Abstract
The production of the peptide hormones ACTH, α-MSH, and β-endorphin requires proteolytic processing of POMC which is hypothesized to utilize dual cysteine and subtilisin-like protease pathways, consisting of the secretory vesicle cathepsin L pathway and the well-known subtilisin-like prohormone convertase (PC) pathway. To gain knowledge of these protease components in human pituitary where POMC-derived peptide hormones are produced, this study investigated the presence of these protease pathway components in human pituitary. With respect to the cathepsin L pathway, human pituitary contained cathepsin L of 27-29 kDa and aminopeptidase B of ∼64 kDa, similar to those in secretory vesicles of related neuroendocrine tissues. The serpin inhibitor endopin 2, a selective inhibitor of cathepsin L, was also present. With respect to the PC pathway, human pituitary expresses PC1/3 and PC2 of ∼60-65 kDa, which represent active PC1/3 and PC2; peptide hormone production then utilizes carboxypeptidase E (CPE) which is present as a protein of ∼55 kDa. Analyses of POMC products in human pituitary showed that they resemble those in mouse pituitary which utilizes cathepsin L and PC2 for POMC processing. These findings suggest that human pituitary may utilize the cathepsin L and prohormone convertase pathways for producing POMC-derived peptide hormones.
Keywords: Proteases, cathepsin L, prohormone convertase, ACTH, β-endorphin, α-MSH, POMC
Introduction
The pituitary peptide hormones ACTH, α-MSH, and β-endorphin play key roles in regulating human endocrine functions (1-5). These hormones are generated from the common POMC (propiomelanocortin) precursor that undergoes proteolytic processing to generate the active peptide hormones (6-9). Elucidation of the prohormone processing proteases has been achieved in model neuroendocrine secretory vesicles from other mammalian species that include bovine, mouse, and rat. These studies have led to the proposed function of dual protease pathways for prohormone processing consisting of the newly identified cysteine protease pathway composed of the secretory vesicle cathepsin L followed by the exopeptidase aminopeptidase B (10-14), and the well-established prohormone convertases (PC1/3 and PC2) (14-16) followed by the exopeptidase carboxypeptidase E (CPE) (16-19).
Evidence for participation of cathepsin L in producing POMC-derived peptide hormones has been demonstrated in cathepsin L knockout mice (20). The peptide hormones ACTH, α-MSH, and β-endorphin were reduced by 75% to 90% in pituitaries of cathepsin L knockout mice (20). The lack of cathepsin L results in accumulation of full-length POMC and a POMC-derived intermediate of 22 kDa, suggesting these as substrates of cathepsin L. Furthermore, expression of cathepsin L in the pituitary AtT-20 cell line (mouse) resulted in production of ACTH and β-endorphin in the regulated secretory pathway, consistent with the localization of cathepsin L with POMC-derived peptide hormones in secretory vesicles. Furthermore, cathepsin L in secretory vesicles participates in the proteolytic processing of proenkephalin to generate the enkephalin neuropeptide in adrenal medulla (bovine and mouse) and brain (mouse) (11,12). These studies provide evidence for a role of secretory vesicle cathepsin L in processing POMC and related prohormones into active peptides.
In vivo studies of PC2 protease gene knockout mice also demonstrate participation of PC2 in production of POMC-derived peptide hormones and neuropeptides (21-24). PC2 null mice show nearly complete absence of α-MSH (21,24), indicating the primary role of PC2 in producing α-MSH as a primary peptide product resulting from PC2. These knockout mice also show elevated ACTH, consistent with ACTH as substrate for PC2 (21). Furthermore, PC2 null mouse pituitaries show elevated β-endorphin(1-31), which evidently serves as substrate for PC2 (21,23,25). In addition, PC1 deficient mice show increased β-endorphin (25), a partial decrease in ACTH, and no change in α-MSH (25, 26).
Significantly, the presence of these protease components should be demonstrated in human pituitary for their possible participation in generating human ACTH, α-MSH, and β-endorphin in normal human pituitary gland. Therefore, this study demonstrated that human pituitary contains cathepsin L and aminopeptidase B, combined with the PC1/3 and PC2 proteases with carboxypeptidase E (by western blots). Analyses of POMC-derived intermediates in human pituitary (western blots) showed that they resemble those in mouse pituitary that utlizes cathepsin L, PC2, and PC1/3 proteases for processing POMC into its peptide hormone products. The presence of secretory vesicle cathepsin L and prohormone convertase protease components in human pituitary supports their predicted roles for production of ACTH, α-MSH, and β-endorphin peptide hormones from POMC.
Results
Cathepsin L protease pathway components in human pituitary
The cysteine protease cathepsin L, combined with a subsequent aminopeptidase B step, have been demonstrated to represent a new protease pathway (fig. 1) in secretory vesicles for production of POMC-derived peptide hormones in mice (14). To assess the presence of these proteases in human pituitary, western blots with specific antisera to each protease components were performed (fig. 2). Results illustrated the detection of 27-29 kDa cathepsin L, which represents the active form of cathepsin L detected in chromaffin secretory vesicles (bovine). Cathepsin L is known to be present in both secretory vesicles and lysosomes (14, 20) based on studies in several mammalian species. But since cathepsin L has not been demonstrated in human secretory vesicles, this study examined the colocalization of cathepsin L with ACTH and β-endorphin in human pituitary sections by immunofluorescence confocal microscopy (fig. 3). Results demonstrated the colocalization of ACTH with cathepsin L, and colocalization of β-endorphin with cathepsin L. Because ACTH and β-endorphin are present in secretory vesicles, these data suggest the presence of cathepsin L in ACTH- and β-endorphin-containing secretory vesicles.
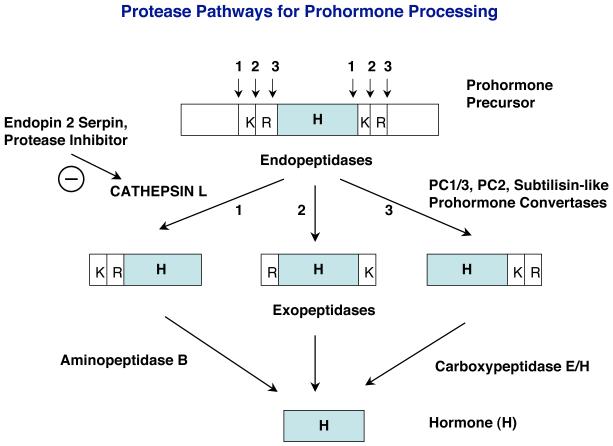
Figure 1. Dual protease pathways for prohormone processing.
Distinct cysteine protease and subtilisin-like protease pathways have been demonstrated for proneuropeptide processing in model neuroendocrine systems largely from bovine and mouse. Recent studies have identified secretory vesicle cathepsin L as a processing enzyme for the production of the endogenous enkephalin and other neuropeptides. The specificity of cathepsin L to cleave at the NH2-terminal side of dibasic residue processing sites of proneuropeptides yields peptide intermediates with NH2-terminal residues, which can be removed by aminopeptidase B. (Cathepsin L may also cleave between the dibasic residues, which then results in neuropeptide intermediates requiring both exopeptdases of aminopeptidase B and carboxypeptidase E for removal of basic residues at NH2- and COOH-termini to generate neuropeptides.) The serpin endopin 2 is an efficient inhibitor of cathepsin L; endopin 2 is colocalized with cathepsin L in secretory vesicles. The well-established subtilisin-like protease pathway involves the prohormone convertases PC1/3 and PC2. The PC enzymes preferentially cleave at the COOH-terminal side of dibasic processing sites, which results in peptide intermediates with basic residue extensions at their COOH-termini that are removed by carboxypeptidase E/H.
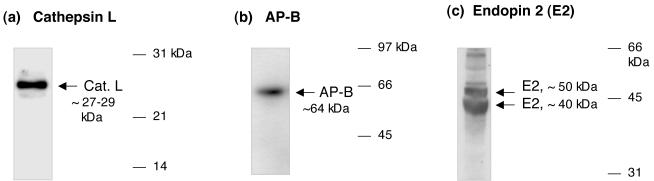
Figure 2. Cathepsin L, endopin 2, and aminopeptidase B pathway components in human pituitary.
(a) Cathepsin L in pituitary. Cathepsin L in human pituitary was detected by western blots as a 27-29 kDa enzyme protein, which corresponds to secretory vesicle cathepsin L identified in secretory vesicles that contain neuropeptides, peptide hormones and neurotransmitters (11).
(b) Aminopeptidase B in pituitary. Aminopeptidase B (AP-B) in human pituitary was detected by western blots as a 64 kDa enzyme protein, which corresponds to AP-B characterized in neuropeptide-containing secretory vesicles and neuroendocrine tissues (13).
(c) Endopin 2 in pituitary. Endopin 2 is a serpin protease inhibitor demonstrated to efficiently inhibit cathepsin L (28,29). Western blot of endopin 2 demonstrates its presence in human pituitary. The anti-endopin 2 serum does not crossreact with the related human α1-antichymotrypsin. Therefore, these western blots likely represent endopin 2 in human pituitary.
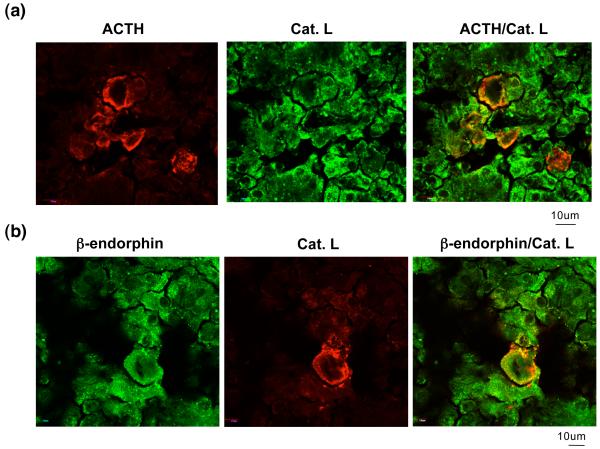
Figure 3. Cathepsin L colocalization with ACTH and β-endorphin in human pituitary by immunofluorescence confocal microscopy.
(a) ACTH and cathepsin L colocalization. Immunofluorescence confocal microscopy was utilized to illustrate the colocalization of ACTH with cathepsin L in human pituitary tissue sections. ACTH (red fluorescence) was visualized in distinct cells, and cathepsin L (Cat. L, green fluorescence) was observed in many cells. Importantly, ACTH-containing cells showed areas containing both ACTH and cathepsin L, visualized by the merged images indicating yellow-orange and the areas of colocalization. Because ACTH is known to be present in secretory vesicles, its colocalization with cathepsin L suggests the presence of cathepsin L in ACTH-containing secretory vesicles.
(b) Beta-endorphin and cathepsin L colocalization. Immunofluorescence confocal microscopy was utilized to show the colocalization of β-endorphin with cathepsin L in human pituitary tissue sections. Beta-endorphin (red fluorescence) was visualized in pituitary cells in a punctate pattern, consistent with its secretory vesicle localization. Importantly, b-endorphin-containing cells showed areas of colocalization with cathepsin L, indicated by the areas of yellow-orange fluorescence in the merged image. These results suggest the colocalization of cathepsin L with β-endorphin in secretory vesicles.
Endopin 2, a serpin protease inhibitor of cathepsin L (28,29), was was found to be present in human pituitary as bands of ∼50 and ∼40 kDa, illustrated in western blots (fig. 2). Recombinant endopin 2 expressed in E. coli shows an apparent molecular weight on SDS-PAGE gels of ∼45-48 kDa (28, 29). Furthermore, human pituitary contains aminopeptidase B (AP-B) of ∼64 kDa, which corresponds to AP-B in several neuroendocrine tissues of bovine and rat (13).
The presence of cathepsin L, endopin 2, and aminopeptidase B in human pituitary indicates that the protease and protease inhibitor components of the cathepsin L protease pathway are present in human pituitary. Moreover, cathepsin L is colocalized with ACTH and β-endorphin in secretory vesicles. These protease pathway components have been demonstrated in other mammalian species (including mouse, rat, and bovine) to be involved in the production of POMC-derived peptide hormones.
Prohormone convertases 1 and 2 (PC1/3 and PC2), with carboxypeptidase E (CPE) in human pituitary
The PC1/3 and PC2 proteases, combined with carboxypeptidase E (CPE), represents the subtilisin-like protease pathway for prohormone processing (fig. 1). Western blots demonstrated the presence of PC1/3 of ∼60-65 kDa, and PC2 of ∼60-65 kDa (fig. 4), which are similar to the active forms of these protease purified from chromaffin secretory vesicles (30) and detected in rat and mouse (31-34). Following prohormone processing by PC/13 and/or PC2, carboxypeptidase E (CPE) functions to remove COOH-terminal basic residues from peptide intermediates (17,18,35). CPE was shown to be present in human pituitary with an apparent molecular weight of 55 kDa, similar to the that in rat and bovine pituitary (35,36). These findings indicate that human pituitary contains the PC1/3 and PC2 subtilisin-like protease pathway with CPE that function in prohormone processing.
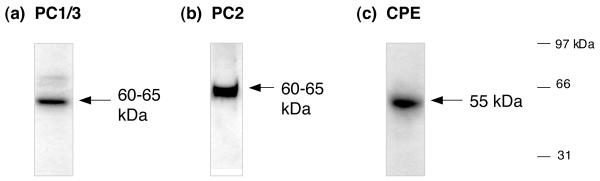
Figure 4. PC1/3 and PC2 prohormone convertases with carboxypeptidase E (CPE) components in human pituitary.
(a) PC1/3. PC1/3 in human pituitary was detected by western blots as an enzyme of ∼60 kDa, corresponding to PC1/3 characterized in secretory vesicles and in neuroendocrine tissues (30,32).
(b) PC2. PC2 in human pituitary was detected western blots as an enzyme of ∼60 kDa, similar to PC2 in secretory vesicles and neuroendocrine tissues (30,31).
(c) Carboxypeptidase E (CPE). CPE was observed in human pituitary by western blots as a protein band of 55 kDa, similar to CPE characterized in secretory vesicles and neuroendocrine tissues in bovine, rat, mouse, and other non-human mammalian species.
POMC-derived peptide products in human pituitary
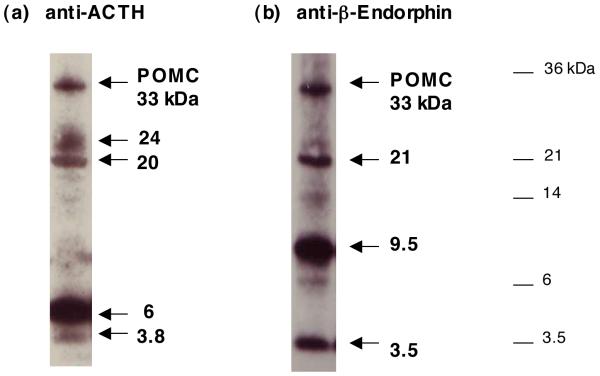
Analyses of peptide intermediates and hormones derived from POMC was achieved by western blots with anti-ACTH and anti-β-endorphin (fig. 5). POMC and POMC-derived ACTH-related products were illustrated as POMC of 33 kDA that represent POMC’s relative mobility on SDS-PAGE (21). POMC-derived products that were detected by anti-ACTH were composed of peptides with apparent mobility and molecular weight of 24, 20, 6, and 3.8 kDa (fig. 5). Based on predicted processing of POMC at dibasic and tetrabasic sites, the 24 kDa product of POMC may represent the POMC domains of N-POMC/γ-MSH/JP/ACTH/portion of γ-LPH (illustrated in fig. 6). The 20/21 kDa intermediate may represent N-POMC/γ-MSH/JP/ACTH or ACTH/β-lipotropin (fig. 6). The 6 kDA band corresponds to standard ACTH examined on these gels. In addition, the 3.8 kDa band corresponds most closely in apparent molecular weight with CLIP, the C-terminal domain of ACTH (fig. 6).
Figure 5. Anti-ACTH and anti-β-endorphin western blots in human pituitary for analyses of POMC-derived peptides.
(a) ACTH-containing products of POMC. ACTH-related peptide derived from POMC was assessed in human pituitary by anti-ACTH western blots. Blots detected ACTH-related peptides of 24, 20, 6, and 3.8 kDa. The 6 kDa band comigrates with standard ACTH (data not shown). The 33 kDa band corresponds to the mobility of recombinant POMC studied previously (21).
(b) Beta-endorphin containing products of POMC. Beta-endorphin related peptide products derived from POMC was assessed in human pituitary by anti-β-endorphin western blots, which detected peptide products of 21, 9.5, and 3.5 kDa. The 3.5 kDa band comigrates with standard β-endorphin (data not shown). The 33 kDa band corresponds to POMC, as illustrated previously for recombinant POMC (21).
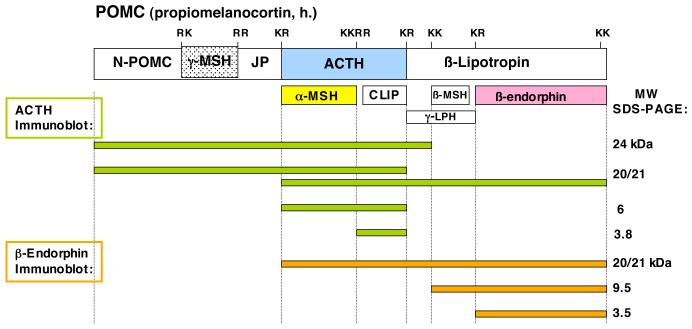
Figure 6. POMC-derived peptide products in human pituitary.
Mapping of the estimated POMC-derived peptide products detected by western blots with anti-ACTH and anti-β-endorphin has been predicted based on processing at dibasic, as well as the tetrabasic, processing sites of POMC. Based on western blot data, ACTH-related products (shown as green bars) are predicted as the POMC-derived products of 24 kDa N-POMC/γ-MSH/JP/ACTH, 20/21 kDa N-POMC/γ-MSH/JP/ACTH or ACTH/β-lipotropin, 6 kDa ACTH, and 3.8 kDa CLIP. Beta-endorphin-related products (shown as orange bars) are predicted as the POMC-derived products of 20/21 kDa ACTH/β-lipotropin, 9.5 kDa β-MSH/β-endorphin, and 3.5 kDa β-endorphin.
Western blots of β-endorphin positive bands illustrated POMC-derived products with apparent molecular weights of 20/21, 9.5, and 3.5 kDa (fig. 5). The 20/21 kDa band is likely to correspond to ACTH/β-lipotropin (fig. 6). The 9.5 kDa band most closely corresponds to a portion of β-lipotropin, and the 3.5 kDa fragment corresponds to standard β-endorphin (fig. 6).
With respect to final peptide products derived from POMC by proteolytic processing, the concentrations of ACTH, α-MSH, and β-endorphin in human pituitary were measured by radioimmunoassay (Table 1). ACTH was present at the highest level in human pituitary at a concentration of 4.94 pmol/μg protein. Lower levels of α-MSH were present at 0.138 pmol/μg protein. Beta-endorphin was measured at the lowest level of 0.382 fmol/μg protein. These different levels of peptide hormones derived from POMC suggest differential processing of POMC into distinct amounts of ACTH, α-MSH, and β-endorphin that are derived from the common POMC precursor.
Table 1. ACTH, β-Endorphin, and α-MSH Content in Human Pituitary.
| Peptide Hormone |
Tissue Concentration |
|---|---|
| ACTH | 4.94 ± 1.73 pmol/μg |
| β-Endorphin | 0.382 ± 0.073 fmol/μg |
| α-MSH | 0.138 ± 0.049 pmol/μg |
Pituitary tissue acid extracts were prepared as described in the methods, and the POMC-derived peptide hormones ACTH, β-endorphin, and α-MSH were measured by radioimmunoassay. Peptide hormone levels are expressed as pmol or fmol of hormone per μg protein, as the mean ± s.e.m. (n = 6).
Discussion
This study investigated human pituitary for the presence of protease pathway components that are known to be involved in producing the POMC-derived peptide hormones ACTH, alpha-MSH, and beta-endorphin (20-26). It is essential to demonstrate that human pituitary contains proteases that have been demonstrated to participate in producing these POMC-derived peptide hormones in other mammalian species. Significantly, human pituitary contains all the known protease components of the dual cathepsin L and prohormone convertase pathways for prohormone processing. The cathepsin L pathway, which includes aminopeptidase B and endopin 2, has been recently demonstrated to participate as a major mechanisms for producing POMC-derived peptide hormones in cathepsin L knockout mice and in in vitro cellular protease expression studies (20). Comparison of the POMC-derived intermediates in human pituitary with those in mouse pituitary provide insight into the roles of the cathepsin L and prohormone convertase proteases in producing human pituitary peptide hormones.
Prohormone processing enzymes were demonstrated in human pituitary by western blots for each of the protease components. Results showed that human pituitary contains cathepsin L of 27-29 kDa, and aminopeptidase B of ∼64 kDa, which are similar to properties of these proteases in secretory vesicles of bovine, rat and mouse neuroendocrine tissues (10-14). Furthermore, PC1/3 and PC2 of 60-65 kDa, respectively, are present in human pituitary, similar to the molecular sizes (by SDS-PAGE) of active PC1/3 and PC2 purified from chromaffin secretory vesicles (30).
Importantly, POMC-derived intermediates and peptide hormone products in human pituitary are comparable with those in mouse pituitary which have been demonstrated to function as substrates or peptide products of cathepsin L (20), and of PC2 as well as PC1/3 (21-26), in mice deficient in each of these proteases. These findings illustrate that human pituitary expresses the dual cysteine and subtilisin-like protease pathways demonstrated in mice and other species to participate in the production of the peptide hormones ACTH, α-MSH, and β-endorphin from POMC.
Specifically, examination of POMC-derived intermediates and peptide products in human pituitary demonstrated the presence of endogenous POMC-derived intermediate and final peptide hormone products. The final POMC-derived peptides ACTH and β-endorphin were detected in human pituitary by western blots and RIA, and the presence of α-MSH was measured by RIA. POMC-derived intermediates that include the ACTH domain in human pituitary were observed as 24 kDa, 20/21 kDa and 3.8 kDa bands in anti-ACTH western blots. The 24 kDa product is predicted to represent N-POMC/γ-MSH/JP/ACTH/portion of γ-LPH, and the 3.8 kDa band may correspond to CLIP. The 20/21 kDa product may represent N-POMC/γ-MSH/JP/ACTH, or the fragment corresponding to ACTH/β-LPH since this band was also detected by anti-β-endorphin. Other POMC-derived intermediates that include the β-endorphin domain include a 20/21 kDa band that may correspond to ACTH/β-lipotropin and a 9.5 kDa band that is predicted to correspond to β-MSH/β-endorphin.
Comparison of POMC-derived products in human pituitary with those in mice deficient in cathepsin L or PC2 suggests that the majority of the POMC-derived peptides in human pituitary serve as substrate or product of these processing proteases (Table 2). POMC and the 20/21 ACTH-containing intermediate serve as substrates for cathepsin L (Table 2) (20). The final peptide hormones ACTH, α-MSH, and β-endorphin represent peptide products of cathepsin L, as they are substantially reduced in cathepsin L knockout mice (20). Furthermore, POMC and the 20/21, 9.5, 6.5, and 3.5 kDa products of POMC serve as substrates for PC2 (Table 2), since they accumulate in pituitaries of PC2 knockout mice (21). Alpha-MSH represents a peptide product of PC2 based on studies of PC2 deficient mice that lack substantial amounts of α-MSH (21). ACTH and β-endorphin(1-31) represent substrates of PC2, since they are elevated in PC2 deficient mice (21,23). Furthermore, in vitro cellular expression of cathepsin L (12), as well as PC2 and PC1/3 (37), have demonstrated their participation in processing POMC into intermediates and into the peptide hormone products ACTH, β-endorphin, and α-MSH that are observed in vivo. Thus, analyses of POMC products in human pituitary indicate that they may represent peptide products or substrates of cathepsin L and PC2.
Table 2. Human Pituitary POMC-Derived Products: Comparison with POMC Products Examined in Knockout Mice Deficient in Cathepsin L or PC2.
| Human Pituitary POMC-Derived Products |
POMC Products that Accumulate in: . |
||||
|---|---|---|---|---|---|
| MW, SDS-PAGE |
ACTH |
β-Endorphin |
Cathepsin L KO mice |
or | PC2 KO mice |
| 33 kDa | + | + | + (S) | + (S) | |
| 24 kDa | + | - | - | - | |
| 20/21 kDa | + | + | + (S) | + (S) | |
| 9.5 kDa | - | + | - | + (S) | |
| 6 kDa | + | - | - | + (S) | |
| 3.8 kDa | + | - | - | - | |
| 3.5 kDa | - | + | - | + (S) | |
The apparent molecular weight (MW) of POMC-derived peptide products detected by western blots with antisera to ACTH and β-endorphin are listed. POMC-derived products studied in cathepsin L and PC2 knockout (KO) mice are shown for comparison. POMC-derived bands that accumulated in the protease knockout mice (20,21) implicate their function as substrates (S) for cathepsin L and/or PC2. Thes similar POMC-derived products in human pituitary that accumulate in protease gene knockout mice deficient in cathepsin L or PC2 implicate the involvement of these two prohormone processing enzymes in human pituitary.
Cellular studies of protease colocalization with POMC-derived peptide hormones are consistent with roles of cathepsin L, PC2, and PC1/3 in producing these peptide hormones. More specifically previous in vitro and in vivo studies have illustrated the colocalization of the proteases PC1/3, PC2, cathepsin L, aminopeptidase B, carboxypeptidase E with POMC-derived peptide hormones in pituitary (10-26). Recent studies of secretory vesicle cathepsin L demonstrates its colocalization with POMC-derived ACTH, β-endorphin, and α-MSH in pituitary (20). In the anterior pitutiary, cathepsin L was expressed in ACTH-containing cells. In the intermediate pituitary, cells strongly expressed beta-endorphin and alpha-MSH which were colocalized with cathepsin L. Moreover, cathepsin L and the lysosomal marker Lamp-1 immunostaining in the mouse pituitary showed weak colocalization (20). These findings supported the hypothesis for cathepsin L localization in peptide hormone-containing secretory vesicles in pituitary.
Previously, the presence of the prohormone convertases PC1/3 and PC2 has also been demonstrated in corticotroph cells in normal human pituitaries (38). The enzymes aminopeptidase B and carboxypeptidase E are utilized following the actions of the proteases cathepsin L, PC1/3 and PC2, respectively to generate active neuropeptides (14). A study of anterior pituitary showed expression of carboxypeptidase E in most cells of human pituitary (39). The presence of aminopeptidase B has also been established in rat and bovine pituitary secretory vesicles, and colocalization of cathepsin L and aminopeptidase B has been observed in secretory vesicles of adrenal medulla (12).
Overall, results from this study demonstrate the expression in normal human pituitary of proteases demonstrated to be involved in the production of ACTH, α-MSH, and β-endorphin in mouse and other non-human mammalian species. Thus, like other neuroendocrine tissues from other species, human pituitary also expresses the recently discovered cysteine protease cathepsin L and aminopeptidase B protease pathway for prohormone processing (10-14,20), as well as the well known PC1/3 and PC2 prohormone convertases with carboxypeptidase E pathway (14-18). These proteases are predicted to participate in the production of the human pituitary peptide hormones ACTH, α-MSH, and β-endorphin that are generated by proteolytic processing of the POMC prohormone precursor. Thus, this study has provided knowledge of the cathepsin L and prohormone convertase protease pathways in normal, healthy human pituitary that produces POMC-derived peptide hormones. It will be of interest in future studies to explore the profile of these processing protease pathway components in human disease conditions that include cancer tumors and Cushing’s disease.
Materials and Methods
Analyses of protease pathway components in pituitary homogenates by western blots
Frozen human pituitaries were obtained from the National Hormone and Peptide Program, (Harbor-UCLA Medical Center, Torrance, CA) and homogenized in buffer (100 mM Tris-HCl, p. 7.4, 50 mM NaCl, 1 mM EDTA) containing a cocktail of protease inhibitors (10 μM pepstatin A, 10 μM leupeptin, 10 μM chymostatin, 10 μM E64c and 100 μM AEBSF). Protein concentration was measured by the Bio-Rad Dc method (Bio-Rad, Hercules, CA). Homogenate samples (20-40 μg protein) were subjected to SDS-PAGE and western blot analyses for the presence of the protease pathway components cathepsin L, endopin 2, aminopeptidase B (AP-B), PC1/3, PC2, and carboxypeptidase E (CPE). Antisera for western blots were commercially obtained for cathepsin L (Athens Research Technology, Athens, GA), PC1/3 (Chemicon/Millipore, Billerica, MA), and PC2 (Chemicon/Millipore, Billerica, MA). Antiserum to endopin 2 was generated to recombinant endopin 2 as described previously (27,28). Antiserum to AP-B (aminopeptidase B) was generated against a peptide domain of AP-B, as described previously (13), was utilized in this study. Antiserum to CPE utilized in this study has been described previously (35). Western blots were performed on NuPAGE 12% Bis-Tris Gels (Invitrogen, Carlsbad, CA) transferred to Amersham Hybond ECL nitrocellulose membranes (GE Healthcare, Piscataway, NJ), analyzed with Amersham ECL Anti-rabbit IgG Horseradish Peroxidase-linked secondary antibodies (GE Healthcare), and developed with Pierce SuperSignal West Femto Maximum Sensitivity Substrate Kit (Thermo Scientific, Rockford, IL) onto Amersham Hyperfilm ECL film (GE Healthcare). Molecular weight standards: Lonza Proseive Prestained Marker (Lonza, Allendale, NJ), and Mark 12 unstained standard (Invitrogen) were used for analysis. Details of western blot procedures have been described previously (27,28). The specificities of antisera utilized for these western blots of protease and protease inhibitor components of this study has been previously examined in prior studies (11, 13, 19, 20, 40, 41).
Analyses of POMC-derived products by anti-ACTH and anti-β-endorphin western blots
Homogenates of individual human pituitary glands (three glands were utilized) were subjected to western blots with anti-ACTH (1:5000 dilution, antisera to ACTH was from the National Pituitary Agency, NIDDK (NHPP-NIDDK, Harbor-UCLA Medical Center, Torrance, CA) and anti-β-endorphin (1:1000 dilution, antisera for β-endorphin was from the National Pituitary Agency, (NHPP-NIDDK, Torrance, CA). NHPP-NIDDK has indicated that these pituitaries are from individuals with no pathologies. Samples were subjected to SDS-PAGE on 12% NUPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to Hybond ECL nitrocellulose membrane (Amersham Biosciences, GE Healthcare, Piscataway, NJ). Immunopositive bands were detected with anti-ACTH or anti-β-endorphin visualized by anti-rabbit conjugated to horseradish peroxidase (Amersham Biosciences, GE Healthcare, Piscataway, NJ), detected with the Femto detection kit (SuperSignal Femto Substrate kit, Pierce, Thermo Scientific, Rockford, IL). Replicate western blots (two-three) for each experiment was conducted.
Colocalization of ACTH and β-endorphin with cathepsin L in human pituitary secretion by immunofluorescence confocal microscopy
Frozen human pituitary from a normal individual was obtained from the Harvard Brain Tissue Ressource Center (Belmont, MA, USA). The human pituitary was cut into 30 μm slices with a cryostat and then post-fixed overnight with paraformaldehyde (PFA) 4% in phosphate buffered saline (PBS). The slices are then permeabilized for 1 hour with a mixture containing Triton X-100 (0.1%) and bovine serum albumin (BSA, 3%) in PBS. The slices were immunostained in PBS-3% BSA overnight at 4°C with the primary antibodies: rabbit anti-cathepsin L (1:750, Athens Research and Technologies, Athens, GA, USA), mouse anti-ACTH (1:1000, Abcam, Cambridge, MA, USA), and guinea pig anti-β-endorphin (1:1500, Bachem, Torrance, CA, USA). Cathepsin L, ACTH and β-endorphin were detected with goat secondary antibodies directed to rabbit, mouse, or guinea primary antibodies, respectively. The secondary goat antibodies were labeled with Alexa fluor 488 or 594 (Invitrogen, Molecular Probes, Carlsbad, CA, USA) and were utilized at a diltution of 1:200 in PBS-3% BSA for 2 hours at room temperature. Sections were coverslipped with mounting medium and examined with the Olympus IX 70 microscope (Olympus, Center Valley, PA, USA) for immunofluorescent confocal microscopy, and images were analyzed with Delta Vision Spectris Image Deconvolution System (Applied Pecision, LLC, Issaquah, WA, USA).
For control experiments, staining with secondary antibodies alone was performed and no fluorescence was detected. For further controls, the cathepsin L antibody was preincubated with recombinant human cathepsin L (gift from Dr. W.D. Lu, UC San Diego) before the immunostaining procedure and a lack of fluorescence was observed. The specificity of the anti-β-endorphin antibody has been determined (by Bachem-Peninsula Laboratories) showing no crossreactivity with ACTH, alpha-MSH, or Met-enkephalin. The antibody against ACTH used in this study is a monoclonal antibody reacting with the amino acids 1-24 of ACTH, a sequence which does not contain β-endorphin. Additionally, the absence of cross-immunoreactivity was confirmed for the secondary and primary antibodies used in this experiment.
Radioimmunoassays for ACTH, α-MSH, and β-endorphin
Measurement of ACTH, α-MSH, and β-endorphin in extracts of human pituitary was conducted as described previously for mouse pituitary (21). Pituitary homogenates (six individual pituitary glands were individually homogenized), prepared as described for western blots, were subjected to acid extraction by adding acetic acid to 1 N, heating at 95° C for 10 minutes, centrifugation at 13,000 × g for 10 minutes, and collection of the soluble acid extract for radioimmunoassays (RIA).
RIA measurements of α-MSH, as well as ACTH and ß-endorphin1-31 were conducted as previously described (21). The ACTH RIA utilized anti-ACTH serum from NIDDK/NIH, and 125I-ACTH from Peninsula Laboratories (San Carlos, CA), performed as we have described previously (21). The RIA for human ACTH does not crossreact with full-length POMC (bovine); the high 80% homology of human POMC with bovine POMC indicates their similar properties with respect to antibody-antigen relationships. The ACTH RIA does not recognize α-MSH, β-MSH, γ-MSH, or ß-endorphin1-31 that are derived from POMC.
A specific RIA was utilized to measure levels of human α-MSH in acid extracts, with RIA kits and protocols from Phoenix Pharmaceuticals, Inc. (Mountain View, CA). The α-MSH RIA does not crossreact with full-length POMC that was prepared by expression and purification of recombinant bovine POMC as we have described previously (21). In addition, the RIA for α-MSH does not crossreact with other POMC-derived peptides that consist of ACTH, ß-endorphin1-31, ß-MSH, γ-MSH, or (Met)enkephalin.
The POMC product ß-endorphin1-31 was also measured in acid extracts by RIA, obtained as a kit (from Phoenix Pharmaceuticals, Inc., Mountain View, CA), which does not recognize POMC. The RIA for ß-endorphin1-31 also does not crossreact with α-MSH, ACTH, ß-MSH, γ-MSH, ß-LPH, (Met)enkephalin, (Leu)enkephalin, ß-endorphin1-27, and ß-endorphin1-26.
The RIA assays provided reproducible measurements of peptide hormones. These RIA assays showed an average coefficient of variance (CV, standard deviation/mean) of 3.5%, indicating reliability of assay measurments of ACTH, β-endorphin, and α-MSH peptides.
Acknowledgments
The authors appreciate support of this research by the National Institutes of Health. Assistance for this project by D. Tierney is acknowledged.
References
- 1.Thomas M, Keramidas M, Monchaux E, Feige JJ. Microscopy Research Technique. 2003;61:247–251. doi: 10.1002/jemt.10333. [DOI] [PubMed] [Google Scholar]
- 2.Krude H, Gruters A. Trends in Endocrinol. Metabol. 2000;11:15–22. doi: 10.1016/s1043-2760(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 3.Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, Cone RD, Elmquist JK. Ann. NY Acad. of Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 4.Kokkoris P, Pi-Sunyer FX. Endocrinol. Metabol. Clin. N. Amer. 2003;32:895–914. doi: 10.1016/s0889-8529(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 5.Slominski AT. J. Investigative Dermatol. 2006;126:1934–1936. doi: 10.1038/sj.jid.5700342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi H, Hakamata Y, Watanabe Y, Kikuno R, Miyata T, Numa S. Nucl. Acids Res. 1983;11:6847–6858. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritchard LE, White A. Endocrinology. 2007;148:4201–4207. doi: 10.1210/en.2006-1686. [DOI] [PubMed] [Google Scholar]
- 8.Castro MG, Morrison E. Crit. Rev. Neurobiol. 1997;11:35–37. doi: 10.1615/critrevneurobiol.v11.i1.30. [DOI] [PubMed] [Google Scholar]
- 9.Bertagna X. Endocrinol. Metabol. Clinics N. Amer. 1994;23:467–485. [PubMed] [Google Scholar]
- 10.Yasothornsrikul S, Aaron W, Toneff T, Hook V. Biochemistry. 1999;38:7421–7430. doi: 10.1021/bi990239w. [DOI] [PubMed] [Google Scholar]
- 11.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Proc. Natl. Acad. Sci. USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook V. J. Biol. Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- 13.Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. J. Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 14.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Ann. Rev. Pharmacol. Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidah NG, Chretien M. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhou A, Webb G, Zhu X, Steiner DF. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 17.Fricker LD. Carboxypeptidase E. Ann. Rev. Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 18.Hook VY, Eiden LE, Brownstein MJ. Nature. 1982;295:341–342. doi: 10.1038/295341a0. [DOI] [PubMed] [Google Scholar]
- 19.Hook VYH, Meze E, Fricker LD, Pruss RM, Siegel RE, Brownstein MJ. Proc. Natl. Acad. Sci. USA. 1985;82:4745–4749. doi: 10.1073/pnas.82.14.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funkelstein L, Toneff T, Mosier C, Hwang SR, Beuschlein F, Lichtenauer UD, Reinheckel T, Peters C, Hook V. J. Biol. Chem. 2008 Oct 10; doi: 10.1074/jbc.M709010200. 2008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. J. Neurochem. 2003;86:556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- 22.Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I. Endocrinology. 2004;145:519–528. doi: 10.1210/en.2003-0829. [DOI] [PubMed] [Google Scholar]
- 23.Allen RG, Peng B, Pellegrino MJ, Miller ED, Grandy DK, Lundblad JR, Washbrun CL, Pintar JE. J. Neurosci. 2001;21:5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan H, Che FY, Png B, Steiner DF, Pintar JE, Fricker LD. J. Neurochem. 2006;98:1763–77. doi: 10.1111/j.1471-4159.2006.04067.x. [DOI] [PubMed] [Google Scholar]
- 25.Pan H, Nanno D, Che FY, Zhu X, Salton SR, Steiner DF, Fricker LD, Devi LA. Biochemistry. 2005;44:4939–4948. doi: 10.1021/bi047852m. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. Proc. Natl. Acad. Sci. USA. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SR, Steineckert B, Hook VY. Biochemistry. 2000;39:8944–8952. doi: 10.1021/bi9929655. [DOI] [PubMed] [Google Scholar]
- 28.Hwang SR, Steineckert B, Toneff T, Bundey R, Logvinova AV, Goldsmith P, Hook VY. Biochemistry. 2002;41:10397–10405. doi: 10.1021/bi020088o. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SR, Stoka V, Turk V, Hook VY. Biochemistry. 2005;44:7757–7767. doi: 10.1021/bi050053z. [DOI] [PubMed] [Google Scholar]
- 30.Azaryan AV, Krieger TJ, Hook VY. J. Biol. Chem. 1995;270:8201–8208. doi: 10.1074/jbc.270.14.8201. [DOI] [PubMed] [Google Scholar]
- 31.Lamango NS, Zhu X, Lindberg I. Arch. Biochem. Biophys. 1996;330:238–250. doi: 10.1006/abbi.1996.0249. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Lindberg I. J. Biol. Chem. 1993;268:5615–5623. [PubMed] [Google Scholar]
- 33.Peters EM, Tobin DJ, Seidah NG, Schallreuter KU. J. Investig. Dermatol. 2000;114:430–437. doi: 10.1046/j.1523-1747.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 34.Bennett DL, Bailyes EM, Nielsen E, Guest PC, Rutherford NG, Arden SD, Hutton JC. J. Biol. Chem. 1992;267:15229–15236. [PubMed] [Google Scholar]
- 35.Hook VY, Mezey E, Fricker LD, Pruss RM, Siegel RE, Brownstein MJ. Proc. Natl. Acad. Sci. USA. 1985;82:4745–4749. doi: 10.1073/pnas.82.14.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson HW, Hutton JC. Biochem. J. 1987;245:575–582. doi: 10.1042/bj2450575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG. Proc. Natl. Acad. Sci. USA. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takumi I, Steiner DF, Sanno N, Teramoto A, Osamura Y. Modern Pathology. 1998;11:232–238. [PubMed] [Google Scholar]
- 39.Fan X, Olson SJ, Johnson MD. J. Histochem. Cytochem. 2001;49:783–790. doi: 10.1177/002215540104900612. [DOI] [PubMed] [Google Scholar]
- 40.Azaryan AV, Krieger TJ, Hook VYH. J. Biol. Chem. 1995;270:8291–8208. doi: 10.1074/jbc.270.14.8201. [DOI] [PubMed] [Google Scholar]
- 41.Hwang SR, Bundey R, Toneff T, Hook V. Neuroendocrinol. 2008 Oct 8; doi: 10.1159/000162916. 2008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]