KL. Bioorg Med Chem. 2006;14:7967. doi: 10.1016/j.bmc.2006.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan B-C, Chen Z-H, Chu E, Wang Chu M-Y, Chu S-H. Nucleosides Nucleotides. 1998;17:2367. [Google Scholar]
- 20.Goldman IM. J. Org. Chem. 1969;34:3285. doi: 10.1021/jo01263a014. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno Y, Watanabe Y, Ikeda K. Chem. Pharm. Bull. 1974;22:1198. doi: 10.1248/cpb.22.1198. [DOI] [PubMed] [Google Scholar]
- 22.Jaime-Figueroa S, Zamilpa A, Guzman A, Morgans J, David J. Synth. Commun. 2001;31:3739. [Google Scholar]
- 23.Seley KL, Zhang L, Hagos A. Org Lett. 2001;3:3209. doi: 10.1021/ol0165443. [DOI] [PubMed] [Google Scholar]
- 24.Seley KL, Zhang L, Hagos A, Quirk S. J Org Chem. 2002;67:3365. doi: 10.1021/jo0255476. [DOI] [PubMed] [Google Scholar]
- 25.Seley KL, Januszczyk P, Hagos A, Zhang L, Dransfield DT. J Med Chem. 2000;43:4877. doi: 10.1021/jm000326i. [DOI] [PubMed] [Google Scholar]
- 26.Elrod P, Zhang J, Yang X, Yin DH, Hu Y, Borchardt RT, Schowen RL. Biochemistry. 2002;41:8134. doi: 10.1021/bi025771p. [DOI] [PubMed] [Google Scholar]