Abstract
To evaluate the impact of contemporary therapy on the clinical outcome of children with pre-B acute lymphoblastic leukemia (ALL) and the t(1;19)/TCF3/PBX1, we analyzed 735 patients with B-cell precursor ALL treated in four successive protocols at St. Jude Children’s Research Hospital. The 41 patients with the t(1;19) had a comparable event-free survival to that of the 694 patients with other B-cell precursor ALL (p=0.63; 84.2% ± 7.1% [SE] vs. 84.0% ± 1.8% at 5 years). However, patients with the t(1;19) had a lower cumulative incidence of any hematological relapse (p=0.06; 0 vs. 8.3% ± 1.2% at 5 years) but a significantly higher incidence of central-nervous-system (CNS) relapse (p<0.001; 9.0% ± 5.1% vs. 1.0% ± 0.4% at 5 years). In a multivariate analysis, the t(1;19) was an independent risk factor for isolated CNS relapse. These data suggest that with contemporary treatment, patients with the t(1;19) and TCF3/PBX1 fusion have a favorable overall outcome but increased risk of CNS relapse.
Keywords: t(1;19), TCF3-PBX1, Pre-B ALL, Pediatric ALL, CNS relapse
INTRODUCTION
The t(1;19)(q23;p13), leading to the production of the TCF3/PBX1 fusion transcript, is one of the most common translocations in pediatric B-cell precursor acute lymphoblastic leukemia (ALL).(1–3) The N-terminal transactivation domain of TCF3(E2A), a helix-loop-helix protein-coding gene on chromosome 19, is fused to the C-terminal DNA-binding homeodomain of PBX1, the pre-B-cell transforming gene on chromosome 1. The chimeric protein interferes with hematopoietic differentiation by disrupting both the expression of HOX genes and the targets of the TCF3 transcription factor.(4) TCF3/PBX1 genotype has been correlated with high initial leukocyte count, non-white race, and absence of hyperdiploidy.(5) Early studies showed that patients with the t(1;19) have an inferior outcome compared with patients lacking this translocation.(1–3, 6–8) We have previously reported that intensified chemotherapy improved outcome of patients with the t(1;19),(9) an observation later confirmed by other groups.(10, 11) The improved treatment outcome with contemporary therapy has led many study groups to exclude this genotype from routine risk assignment in recent clinical trials. In this report, we show that while patients with the t(1;19) have an overall favorable outcome when treated with contemporary therapy, they have a significantly higher incidence of central-nervous-system (CNS) relapse. Our finding argues for the need to identify this genotype for intensifying CNS-directed therapy to further improve outcome in this group of patients.
PATIENTS AND METHODS
Between December 1991 and January 2008, 874 patients with ALL were enrolled onto four successive clinical trials (Total Therapy studies XIIIA,(12) XIIIB,(13) XIV (14) and XV) at St. Jude Children’s Research Hospital. The treatment protocols were approved by the institutional review board at St. Jude, and Total XV was registered at ClincalTrials.gov, number NCT00137111. Signed informed consent was obtained from the patient’s parents or guardian with assent from the patients, as appropriate. Immunophenotype, cytogenetic and molecular genetic analyses were performed using standard methods, as described previously.(5) Treatment plans have been described in details previously.(12–14) In study XIIIA, all patients received triple intrathecal therapy on day 1, 22, and at the end of remission induction. Patients with CNS-2, CNS-3, or traumatic lumbar puncture with blasts received additional intrathecal treatments on days 8 and 15 of induction, and every 4 weeks during the first year of continuation therapy. Intensified intrathecal therapy was also administered to patients with high risk features (T-cell ALL with leukocyte count of at least 50×109/L, B-cell precursor ALL with leukocyte count of at least 100 × 109/L, or the presence of t(9;22)[BCR-ABL1]), regardless of CNS status. Cranial irradiation plus 5 intrathecal treatments was given to patients who had CNS-3 status (24 Gy) or high-risk leukemia (18 Gy). The number of intrathecal therapy was 13 for lower-risk cases and ranged from 21 to 26 for higher-risk cases. In study XIIIB, we intensified systemic therapy by substituting dexamethasone for prednisone in post remission therapy. CNS-directed therapy was similar to that in study XIIIA except that cranial irradiation was limited to patients who had CNS-3 status (24 Gy), or with T-cell ALL and initial leukocyte count of 100 × 109/L or greater (18 Gy). The total number of intrathecal treatments ranged from 13 in lower-risk cases to 26 in high-risk cases who received cranial irradiation. In study XIV, post-remission therapy was similar to that in Total XIIIB with some modifications including the use of higher dose methotrexate (5 g/m2 for higher-risk cases and 2.5 g/m2 for lower-risk cases), and dexamethasone. The total number of triple intrathecal treatments ranged from 16 in lower-risk cases to 23 in higher-risk cases with CNS-2, CNS-3 or traumatic lumbar puncture with blasts status at diagnosis. None of the patients received prophylactic cranial irradiation, regardless of CNS status or other features at diagnosis.
In study XV we revised the system of risk-assessment in which patients were assigned to low-, standard- and high-risk groups, based on sequential measurements of minimal residual disease. All patients received intrathecal therapy on day 1, 19 and at the end of remission induction. On days 8 and 26, additional intrathecal treatments were given to patients with high-risk features of CNS relapse (CNS-2, CNS-3, traumatic lumbar puncture with blasts, T-cell ALL with leukocyte count > 50×109/L, B-cell precursor ALL with leukocyte count > 100 × 109/L, or the presence of t(9;22)[BCR-ABL1], MLL rearrangement, or hypodiploidy < 45 chromosomes). Triple intrathecal chemotherapy was given every 8 weeks in low-risk cases and every 4 weeks in standard-or high-risk cases up to one year. Patients at high risk of CNS relapse continued to receive intrathecal therapy every 8 weeks until week 96 of continuation therapy. Depending on their CNS status, low-risk patients received 13 to 18, and standard-and high-risk cases 16 to 25, intrathecal treatments. No patient received prophylactic cranial irradiation.
We compared the outcome between 41 patients with pre-B ALL and the t (1;19)/TCF3-PBX1 and 694 patients with other B-cell precursor ALL. The TCF3-PBX1 fusion was confirmed with RT-PCR and FISH in all 33 cases tested. Molecular testings were not performed in the first 8 cases because the probes were not available at that time. Because all cases with the (1;19) have B-cell precursor phenotype, analysis was limited to patients with this immunophenotype and the 139 patients with T-cell ALL were excluded from the analyses. The median follow up time was 6.9 years (range 0.22 to 16.0 years). Overall survival and event-free survival (EFS) were estimated by the Kaplan-Meier method and compared with the Mantel-Haenszel (log-rank) test. The cumulative incidence of relapse was estimated by the method of Kalfleisch and Prentice and the functions were compared using the Gray’s test to adjust for competing events. The duration of EFS was defined as the time from diagnosis until the date of treatment failure (induction failure, relapse, death, or the development of a second malignancy) or until the date of last contact. Patients who did not attain a complete remission were considered failures to respond to therapy at time zero. The length of time at risk for relapse was computed from the date of achieving a complete remission to the date of relapse or the date of last contact, whichever came first. Death from non-relapse cause and second malignancy were included in the analysis and treated as competing events. The Cox proportional hazards model was used to identify independent prognostic factors.
RESULTS
The t(1;19)/TCF3-PBX1 fusion was detected in 41 (5.6%) of 735 children with B-cell precursor ALL. The TCF3-PBX1 genotype was significantly associated with black race, higher presenting leukocyte count, and DNA index <1.16 (Table 1). The incidence of overt CNS disease at presentation (CNS-3), blasts in cerebrospinal fluid (CSF) with < 5 leukocytes/μL (CNS-2), and traumatic lumbar puncture with blasts were comparable between patients with the t(1;19) and those with other B-cell precursor ALL. Patients with the t(1;19) and those with other B-cell precursor ALL had similar early response rates to remission induction therapy as assessed by the levels of minimal residual disease (MRD) measured by flow cytometry and/or PCR (undetectable or <0.01% at day 19 of remission induction in 48% vs. 46%; and at the end of 6-week induction in 91% vs.81%, respectively).
Table 1.
| Clinical Features | N (%) | TCF3PBX1 (%) | Other Pre_B ALL (%) | P values** |
|---|---|---|---|---|
| Age at diagnosis | 0.4975 | |||
| Infant | 16 (2) | 0 (0) | 16 (2) | |
| 1–10 years old | 536 (73) | 28 (68) | 508 (73) | |
| Older than 10 | 183 (25) | 13 (32) | 170 (25) | |
| WBC at diagnosis | <0.0001 | |||
| WBC<10k | 371 (51) | 7 (17) | 364 (52) | |
| WBC 10k–50k | 214 (29) | 16 (39) | 198 (29) | |
| WBC 50k–100 | 84 (11) | 13 (32) | 71 (10) | |
| WBC>100k | 66 (9) | 5 (12) | 61 (9) | |
| DNA index | <0.0001 | |||
| DNA_indx>=1.16 | 198 (27) | 0 (0) | 198 (29) | |
| Else | 537 (73) | 41 (100) | 496 (71) | |
| Sex | 0.3360 | |||
| Male | 381 (52) | 18 (44) | 363 (52) | |
| Female | 354 (48) | 23 (56) | 331 (48) | |
| Race | <0.0001 | |||
| White | 567 (77) | 20 (49) | 547 (79) | |
| Black | 113 (15) | 19 (46) | 94 (13) | |
| Other | 55 (8) | 2 (5) | 53 (8) | |
| CNS Status | 0.9667 | |||
| CNS1 combined with traumatic without blast | 514 (70) | 29 (71) | 485 (70) | |
| CNS3-blasts >=5 WBC | 15 (2) | 1 (2) | 14 (2) | |
| CNS2-blasts <5 WBC | 161 (22) | 9 (22) | 152 (22) | |
| traumatic blast+ | 44 (6) | 2 (5) | 42 (6) | |
| Day 19 MRD | 0.2509 | |||
| <0.01% | 207 (46) | 10 (48) | 197 (46) | |
| 0.01<=MRD19<1% | 164 (36) | 10 (48) | 154 (36) | |
| >=1% | 80 (18) | 1 (2) | 79 (18) | |
| End of induction MRD | 0.7086 | |||
| <0.01% | 370 (81) | 20 (91) | 350 (81) | |
| 0.01<=MRD46<1% | 68 (15) | 2 (9) | 66 (15) | |
| >=1% | 16 (4) | 0 (0) | 16 (4) | |
| Total Therapy | ||||
| XIIIA | 145 (20) | 8 (20) | 137 (20) | |
| XIIIB | 204 (28) | 10 (24) | 194 (28) | |
| XIV | 41 (5) | 1 (2) | 40 (6) | |
| XV | 345 (47) | 22 (54) | 323 (46) | |
EFS was virtually identical in both groups of patients (p=0.63; 84.2% ± 7.1 [SE] in the t(1;19) cases and 84.0% ± 1.8% in the other B-cell precursor cases at 5 years). There was also no difference in cumulative incidence of any relapse (p=0.80; 12.4% ± 5.9% in the t(1;19) cases and 9.5% ± 1.3% in the other B-cell precursor cases). While no hematologic relapse occurred among the t(1;19) cases, the cumulative risk of any hematologic relapse at 5 years was 8.3% ± 1.2% in the other B-cell precursor cases (p=0.06).
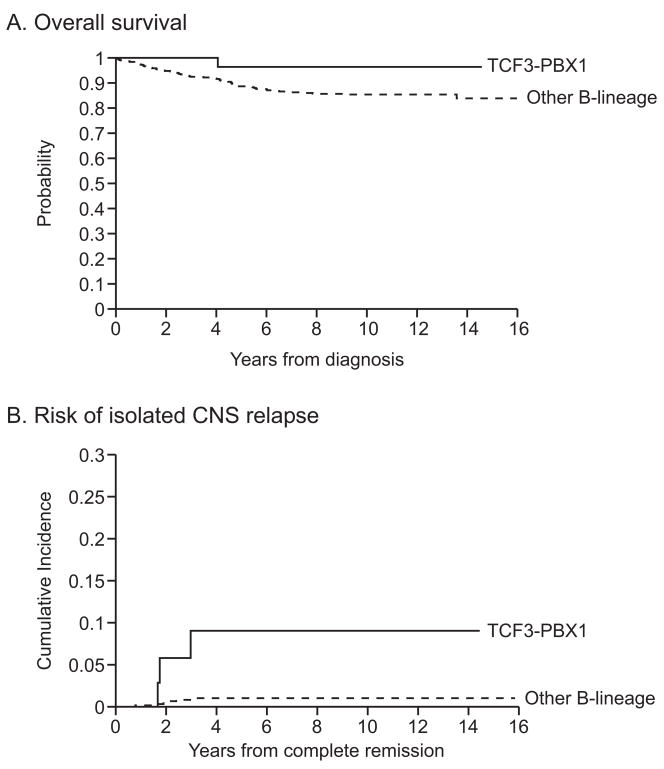
Of the 41 t(1;19) cases, 3 had isolated CNS relapses, 1 had combined CNS and ocular relapse, and 1 had a secondary malignancy (chronic myeloid leukemia). Hence, the t(1;19) was associated with an increased risk of isolated CNS relapse (p=0.0002; 9.0% ± 5.1% vs 1.0% ± 0.4% at 5 years in other B-cell precursor cases), and of any CNS (isolated plus combined) relapse (p=0.005; 12.4 ± 5.9% vs 2.7% ± 0.7%; Fig. 1). In a multivariate analysis including age, presenting leukocyte count, race, and CNS status at diagnosis, the t(1;19) was an independent risk factor for isolated CNS relapse (hazard ratio, 7.4; 95% confidence interval, 1.8 to 39.9; p=0.0059) or any CNS relapse (hazard ratio, 3.4; 95% confidence interval, 1.03 to 11.3; p=0.045). Similar conclusions were drawn when the effect of minimal residual disease level at day 19 of remission induction was also adjusted in multivariate analysis.
Fig. 1.
Comparisons of overall survival (A) and cumulative risk of CNS relapse (B) between patients with the t(1;19) and those with other B-lineage ALL.
The different pattern of relapse translated into a slight overall survival advantage in the t(1;19) cases as compared to other B-cell precursor cases (96.4% ± 3.7% vs.88.7% ± 1.5% at 5 yrs; Fig. 1).
The use of prophylactic cranial irradiation has decreased in our successive clinical trials (13% in study XIIIA, 3.9% in XIIIB and 0 in studies XIV and XV among cases with B-cell precursor ALL). Five (12%) of our 41 patients with t(1;19) received prophylactic cranial irradiation and none of these five patients developed CNS relapse. The 3 isolated CNS relapse and 1 combined CNS and ocular relapse in the t(1;19) cases developed among the 36 patients who did not receive prophylactic irradiation. Likewise, among the cases without t(1;19), none of 22 patients who had received prophylactic irradiation developed any CNS (isolated plus combined) relapse whereas the 672 patients who did not receive irradiation had a 5-year cumulative risk of relapse of 2.7% ± 0.7%. After adjusting for the effect of prophylactic CNS irradiation, the presence of t(1;19) was associated with an increased risk of any CNS relapse (p=0.009). In a multivariate analysis, after adjusting for other risk factors as well as irradiation, the translocation is still associated with increased risk of CNS relapse (p=0.001)
DISCUSSION
Our finding suggests that contemporary effective systemic therapy has led to improved hematological control but allowed the development of CNS relapse in patients with the t(1;19). Kager et al. (11) reported 3 bone marrow relapses and one combined CNS and bone marrow relapse among 31 patients with t(1;19). Baruchel et al. (15) reported 13 bone marrow relapses and 4 CNS relapses among 110 patients with the t(1;19). These results ostensibly weaken our finding. However, bone marrow relapse and CNS relapse are competitive events. As systemic control improves, the risk of CNS relapse could potentially be increased. In this regard improved systemic therapy has led to increased CNS relapse in patients with acute promyelocytic leukemia or Philadelphia chromosome-positive leukemia.(16, 17) Our result underscores the importance of identifying patients with the t(1;19) to intensify not only the systemic therapy but also CNS-directed therapy to further improve treatment outcome in them.
Among this relatively small number of patients with the t(1;19), we were not able to identify any risk factors including the presence or absence of balanced translocation that would predict event-free survival (data not shown).(18–20) However, the presence of blast cells in the CSF at diagnosis (CNS-2, CNS-3, or traumatic lumbar puncture with blasts) was associated with a particularly high risk of CNS relapse. The cumulative risk of isolated CNS relapse among the 12 patients with t(1;19) and blasts in the CSF was 27.3% ± 14.2% at 5 years compared with 5.0% ± 5.0% in the other 29 t(1;19) cases (p=0.05). Indeed, the rate of isolated CNS relapse in this 12 patients was much higher than the 2.7 + 1.2% in the 208 patients without t(1;19) who had blasts in the CSF (p<0.0001). In this regard, the prognostic impact of blasts in the cerebrospinal fluid or traumatic lumbar puncture with blasts has varied in clinical trials, depending on the patient groups studied, the use of cranial irradiation, and the intensity of intrathecal and systemic therapy.(21)
All study groups have substantially decreased the use of prophylactic cranial irradiation, and some groups even limited the use of this treatment modality only to patients with CNS- status at diagnosis.(21) Our study showed that the reduced use of cranial irradiation has not adversely affected the overall rates of CNS relapse, but could have uncovered subgroups at high risk such as the t(1;19) cohort. Although all our patients with isolated CNS relapse remain in second remission after a single course of therapeutic irradiation, efforts are being made to reduce CNS relapse and the need of salvage therapy. Thus, in our ongoing study XVI, we have increased the frequency of early intrathecal treatments in patients with t(1;19)/TCF3-PBX1.
Acknowledgments
Supported in part by a Cancer Center Support Grant (CA21765) from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). Dr. Pui is the American Cancer Society Professor
Reference List
- 1.Carroll AJ, Crist WM, Parmley RT, Roper M, Cooper MD, Finley WH. Pre-B cell leukemia associated with chromosome translocation 1;19. Blood. 1984 Mar;63(3):721–4. [PubMed] [Google Scholar]
- 2.Pui CH, Williams DL, Kalwinsky DK, Look AT, Melvin SL, Dodge RK, et al. Cytogenetic features and serum lactic dehydrogenase level predict a poor treatment outcome for children with pre-B-cell leukemia. Blood. 1986 Jun;67(6):1688–92. [PubMed] [Google Scholar]
- 3.Crist WM, Carroll AJ, Shuster JJ, Behm FG, Whitehead M, Vietti TJ, et al. Poor prognosis of children with pre-B acute lymphoblastic leukemia is associated with the t(1;19)(q23;p13): a Pediatric Oncology Group study. Blood. 1990 Jul 1;76(1):117–22. [PubMed] [Google Scholar]
- 4.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350(15):1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Sandlund JT, Pei D, Rivera GK, Howard SC, Ribeiro RC, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290(15):2001–7. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 6.Shikano T, Kaneko Y, Takazawa M, Ueno N, Ohkawa M, Fujimoto T. Balanced and unbalanced 1;19 translocation-associated acute lymphoblastic leukemias. Cancer. 1986 Nov 15;58(10):2239–43. doi: 10.1002/1097-0142(19861115)58:10<2239::aid-cncr2820581013>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Secker-Walker LM, Berger R, Fenaux P, Lai JL, Nelken B, Garson M, et al. Prognostic significance of the balanced t(1;19) and unbalanced der(19)t(1;19) translocations in acute lymphoblastic leukemia. Leukemia. 1992 May;6(5):363–9. [PubMed] [Google Scholar]
- 8.Pui CH, Raimondi SC, Hancock ML, Rivera GK, Ribeiro RC, Mahmoud HH, et al. Immunologic, cytogenetic, and clinical characterization of childhood acute lymphoblastic leukemia with the t(1;19) (q23; p13) or its derivative. J Clin Oncol. 1994 Dec;12(12):2601–6. doi: 10.1200/JCO.1994.12.12.2601. [DOI] [PubMed] [Google Scholar]
- 9.Rivera GK, Raimondi SC, Hancock ML, Behm FG, Pui CH, Abromowitch M, et al. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet. 1991 Jan 12;337(8733):61–6. doi: 10.1016/0140-6736(91)90733-6. [DOI] [PubMed] [Google Scholar]
- 10.Uckun FM, Sensel MG, Sather HN, Gaynon PS, Arthur DC, Lange BJ, et al. Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report from the Children’s Cancer Group. J Clin Oncol. 1998 Feb;16(2):527–35. doi: 10.1200/JCO.1998.16.2.527. [DOI] [PubMed] [Google Scholar]
- 11.Kager L, Lion T, Attarbaschi A, Koenig M, Strehl S, Haas OA, et al. Incidence and outcome of TCF3-PBX1-positive acute lymphoblastic leukemia in Austrian children. Haematologica. 2007 Nov;92(11):1561–4. doi: 10.3324/haematol.11239. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Mahmoud HH, Rivera GK, Hancock ML, Sandlund JT, Behm FG, et al. Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia. Blood. 1998 Jul 15;92(2):411–5. [PubMed] [Google Scholar]
- 13.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004 Nov 1;104(9):2690–6. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 14.Kishi S, Griener J, Cheng C, Das S, Cook EH, Pei D, et al. Homocysteine, Pharmacogenetics, and Neurotoxicity in Children With Leukemia. J Clin Oncol. 2003 Aug 15;21(16):3084–91. doi: 10.1200/JCO.2003.07.056. [DOI] [PubMed] [Google Scholar]
- 15.Baruchel A, Chevret S, Auvrignon A, Ballerini P, Michel G, Gabert J, et al. A plateau after 30 months of follow-up in B-lineage childhood acute lymphoblastic leukemia with t(1;19) (q23;p13)/E2A-PBX1? Blood. 2005;1441(106):416a. [Google Scholar]
- 16.de BS, Sanz MA, Chevret S, Dombret H, Martin G, Thomas X, et al. Extramedullary relapse in acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Leukemia. 2006 Jan;20(1):35–41. doi: 10.1038/sj.leu.2404006. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer H, Wassmann B, Hofmann WK, Komor M, Scheuring U, Bruck P, et al. Risk and prognosis of central nervous system leukemia in patients with Philadelphia chromosome-positive acute leukemias treated with imatinib mesylate. Clin Cancer Res. 2003 Oct 15;9(13):4674–81. [PubMed] [Google Scholar]
- 18.Shikano T, Kaneko Y, Takazawa M, Ueno N, Ohkawa M, Fujimoto T. Balanced and unbalanced 1;19 translocation-associated acute lymphoblastic leukemias. Cancer. 1986;58(10):2239–43. doi: 10.1002/1097-0142(19861115)58:10<2239::aid-cncr2820581013>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Secker-Walker LM, Berger R, Fenaux P, Lai JL, Nelken B, Garson M, et al. Prognostic significance of the balanced t(1;19) and unbalanced der(19)t(1;19) translocations in acute lymphoblastic leukemia. Leukemia. 1992;6(5):363–9. [PubMed] [Google Scholar]
- 20.Pui CH, Raimondi SC, Hancock ML, Rivera GK, Ribeiro RC, Mahmoud HH, et al. Immunologic, cytogenetic, and clinical characterization of childhood acute lymphoblastic leukemia with the t(1;19) (q23; p13) or its derivative. J Clin Oncol. 1994;12(12):2601–6. doi: 10.1200/JCO.1994.12.12.2601. [DOI] [PubMed] [Google Scholar]
- 21.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–68. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]