Abstract
Cognitive control of behavior continues to improve through adolescence in parallel with important brain maturational processes including synaptic pruning and myelination, which allow for efficient neuronal computations and the functional integration of widely distributed circuitries supporting top-down control of behavior. This is also a time when psychiatric disorders, such as schizophrenia and mood disorders, emerge reflecting a particularly vulnerability to impairments in development during adolescence. Oculomotor studies provide a unique neuroscientific approach to make precise associations between cognitive control and brain circuitry during development that can inform us of impaired systems in psychopathology. In this review, we first describe the development of pursuit, fixation, and visually-guided saccadic eye movements, which collectively indicate early maturation of basic sensorimotor processes supporting reflexive, exogenously-driven eye movements. We then describe the literature on the development of the cognitive control of eye movements as reflected in the ability to inhibit a prepotent eye movement in the antisaccade task, as well as making an eye movement guided by on-line spatial information in working memory in the oculomotor delayed response task. Results indicate that the ability to make eye movements in a voluntary fashion driven by endogenous plans shows a protracted development into adolescence. Characterizing the transition through adolescence to adult-level cognitive control of behavior can inform models aimed at understanding the neurodevelopmental basis of psychiatric disorders.
Keywords: Oculomotor, Working memory, Inhibition, Saccades, Cognition, DLPFC, FEF
1. Introduction
The neurodevelopmental basis of psychopathology is not widely recognized. Disorders such as schizophrenia, bipolar disorder, anxiety disorder, and anorexia nervosa often emerge during adolescence from systems that appeared to have been developing within normative ranges. Disorders such as autism, attention deficit hyperactivity disorder (ADHD), and Tourette's syndrome, while present early in development, show unique developmental progressions. Each of these disorders is now understood as having a neurobiological basis in which development plays a significant role. While most of the work on the neurobiological basis of psychopathology has focused on the mature system, investigation of the developmental trajectories of such disorders can provide crucial information regarding their etiology and, importantly, insight on appropriate windows for intervention and the effects of treatment.
Eye-movement tasks are a unique neuroscientific tool that allows us to examine the relationship between brain and behavior and its development, critically important to our understanding of the neurobiological basis of psychiatric illnesses. Oculomotor methods have proven to be sensitive to impaired executive function in a wide range of psychopathologies that are believed to have a neurodevelopmental basis, such as schizophrenia, ADHD, autism, and others (Everling & Fischer, 1998; Sweeney, Takarae, Macmillan, Luna, & Minshew, 2004) (see Section by Rommelse et al., in this volume). Specifically, voluntary control of saccades is particularly sensitive to psychopathology (Sweeney et al., 2004). These impairments are believed to reflect abnormalities in circuitry supporting executive control of responses that is also core to psychopathology.
This review will focus on the developmental transition from adolescence to adulthood of eye-movement performance on tasks of sensorimotor and cognitive control. During this period, performance on various eye-movement tasks begins to reach stabilization, paralleling developmental changes in the brain. Specific brain maturational processes will be described first because they provide the bases for developmental improvements in behavior. We then review the literature on the development of basic eye-movement processes as well as those that require cognitive control, including response inhibition, working memory, and reward processing. We conclude with a summary of which processes are mature and which have a protracted development which support the transition to adult-level eye-movement control.
2. The oculomotor system: A Model for characterizing cognitive development
The oculomotor system is an ideal system for investigating the neural basis of reflexive and voluntary behavior and for characterizing developmental improvements in behavior that are linked to brain maturational processes. Oculomotor tasks have been used extensively in investigations of the brain bases of higher cognitive processes such as memory, planning, expectation, and reading (Basso, 1998; Evarts, Shinoda, & Wise, 1984; Hutton & Ettinger, 2006; Land & Furneaux, 1997) in healthy populations. Given that such processes are also often disrupted in psychiatric illness, oculomotor tasks have been used to investigate the biological basis of clinical impairments (Everling & Fischer, 1998; Luna & Sweeney, 1999; Luna & Sweeney, 2001). By adding cognitive demands to a task, voluntary eye movements require the use of high-level cognitive processes as they generate neuronal activity throughout the brain in anticipation of a planned response, allowing brain regions involved in cognitive processes to be identified (Basso, 1998). Delineating the emergence of this brain circuitry through development can thus provide us with valuable information about the brain/behavior interaction underlying cognitive maturation.
Eye-movement studies also have a good fit with pediatric populations. Oculomotor tasks are simple and can readily be performed successfully by children (Cohen & Ross, 1978; Ross, Radant, & Hommer, 1993). Performance on these tasks is less likely to be aided by verbal or learning strategies which often overestimate developmental progression in neuropsychological tests. Moreover, the stimulus–response relationship of a saccade to a visual stimulus is direct, in contrast to paper and pencil or manual responses where transformations to adapt to different input/output modalities are applied. Additionally, eye-movement responses can be measured with extreme precision and are unusually rich in terms of derivable parameters compared to other modes of responses (e.g., manual responses) (see Smyrnis review, in this volume).
Furthermore, the oculomotor system is well suited to investigate brain/behavior relationships because single-cell studies in non-human primates have delineated its neurophysiology, neuroanatomy, and neurochemistry to a greater degree than other systems (Bon & Lucchetti, 1990; Bruce & Goldberg, 1985; Robinson, Goldberg, & Stanton, 1978). As such, the oculomotor system provides a unique model for making links between brain and behavior. Performance on these tasks has also been well documented in normal adults (Leigh & Zee, 1991) and brain lesion patients (Guitton, Buchtel, & Douglas, 1985; Henik, Rafal, & Rhodes, 1994; Paus et al., 1991; Pierrot-Deseilligny, Rivaud, Gaymard, Muri, & Vermersch, 1995) (see Mueri & Nyffeler review, in this volume). Additionally, oculomotor tasks are known to result in robust brain activation in adult subjects, engaging a distributed network including the frontal eye field (FEF), posterior parietal cortex (PPC), the supplementary eye fields (SEF), dorsolateral prefrontal cortex (DLPFC; see glossary) basal ganglia, thalamus, superior colliculus (see glossary), and cerebellum (see glossary) (Luna et al., 1998; Muri et al., 1996; Petit, Clark, Ingeholm, & Haxby, 1997; Sweeney et al., 1996). The oculomotor system is thus particularly well suited for functional neuroimaging studies and to test hypotheses about changes in brain systems during development.
Additionally, oculomotor tasks are exquisitely adaptable. Different oculomotor paradigms have been developed that tap into discrete behavioral and cognitive processes. Pursuit tasks require the tracking of a visual stimulus which allows prediction (see glossary) and adjustment processes to be assessed (see Barnes et al. review in this issue). Fixation tasks test the ability to voluntarily retain gaze on a visual stimulus, thereby reflecting cognitive control. Visually guided saccade tasks require the simple, reflexive foveation of a visual stimulus, which allows basic aspects of attention and sensorimotor control to be assessed (see Hutton review in this Issue). A cognitive load can also be added to eye-movement tasks allowing higher-order cognitive processes to be investigated (see Hutton review in this issue). The antisaccade task (Hallett, 1978) requires the suppression of a prepotent saccadic response and the generation of an endogenous response, modulated by the integration of preparatory activity (see glossary) in frontal and brain stem regions (Everling & Munoz, 2000). The oculomotor delayed response (ODR) task, the prototypical spatial working-memory task used in single-cell studies with non-human primates (Funahashi, Bruce, & Goldman-Rakic, 1989; Hikosaka & Wurtz, 1983), requires the execution of a saccade guided only by the memory of a previously presented target location and is also subserved by a widely distributed fronto-parieto-striatal network (Funahashi et al., 1989; Sweeney et al., 1996). Given that the regional neurophysiology subserving performance on these oculomotor tests has been well studied, changes in performance due to cognitive development can be interpreted within the context of a well-developed neuroscience and neurological framework.
3. Brain maturation
Concurrent with the influences of the environment and learning on age-related improvements in cognitive control, brain maturation processes provide the mechanisms for these processes to affect behavior. During adolescence, the brain undergoes significant specialization that enables the individual to be adaptable to their particular environment. Understanding these changes in brain structure and periods of plasticity can provide insight on the possible neurodevelopmental underpinnings of psychopathology.
Although the skull thickens throughout childhood and is often interpreted as reflecting change in brain size, the gross morphology of the brain is actually in place early in development. The degree of cortical folding (Armstrong, Schleicher, Omran, Curtis, & Zilles, 1995), overall size, weight, and regional functional specialization is adult-like by early childhood (Caviness, Kennedy, Bates, & Makris, 1996; Giedd Snell, et al., 1996; Reiss, Abrams, Singer, Ross, & Denckla, 1996). While the basic aspects of brain development are in place early, key processes which refine the basic structure persist, sculpting the brain to fit the biological and external environments. These processes include synaptic pruning and myelination (Huttenlocher, 1990; Pfefferbaum et al., 1994; Yakovlev & Lecours, 1967) which enhance neuronal processing and support mature cognitive control of behavior.
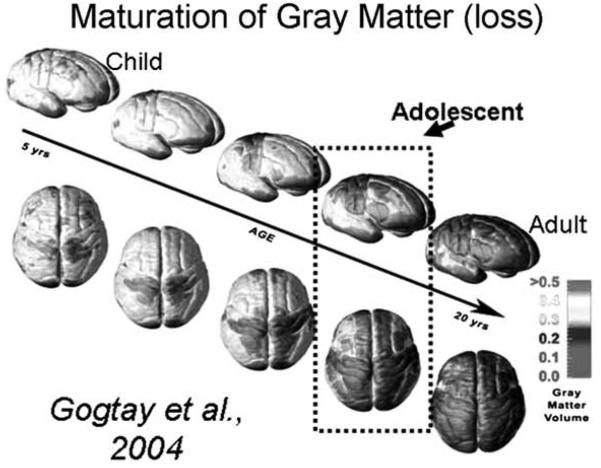
Synaptic pruning refers to the programmed loss of excessive neuronal connections of which experience is thought to be a primary contributor (Rauschecker & Marler, 1987). The loss of non-essential connections results in neural systems that support complex computations within regional circuitry, as well as enhancing the capacity and speed of information processing (Huttenlocher, 1990; Huttenlocher & Dabholkar, 1997). Structural neuroimaging studies have indicated reductions in gray matter throughout cortical association areas, notably the frontal and temporal regions (Giedd et al., 1999; Gogtay et al., 2004; Paus et al., 1999; Toga, Thompson, & Sowell, 2006), as well as the basal ganglia (Sowell, Thompson, Holmes, Jernigan, & Toga, 1999), thought in part to reflect loss of synaptic connections. Notably, the last parts of the brain to show persistent decreases in gray matter volume are association areas in each brain lobe and not a hierarchical protracted development of frontal regions as had been traditionally thought (see Fig. 1). These results indicate that the transition to adult-level control of behavior is supported by the ability to efficiently integrate information throughout the brain, which would support the complex computations needed for executive control of responses.
Fig. 1.
View of cortical surface of the brain. Colors represent degree of thinning of gray matter. Blue indicates mature adult-levels have been reached. We have added a box around the brains that represent adolescence (figure from Gogtay et al. (2004). PNAS, 101, 8174–8179).
Myelination refers to the process of electrically insulating nerve tracts, which has the effect of significantly increasing the speed of neuronal transmission (Drobyshevsky et al., 2005). Increased speed of neuronal transmission allows for distant regions to integrate function more efficiently. Importantly, this supports the integration of widely distributed circuitry needed for complex behavior (Goldman-Rakic, Chafee, & Friedman, 1993). Specifically, these structural changes are believed to underlie the functional integration of frontal regions with the rest of the brain supporting top-down control of behavior (Chugani, 1998; Luna & Sweeney, 2004; Thatcher, Walker, & Giudice, 1987). While some subcortical areas such as the brain stem myelinate early (Sano, Kaga, Kuan, Ino, & Mima, 2007), neocortical areas continue to myelinate past adolescence and may reflect both reduced synaptic connections and increased myelination. Diffusion Tensor Imaging (DTI; see glossary) is an MRI method that images the coherence of the trajectory of water molecules. Given that there is higher coherence of water molecule trajectories within tracts, this method can identify white matter tracts and provide a measure of white matter integrity of which myelination is a primary factor (Conturo, McKinstry, Akbudak, & Robinson, 1996; Moseley et al., 1990). DTI studies indicate a continued increase in frontal white matter integrity throughout childhood, providing evidence for continued myelination with age (Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999). Similar to findings regarding gray matter thinning, myelination also does not occur last in frontal regions but throughout the brain. These findings suggest that the functional integration of widely distributed circuitry characterizes late development into adulthood (Luna & Sweeney, 2004).
Taken together, these studies indicate that the brain systems crucial for exerting cognitive control over behavior are still maturing during adolescence. An immature system is able to exert cognitive control, but fails to do so in a consistent manner and with limited flexibility and motivational control. In other words, although the basic elements are established, refinements persist, which support the necessary efficiency in circuit processing to establish reliable executive control evident in adulthood.
4. Development of the pursuit system
The smooth pursuit system is distinct from the saccadic system (described below) in that it supports voluntarily foveation of a stimulus that is moving. This is the system that allows us, for example, to catch a ball speeding toward us, or to cross the street without getting run over by a moving vehicle. Different from the rapid eye movements in the saccade system, pursuit involves slow eye movements (as well as small compensatory saccades) that approximate the velocity of a moving target in order to focus the visual image on the fovea. Single-cell and human neuroimaging studies have found that smooth pursuit is supported by regions adjacent to the saccade system (Berman et al., 1999; MacAvoy, Gottlieb, & Bruce, 1991) and overlaps with regions supporting the vestibular system, which is integral to pursuit processes (Fukushima, Akao, Kurkin, Kaneko, & Fukushima, 2006). Areas related to pursuit include the cerebellar floccular region, dorsal vermis, caudal fastigial nucleus, medial superior temporal cortical area, caudal FEF, SEF, dorsolateral pontine nucleus, and nucleus reticularis tegmenti pontis (see glossary; for review see Fukushima et al., 2006). Additionally, pursuit also recruits regions of visual cortex (V5; see glossary, a.k.a. area MT) known to support motion processing (Newsome, Wurtz, & Komatsu, 1988), also see reviews by Lencer & Trillenberg; Ilg & Their; Sharpe; and Barnes, in this volume).
While the pursuit system is immature at birth, it undergoes significant improvements in the first year of life. In the first two weeks after birth, there is evidence for the ability to track a moving object using optokinetic nystagmus (see glossary) but not yet smooth pursuit (Haishi & Kokubun, 1998; Rosander, 2007; Shea & Aslin, 1990). In the first 2 months of life, tracking of moving objects is accomplished by a series of saccadic movements (Rosander & von Hofsten, 2002; Roucoux, Culee, & Roucoux, 1983; Shea & Aslin, 1990). The ability to track a moving object with slow, controlled smooth eye movements that are distinct from saccades comes on-line after the first few months of life, but it is slow and inaccurate (Rosander & von Hofsten, 2002; Shea & Aslin, 1990). Increases in pursuit speed show great improvements through infancy supporting the ability to track faster moving stimuli (Roucoux et al., 1983). During the first few months of life, there are also significant improvements in the ability to coordinate head movements with gaze shifts, becoming mature by approximately 7 months of age (Daniel & Lee, 1990). Saccadic aspects of pursuit tracking, which are needed to make adjustments, are present by 6 months (Gredebäck, von Hofsten, Karlsson, & Aus, 2005) and continue to appear adult-like through childhood and adolescence (Ross et al., 1993). Important for pursuit processes is the ability to predict movement in repetitive tracking enhancing pursuit accuracy. Consistent predictive gaze tracking is not present until 8 months of age (Gredebäck et al., 2005) and continues to improve through childhood (Salman, Sharpe, Lillakas, Dennis, & Steinbach, 2006).
The ability to tightly match pursuit eye movements with a moving stimulus (i.e., pursuit accuracy) continues to be immature throughout infancy (Grönqvist, Gredebäck, & Hofsten, 2006; Jacobs, Harris, Shawkat, & Taylor, 1997; Shea & Aslin, 1990; von Hofsten & Rosander, 1997). Pursuit accuracy is achieved by smooth tracking movements that rely on the ability to predict the motion of the stimuli, but small corrections are also used in the form of catch-up saccades (Leigh & Zee, 1999). Pursuit gain (see glossary) is used to assess accuracy independent of catch-up saccades (see glossary) hence reflecting the integrity of the pursuit system independent from that of the saccade system (Leigh & Zee, 1999). While saccadic mechanisms are present since infancy, pursuit accuracy, determined by the gain of smooth eye tracking, continues to improve through childhood into adolescence, especially at higher speeds of pursuit tracking (Haishi & Kokubun, 1995; Katsanis, Iacono, & Harris, 1998; Ross et al., 1993; Rütsche, Baumann, Jiang, & Mojon, 2006) and some studies show continued improvements into mid-adolescence (Salman et al., 2006). Pursuit accuracy requires the prediction of movement and performance monitoring requiring an efficient distributed system that may not reach maturity until adulthood. Young human children (9–11 years old) and monkeys (3–4 years old) have been found to have asymmetric eye movements when performing upward pursuit eye movements, suggesting immaturities in the organization of the floccular–vestibular system as well as compensatory mechanisms supported by the SEF that allow for the cancellation of the downward vestibular ocular reflex (Fukushima, Akao, Takeichi, Kaneko, & Fukushima, 2003; Takeichi et al., 2003). The establishment of mature, adult-level pursuit tracking is believed to reflect the integration of cortical and cerebellar circuitries supporting the predictive processes underlying pursuit accuracy (Rosander, 2007). As such, accuracy of pursuit eye movements reflects the integrity of long-range brain circuits that also underlie complex behavior impaired in psychopathology. There is a large literature that describes pursuit abnormalities in psychopathology, especially schizophrenia and their first degree relatives (Sweeney et al., 1998) (also see review by O'Driscoll).
5. Development of the fixation system
Visual fixation, the ability to resist eye movements in order to retain a stationary visual stimulus in the fovea, is often considered part of the pursuit system because of the need to detect and correct drifts in fixation (threading a needle), although there is also evidence to support that they are distinct systems (Leigh & Zee, 1999). Visual fixation is not a resting or passive process but in fact an active process that plays an important role in both maintaining focused attention and inhibiting inappropriate eye movements. Visual fixation is the process that drives the shifting of attention, including the engagement or locking of attention. Subsequent saccades to new visual targets require that visual fixation be actively inhibited. The retention of fixation, however, does not exclude the presence of microsaccades around the visual target (Engbert, 2006). Non-human primate single-cell studies have demonstrated that active visual fixation also recruits a distributed circuitry including frontal eye field (see glossary; Goldberg, Bushnell, & Bruce, 1986), posterior parietal cortex (Mountcastle, Andersen, & Motter, 1981; Shibutani, Sakata, & Hyvarinen, 1984), and brain stem (Munoz & Wurtz, 1992).
The ability to fixate is present early in life, but the stability and control of fixation continues to improve through adolescence. Results indicate that the distance of fixations around the “center of gravity” and number of intruding saccades decreases while the duration of fixation increases from 4 to 15 years of age, indicating developmental improvement in the stability of fixations (Aring, Grönlund, Hellström, & Ygge, 2007; Ygge, Aring, Han, Bolzani, & Hellström, 2005). Interestingly, the degree of attention engaged in the test stimulus appears to affect age-related differences in fixation. A clear decrease in number of breaks of fixation has been found in 8–10 year olds to distracting peripheral stimuli when the stimulus was meaningless and subjects were verbally instructed to maintain fixation. However, when the central stimulus was engaging (name the animal and press a button), age-related differences disappeared (Paus, 1989; Paus, Babenko, & Radil, 1990). These results suggest that developmental limitations in visual fixation are related to higher order, cognitive control processes such as the ability to inhibit eye-movement responses to distracting peripheral stimuli.
6. Development of the reflexive saccade system
Saccades are rapid eye movements (the fastest movement the human body can make) that allow visual stimuli to be foveated and become the target of attention. Saccades are therefore essential to our interaction with the world. Saccades can be automatic in nature, as when reflexively gazing to a suddenly appearing visual stimulus (e.g., a person walks into your office and you promptly turn to look at him/her). Saccades can also be controlled in a more endogenous and voluntary fashion, and in this manner tap into executive control (e.g., a person walks into your office but you stop the reflexive gazing because you “choose” to continue writing a paper). In this section, we will describe the development of the reflexive system which requires minimal cognitive control. The next section will review the development of voluntary saccades in detail.
Saccade performance is assessed by measuring peak velocity, latency, and accuracy. In general, the saccade system is known to be supported by a widely distributed circuitry of which cerebellar, brain stem, and cortical eye fields in frontal and parietal regions are involved (Bruce & Goldberg, 1985; Goldberg & Bruce, 1990; Keating & Gooley, 1988; Leigh & Zee, 1999; Schlag & Schlag-Rey, 1987). Saccade velocity is determined by burst neurons (see glossary) and omni-pause neurons in the brainstem (Leigh & Zee, 1999) and is considered a basic aspect of sensorimotor function. In infancy, saccade velocity is slower compared to adults (Hainline, Turkel, Abramov, Lemerise, & Harris, 1984). Developmental changes from childhood have not been consistent, however. Some studies have found no age-related effects from childhood to adulthood in saccade velocity (Luna, Garver, Urban, Lazar, & Sweeney, 2004; Munoz, Broughton, Goldring, & Armstrong, 1998), whereas other studies have found that children make faster saccades than adults (Fioravanti, Inchingolo, Pensiero, & Spanio, 1995; Funk & Anderson, 1977; Irving, Steinbach, Lillakas, Babu, & Hutchings, 2006). Across studies, however, age ranges varied and given the modest difference found between ages (typically less than 100 deg/s) there may have been differences in the sensitivity to capture developmental changes. The studies that have not found age differences in peak velocity considered age as a continuous variable (Luna et al., 2004) or used small age bins of 2–3 years (Munoz et al., 1998). The ones that have found faster saccades in children have used large age bins (5 years) (Fioravanti et al., 1995; Irving et al., 2006). One study with a large age range (3–86 years of age) found that saccade velocity increased throughout childhood peaking in a group of 10–15 year olds followed by a decrease with age (Irving et al., 2006). Thus, age appears to have an effect, if minimal, on saccade velocity which may be due to a peak in physical health during adolescence or to a slowing down of basic process due to voluntary control of even basic aspects of behavior evident in adulthood.
Saccade accuracy, the process of stopping a saccade in a location to optimally foveate a visual stimulus, is primarily determined by cerebellar circuits. Hypometria (see glossary), making a saccade short of the optimal location for foveation, is evident in infancy (Aslin & Salapatek, 1975; Harris, Jacobs, Shawkat, & Taylor, 1993; Regal, Ashmead, & Salapatek, 1983) and appears to continue into childhood (Fioravanti et al., 1995; Munoz et al., 1998) when it stabilizes and age effects are no longer predominant (Irving et al., 2006). The fact that adults generate saccades with slower velocities but similar or improved accuracy suggests that increased velocity may not always be a gain.
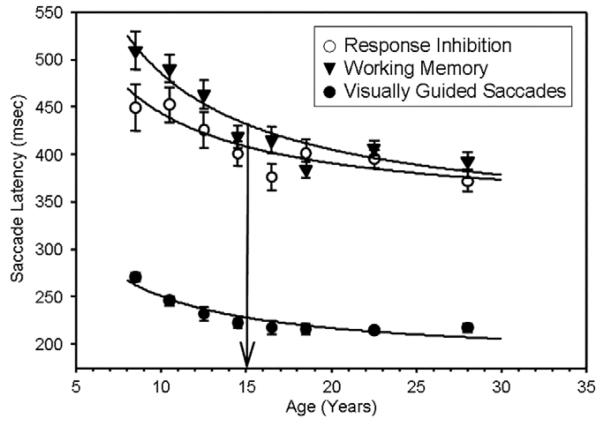
Saccade latency refers to the reaction time to initiate an eye movement. Studies have agreed that latency to initiate reflexive and voluntary eye movements decreases exponentially from birth to approximately 14–15 years of age when it stabilizes throughout adulthood (Fischer, Biscaldi, & Gezeck, 1997; Fukushima, Hatta, & Fukushima, 2000; Irving et al., 2006; Klein & Foerster, 2001; Munoz et al., 1998). Our results in 245 8–30 year old subjects confirm this finding (Luna et al., 2004) (see Fig. 2). These results are similar to developmental studies of saccade latency to cognitively driven saccade responses, such as in the antisaccade task and the memory-guided saccade task (described below), in that while voluntary saccades show much longer latencies, they are similar to reflexive saccades (see glossary) in their protracted development into adolescence. It is important to note that the similarity in development across these tasks is only in the shape of the trajectory, as cognitively driven responses are longer, and only for latency. Accuracy of responses matures early for visually guided saccades (see glossary) in comparison to the protracted development of accuracy of voluntary saccades which continues into adolescence. Studies using manual responses to a range of cognitive tasks also show decreases in reaction time into adolescence (Hale, 1990; Kail & Park, 1992). It is surprising that the reaction time to an automatic/reflexive response such as a visually guided response would parallel those of harder tasks that involve overall longer reaction times and that they would show such delays of development into adolescence. These results indicate that age-related decreases in saccade latency are driven by processes that generalize beyond the oculomotor system and may reflect the speed of information processing supported by enhanced neuronal processing afforded by continued myelination throughout this age period. Circuits crucial to response planning and preparation that support response latency may show specific maturation that becomes adult-like in adolescence, including neocortical to subcortical pathways that allow for top-down control of behavior. As such, developmental studies on saccade latency can be used to probe the integrity of information processing especially in populations with impaired development such as in psychiatric disorders.
Fig. 2.
M ± 1 standard error of the M (SEM) of the latency to initiate a saccade in each task for each age group. Solid circles depict the latency to initiate a saccade to a visual stimulus during the visually guided saccade (VGS) task. Open circles depict the latency to initiate an eye movement to the opposite location of a visual target in the antisaccade (AS) task. Solid triangles depict the latency to initiate an eye movement to a remembered location in the oculomotor delayed response (ODR) task. Thick lines indicate the inverse curve fit on the M latency to initiate an eye-movement response in millisecond by age in years. Arrows depict the ages at which change-point analyses indicate adult levels of performance were reached (from Luna et al. (2004). Child Development, 75, 1357–1372).
Saccades with a latency of 80 to approximately 140 ms are defined as express saccades (see glossary) and believed to be primarily guided by subcortical systems (Dorris, Martin, & Munoz, 1997; Dorris & Munoz, 1995; Guitton et al., 1985; Klein, Foerster, Hartnegg, & Fischer, 2005). Express saccades are considered to be the most reflexive type of eye movement toward a visual stimulus. A large number of express saccades has been found to be associated with a higher tendency to make inhibitory errors in the antisaccade task (see below), suggesting immaturities in the fixation system. However, the number of inhibitory errors is not associated with number of express saccades, indicating that immaturities in the voluntary production of saccades are distinct from the fixation system (Fischer et al., 1997). Unlike with visually guided saccades, which have a longer latency that decreases significantly with age, there is a weak relationship with age and express saccades, showing only modest decreases of their occurrence with age (Fischer et al., 1997; Klein et al., 2005) that can persist past adulthood (Munoz et al., 1998). The lack of developmental changes in the express saccade system suggests that the fixation system supported by subcortical systems matures earlier than the cognitive processes that support voluntary eye-movement responses.
Taken together, the development of pursuit, fixation, and reflexive saccades appear by infancy or childhood, yet show continued refinement into adolescence of cognitive components. The peaking of saccade velocity in adolescence and the stabilizing of saccade accuracy by childhood indicates that subcortical processes may still have some specialization into childhood affecting basic mechanisms, albeit having relatively minimal effects on behavior. The protracted development of the latency to make a reflexive saccade may reflect the age-related enhancement of more generalized systems across the brain such as myelination. The continued improvements in pursuit accuracy and prediction and the ability to suppress distraction to maintain fixation all reflect improvements of more complex systems that integrate larger networks across neocortex, which are known to support cognitive control in general. These we will describe in more detail next.
7. Development of voluntary control of eye movements
Eye movements can also be voluntarily generated by a goal-directed plan, thereby providing a model to study executive/cognitive control of behavior in a direct manner. Cognitive control is exerted in all planned behavior and it is particularly vulnerable to psychopathology where executive dysfunction is a common feature. Fundamental to executive function is the ability to voluntarily suppress prepotent or reflexive/automatic responses in order to make a planned response (response inhibition), working memory, the ability to retain and manipulate information on-line in order to make a plan to direct a response, and attention switching, the ability to change attentional focus in a controlled fashion (Miyake et al., 2000). These processes work in unison to support cognitive control, but can be characterized independently (Asato, Sweeney, & Luna, 2006; Miyake et al., 2000). Response inhibition and working memory have been described as aspects of the same mental process (Miller & Cohen, 2001). While the circuitry that underlies inhibitory and workingmemory tasks overlap, the neuronal computations are distinct. Primary to working memory is the reliance on reverberating circuits that can maintain activity across prolonged periods of time (Funahashi et al., 1989). Primary to inhibition is top-down modulation that permits the shutting down of a reflexive response. Voluntary movements, including inhibitory control, require that a planned response be on-line, while working memory, as defined by (Baddeley, 1992), includes the ability to manipulate information on-line, which would require inhibitory components,. Developmental studies indicate that even in childhood these two processes work closely together to affect performance (Eenshuistra, Ridderinkhof, Weidema, & van der Molen, 2007), as in adulthood (Kane, Bleckley, Conway, & Engle, 2001; Van der Stigchel, Merten, Meeter, & Theeuwes, 2007). Although these processes cannot be completely separated, they are unique computational processes. When considering development and psychopathology, the relative integrity of these two systems can be assessed. Aspects of response inhibition and working memory have been found to develop on different time tables and influence performance in complex executive tasks differently (Asato et al., 2006; Luna et al., 2004; Miyake et al., 2000). As described below, while latency and accuracy of initial responses to working memory and inhibitory oculomotor tasks appear to mature around mid-adolescence, the ability to enhance precision of mnemonic responses continues into adulthood (Luna et al., 2004). There is also evidence that these two processes may be affected differentially in psychopathology such as in ADHD, where the ability to inhibit an eye movement is impaired while the ability to make a memory-guided saccade has been found to be preserved, suggesting that ADHD is associated more with a specific impairment in inhibitory control and less so with working memory, when there are minimal working memory requirements (Ross, Hommer, Breiger, Varley, & Radant, 1994). Schizophrenia, on the other hand, appears to show impairment in both inhibitory eye movements and memory-guided saccades indicating a different vulnerability in cognitive control (Ross, Harris, Olincy, & Radant, 2000). The ability to test these two aspects of cognitive control by manipulating the reliance on each process could potentially be of great use in dissociating impairments in specific circuitry in psychopathology especially in the context of development. Most neuropsychological tasks, however, have both response inhibition and working memory processes tightly associated in the demands of the task (e.g., the Wisconsin Card Sort involves both keeping on-line previous stimulus arrangements and inhibiting the perseveration of responses that are inadequate). This is another area where oculomotor studies are particularly well suited to characterize inhibitory control with minimal working memory demands except for remembering the general instruction (the antisaccade task, below), as well as tasks with minimal inhibitory demands and driven primarily by working memory (the memory-guided saccade task, below). We will now describe the developmental trajectories of performance in these tasks that are able to reveal the basic aspects of the development of cognitive control.
7.1. Development of antisaccades
7.1.1. Development of the ability to suppress a prepotent saccade
The antisaccade (AS; see glossary) task is an oculomotor task that probes the ability to exert cognitive control of behavior by exerting voluntary response inhibition. In this task, subjects must voluntarily inhibit a reflexive eye movement towards a visual stimulus (prosaccade–PS; see glossary) and instead make a planned movement to its mirror location (Hallett, 1978). An antisaccade error (often referred to as response “accuracy”) refers to the inability to suppress the reflexive eye-movement response to the peripheral stimulus. These errors are usually followed by a corrective response to the appropriate location, indicating that the instruction was understood but that the reflexive response was not able to be suppressed (Luna et al., 2004). Investigators studying the development of AS performance have typically compared AS and PS performance in an effort to distinguish developmental change in systems implicated in response suppression (and maintenance of fixation) from systems supporting basic sensorimotor function (Fischer et al., 1997). As we will detail, the bulk of evidence indicates that age-related improvements in AS performance are largely attributable to changes in the ability to consistently exert inhibitory control.
Many studies have used the AS task in large samples of healthy controls and have found strikingly similar results (Fischer et al., 1997; Fukushima et al., 2000; Klein & Foerster, 2001; Luna et al., 2004; Mayfrank, Mobashery, Kimmig, & Fischer, 1986; Munoz et al., 1998; Nelson et al., 2000). From childhood to adolescence there is a reduction in the latency to initiate both prosaccades and antisaccades and in correcting inhibitory errors, supporting developmental increases in speed of processing (see Fig. 2). Importantly, these studies have also found that from childhood to approximately 15 years of age there is a significant reduction in inhibitory errors, which indicates important improvements in cognitive control. Additionally, when a short gap separates fixation offset and target onset, antisaccade errors are increased compared to the case when an overlap in fixation and target exists, which allows the fixation system to support the inhibition of reflexive saccades (Fischer, Gezeck, & Hartnegg, 1997). The relative gain in performance from the overlap compared to the gap condition is called the gap-effect. This gap-effect decreases from childhood to adulthood indicating that children rely more on the protective effect of fixation than mature individuals (Klein, 2001; Klein & Foerster, 2001; Klein et al., 2005).
Fischer et al. (1997) performed the original study where the above findings were evident in 300 8–65 year old subjects (as well as demonstrating a moderate deterioration of performance from 40 to 65 years of age). The strong developmental effects observed in AS performance were subsequently replicated and extended in other studies examining developmental change (Fukushima et al., 2000; Klein & Foerster, 2001; Luna et al., 2004; Mayfrank et al., 1986; Munoz et al., 1998; Nelson et al., 2000). For example, Munoz and colleagues (1998) showed dramatic age-dependence from 8 to 20 years of AS error rates and response times, but little variation with age in PS metrics and dynamics. Additionally, these authors noted that all subjects corrected at least some of their errors, indicating that all subjects, independent of age, were capable of generating post-inhibition voluntary saccades. Their results indicated that children have greater difficulty suppressing short-latency reflexive prosaccades. Fukushima et al. (2000) reached similar conclusions in their study of children (aged 8–12 years) versus adults, reporting stabilization of AS error rates at 10–12 years of age, but continued decreases to adulthood coupled with decreasing saccade latencies. In contrast, PS latencies reached adult levels by 12 years, with their peak velocities showing no change. Fukushima et al. echoed the conclusions reached by prior investigators, arguing that brain systems supporting the inhibition of reflexive prosaccades are still immature at age 12. As in prior studies, Klein (2001) and colleagues observed dramatic developmental change in AS error rates in 6–26 year old participants. However, they added a novel approach by fitting regression models across the age range as a continuous variable. Their findings indicated that a curvilinear model that shows rapid changes through childhood and slower rate of change later in development provided the best fit.
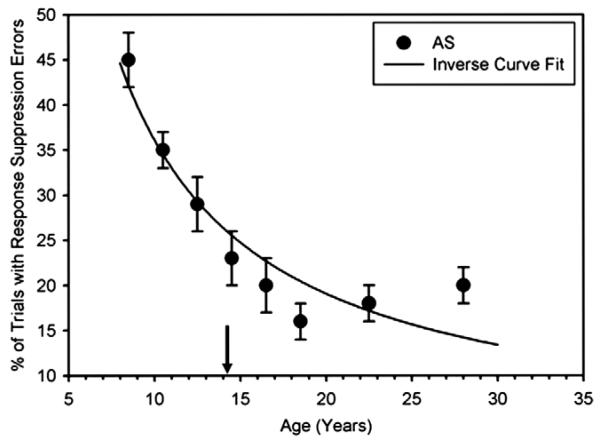
Our own study of 245 8–30 year old sought specifically to characterize the transition to adult-level performance and to better determine the age of adult-level performance (Luna et al., 2004). Similar to Klein et al. (2001), we chose to also use curvilinear regressions in order to investigate the shape of developmental maturation. “Maturation” in this context is used to highlight the specific stage of development of adolescence when development is reaching stabilization of adult levels. Our results indicated that similar to Klein et al., 2001, an inverse regression [Y = b0 + (b1/t)] best represented the age-related changes in saccade latency and proportion of inhibitory errors (see Figs. 2 and 3), indicating that from childhood to adolescence there is a steep improvement in performance which stabilizes through adulthood. In order to determine the age of maturation, we applied change-point analyses, which can be performed only on cross-sectional data, to determine the age at which the distribution of responses ceases to change (see also Klein, Foerster, & Hartnegg, 2007; Klein et al., 2005). Our results indicated that maturity was reached by 14–15 years of age for prosaccade and antisaccade latency, as well as inhibitory control (see Fig. 2). The exact age of maturity, however, varies across studies including those indicating development into the twenties (Klein et al., 2005; Munoz et al., 1998), which may be linked to sample variability.
Fig. 3.
Solid circles depict the M ± 1 standard error of the M (SEM) for the percent of trials with a response suppression failure in the antisaccade (AS) task. Thick lines depict the inverse curve fit on the response suppression failures by age in years. The arrow depicts the age at which change-point analyses indicate adult levels of performance were reached (from Luna et al. (2004). Child Development, 75, 1357–1372).
Thus, studies have consistently demonstrated that improvements in AS performance continue into adolescence. Nevertheless, it is important to note that across all studies the ability to successfully suppress a prepotent saccade was present early (that is, by age 8 children could perform at least one correct AS trial in each of the studies reviewed above). Indeed, the ability to suppress a response toward a suddenly presented stimulus has been documented even in infancy using preferential looking tasks that required the suppression of reflexive head- and eye-movement responses to probes (Johnson, 1995). Rather, developmental improvements in error rates indicate that it is the consistency with which this ability can be applied that is age-dependent. Moreover, across studies there is also agreement that with development there is a dramatic decrease in intra-subject variability so that by adulthood performance is optimal and even across the age group. This decrease in performance variability with age has been documented in previous studies (Klein et al., 2005). At younger ages, there is a wide distribution of performance with some children and adolescents showing mature levels and others showing significantly poorer performance. This may be due to true variability in developmental schedules with some individuals maturing earlier than others or it may reflect that immature performance is variable at the individual level. The latter case supports the proposal that the ability to perform at adult levels is present at early ages but the ability to be consistent is immature. The implications of this proposal are important since they suggest that the ability to inhibit is present early on as well as the circuitry that supports cognitive control processes and that what is immature is the ability to use this cognitive tool and the brain systems that allow the flexible implementation of executive abilities. This proposal has similar implications for psychopathology, where results also indicate the capability of making some correct responses but that overall performance is impaired.
Many of the aforementioned studies included a wide age-range from childhood to senior years into the 70's (Fischer et al., 1997; Klein et al., 2005; Munoz et al., 1998; Olincy, Ross, Youngd, & Freedman, 1997). Unlike the development of the ability to inhibit saccades, which shows dramatic improvements from childhood to adolescence, after the second or third decades of life there are more modest changes with aging. Results indicate a general slowing evident in the latency to initiate an antisaccade (Fischer et al., 1997; Munoz et al., 1998; Olincy et al., 1997) supporting models of general cognitive slowing in aging (Myerson, Hale, Wagstaff, Poon, & Smith, 1990). There is also evidence for a moderate increase in the number of inhibitory errors in the antisaccade task (Klein et al., 2005; Olincy et al., 1997), which in some studies does not reach significance (Fischer et al., 1997; Munoz et al., 1998). While the initial developmental changes from childhood to adulthood reflect a curvilinear relationship (Klein et al., 2005; Luna et al., 2004), from adulthood to elderly stages the relationship between age and antisaccade performance has been found to be linear (Klein et al., 2005). These results suggest that different processes may underlie the initial maturation of inhibitory control and the subsequent loss of optimal performance in aging.
7.1.2. Development of the ability to retain an inhibitory response set
We have begun to investigate the proposal that what underlies the development of inhibitory control is the ability to retain an inhibitory response “state” or “task set (see glossary)”. Sustained voluntary control of behavior has long been thought to rely on the effective instantiation and maintenance of a task set (Logan & Gordon, 2001; Monsell, 1996). The adoption of a task set is thought to enable the configuration of moment-to-moment data processing in a task-specific and goal-appropriate fashion. Models of task set postulate higher-order supervisory control processes that select and modulate downstream task-relevant transient processes (Baddeley, 1996; Desimone & Duncan, 1995; Logan & Gordon, 2001; Norman & Shallice, 1986; Schneider & Shiffrin, 1977; Shiffrin & Schneider, 1977). For example, we propose that the AS task requires the initiation and maintenance of top-down signals supporting the modulation of reflexive or prepotent responses in addition to operations executed on a trial-by-trial basis. Difficulty with maintenance of task sets is entirely consistent with immature AS performance in children and young adolescents. Although younger subjects often show adult-like performance at the single trial level, increased errors associated with failures of inhibition and anticipatory errors are hallmarks of their performance. Additionally, children and young adolescents show immature performance in dual task and task-switching paradigms, which are thought to provide indirect measures of the integrity of task sets required for the coordination of multiple tasks (Dosenbach et al., 2006; Logan & Gordon, 2001; Monsell & Mizon, 2006; Schneider & Logan, 2006), and to do so even when individual task performance is equivalent to that of adults (Karatekin, 2004). Further, with respect to the development of task switching, hierarchical analyses have demonstrated that age-related variance in task-switching performance can be independent from that associated with task sub-processes such as perceptual speed and working memory (Cepeda & Kramer, 2001). In this manner, integral to the protracted development of AS performance into adolescence may be age-related improvements in the ability to maintain an inhibitory set.
7.1.3. Development of brain function underlying response inhibition
Functional neuroimaging work in adult humans, consistent with extensive neurophysiological work in monkeys (Bruce & Goldberg, 1985; Robinson & Goldberg, 1978), has demonstrated that AS task performance produces robust activation in a network of regions including dorsolateral prefrontal cortex (DLPFC), supplementary eye field (SEF; see glossary), frontal eye field (FEF), (lateral) posterior parietal cortex (PPC), striatum, superior colliculus (SC), and cerebellum (Brown, Goltz, Vilis, Ford, & Everling, 2006; Connolly, Goodale, DeSouza, Menon, & Vilis, 2000; Matsuda et al., 2004; Miller, Sun, Curtis, & D'Esposito, 2005; Muri et al., 1996).
Single-cell studies have found that preparatory activity in eye-movement regions predicts successful inhibitory responses (Amador, Schlag-Rey, & Schlag, 2004; Everling & Munoz, 2000). During the preparation to inhibit an eye movement, activity in saccade-re-lated neurons in subcortical structures (SC) and cortical regions (notably, FEF, and PPC) is dampened while activity in fixation-related neurons in these regions (implicated in the suppression of eye movements) is increased (Munoz & Everling, 2004). Recent fMRI (see glossary) studies indicate that DLPFC also shows preparatory activity, but unlike SC, FEF, and PPC, which also showed activity during saccade responses, DLPFC was only recruited in the preparatory phase of AS (versus PS) trial performance indicating that activity in this region likely reflects processing related to biasing the oculomotor network for AS performance (Brown, Vilis, & Everling, 2007). This interpretation is consistent with the extensive number of projections from DLPFC to both cortical and subcortical regions (Fuster, 1997), and also with the finding that neurons within DLPFC that project directly to SC and which are thought to provide saccade suppression signals, show increased activity in preparation for AS versus PS trial performance (Everling, Dorris, Klein, & Munoz, 1999; Everling & Munoz, 2000). These results indicate that the ability to inhibit an impending saccade requires the concerted activity of prefrontal, premotor, and subcortical regions. The ability to make correct antisaccades in childhood implies that this circuitry is capable of functioning in a mature manner early on, albeit inconsistently. However, little data has been gathered to date documenting developmental change in AS-related brain activity.
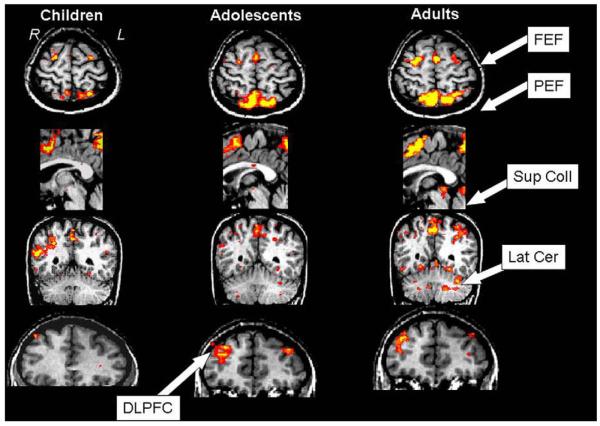
In the only published study to do so, we compared activity observed during blocks of AS performance with that observed during blocks of PS performance, in children aged 8–13 years, adolescents aged 14–17 years, and adults aged 18–30 years (Luna et al., 2001). Key regions implicated in oculomotor control (FEF, PPC, and SC) were more active in adults than in adolescents or children (see Fig. 4). Children, who performed worse than the older groups, showed increased activity of parietal regions indicating a compensatory reliance on visuo-spatial processing. Adolescents, who performed similar to adults, showed increased recruitment of dorsolateral prefrontal cortex, suggesting increased effort to perform at adult levels by relying on this region known to support AS performance (Brown et al., 2007). Adults showed less reliance on prefrontal systems, efficient use of eye-movement regions, and recruitment of additional regions such as the lateral cerebellum. These results are supported by a recent topographical ERP study using the antisaccade task showing that children rely on parietal regions, but by late adolescence a frontal predominance is evident (Klein & Feige, 2005).
Fig. 4.
Mean group activity during a block antisaccade task for children, adolescents, and adults overlaid on top of the structure of a representative subject (from Luna et al. (2001). Neuroimage, 2001 13(5), 786–793).
However, a limitation of block designs is that both error and correct trials are included. Event-related studies in our laboratory enabled us to characterize age-related changes in brain function during correct and error trials separately therefore comparing similar performance (Velanova, Wheeler, & Luna, in press). Results indicated that the FEF, SMA/preSMA, PPC, and putamen show increased activity for correct AS versus error trials (on which prepotent prosaccades were incorrectly executed, prior to a corrective saccade), but no age group-related effects. Instead there are age-related decreases in activity in prefrontal regions again reflecting increased effort in younger subjects. Similar to adult studies where increases in task difficulty result in increased prefrontal recruitment (Carpenter, Just, Keller, & Eddy, 1999), immature subjects have greater difficulty performing this task correctly as is reflected by the larger number of total errors, resulting in the necessity to recruit PFC at higher levels. These results both predict increased activity with age in blocked studies (attributable to age-related increases in the proportion of correct trials) and demonstrate that when eliciting the same response, i.e., a correct or incorrect inhibitory response, the same regions known to support cognitive control of eye movements are used across development. That these regions showed similar levels of activity across age again suggests that brain systems implicated in successful eye-movement control show early maturation.
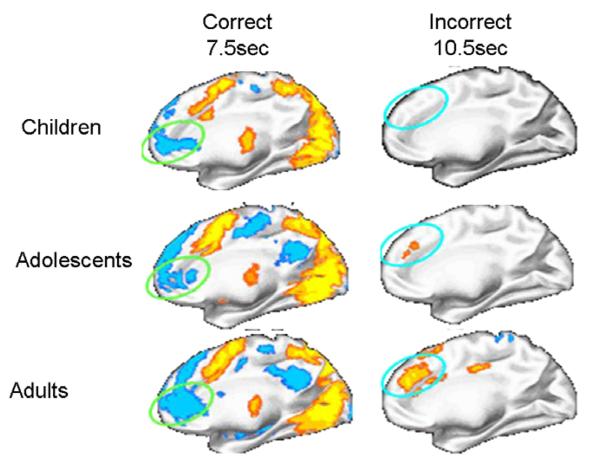
One aspect of inhibitory control that did show strong developmental changes in brain function was error regulation functions, supported by anterior cingulate (see glossary) cortex (ACC). Our recent results indicate that dorsal anterior cingulate (dACC) shows late increased modulation for error versus correct trials that peaked following the response (Velanova, Wheeler, & Luna, in press), similar to findings from other adult studies (Polli et al., 2005). Children and, to a lesser extent, adolescents, failed to show late differential activity of this sort, indicating immaturity (see Fig. 5). Thus, while children and adolescents appear fully mature in their ability to recognize when they have made an error (as indicated by correction rates that paralleled those of adults), their ability to use this information to influence future behavior may be limited. To the extent that dACC provides a signal that can inform subsequent task performance, as a growing body of work indicates it does (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004), our data suggest that children and adolescents receive less support from such feedback signaling, and hence implicates immature error regulation and error-feedback utilization as a source of performance decrements in younger age groups. These results are supported by other studies showing that adults demonstrate increased recruitment of ACC and prefrontal cortex during a stop-signal inhibitory task (Rubia, Smith, Taylor, & Brammer, 2007). Taken together, these results underscore the important role of protracted development in error processing, subserved by immaturities in the functional integration of prefrontal regions underlying the development of cognitive control of behavior. These findings further suggest that abnormalities in the transition to adult-level error monitoring may be limited in psychopathology.
Fig. 5.
Activation maps displayed on the partially inflated medial cortical surface of the right hemisphere for inhibitory errors in the AS task for children, adolescents, and adults. Results indicate similarities across age groups during the initial stage of error processing in the medFG/rACC. However, only adults show recruitment of dACC in later stages of error processing. Blue indicated deactivation. Red/Yellow indicated activation (adapted from Velanova et al. (2008). Cerebral Cortex, February 14 [Epub ahead of print]). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Additionally, children showed increased recruitment of DLPFC relative to adolescents and adults for both correct and error AS trials. Similar to adult studies showing increased activity in prefrontal cortex with increased task difficulty (Velanova et al., 2003; Wheeler & Buckner, 2003; Wheeler et al., 2005), our results could reflect the additional recruitment in younger subjects of task-general frontal control systems that permit, for example, improved task focus or that reflect additional processing required to manage task performance independent of trial accuracy. In particular, and in accord with Brown et al. (2007) results, we suggest that such additional processing in DLPFC might reflect “compensatory” control exerted to overcome inefficiency in the biasing of response pathways suitable for inhibiting a prepotent response.
7.1.4. Incentive processing and the development of voluntary saccades
The influence of incentives (i.e., rewards, punishment) on saccade parameters has been exceptionally well characterized at the single-unit level in non-human primates. Results indicate that reward-related responses are supported by a distributed brain circuitry including basal ganglia, ventral tegmental area, nucleus accumbens, amygdala, and orbital frontal cortex (Amador, Schlag-Rey, & Schlag, 2000; Hikosaka, Takikawa, & Kawagoe, 2000; Kawagoe, Takikawa, & Hikosaka, 1998). Reward incentive enhances activity of critical brain regions that support antisaccade and memory-guided saccade performance (Amador et al., 2000; Hikosaka et al., 2000; Johnston & Everling, 2006).
Brain systems supporting reward processing have a protracted development through adolescence, during which time there is evidence for increased risk-taking behavior and the emergence of psychopathology (Chambers, Taylor, & Petenza, 2003). Impairments in reward processing have been associated with gambling, depression, and substance abuse, which often appear in adolescence (Angold, Costello, & Worthman, 1998; Barlow, 1988; Bechara, Damasio, Damasio, & Anderson, 1994; Kessler et al., 1994; Lafer, Renshaw, & Sachs, 1997; Leshner & Koob, 1999). Basal ganglia (Giedd, Vaituzis, et al., 1996; Sowell et al., 1999; Toga et al., 2006) and orbitofrontal cortex (Gogtay et al., 2004) are found to show protracted thinning of gray matter into adolescence, a presumed consequence of synaptic pruning. The under-specialized reward system may be limited in adolescence in the ability to properly assess the valence (rewards and punishment) and value of incentives. Additionally, during adolescence there is greater activity in dopamine systems that surpasses that of inhibitory 5-HT systems resulting in a potential imbalance in reward and suppression mechanisms (Andersen, 2005; Chambers et al., 2003; Lambe, Krimer, & Goldman-Rakic, 2000; Takeuchi et al., 2000). The effects of reward on the cognitive control of eye movements could provide a model to test incentive processing in development and psychopathology.
Recent work has investigated the influence of incentives on the suppression of saccades from a developmental perspective. Studies using reward probes during an antisaccade task have found that incentives (rewards and punishment) increased the number of correct antisaccades in healthy adults and adolescents, although the effect was less evident in adolescents with anxiety and mood disorders suggesting abnormalities in the reward system (Hardin, Schroth, Pine, & Ernst, 2007; Jazbec, McClure, Hardin, Pine, & Ernst, 2005; Jazbec et al., 2006). The latency and peak velocity of erroneous antisaccades were also modulated by incentives in adolescents but not adults. The next step in understanding the effects of motivation on behavior is to directly investigate how reward modulates cognitively driven responses at different ages. Additionally, reward processing involves different stages of processing that could have different developmental profiles that could help disentangle the discrepant results in the literature. We have begun such studies which are already indicating immaturities in how motivation affects cognitive control in adolescence.
7.2. Development of memory-guided saccades
7.2.1. Development of memory-guided performance
Working memory (WM) refers to the cognitive ability to maintain and manipulate information ‘on-line’ about stimuli that are no longer present in the external environment (Baddeley, 1986). WM supports volitional or goal-directed responses and is known to be a critical component of higher-order executive function (Bjorklund & Harnishfeger, 1990; Case, 1992; Dempster, 1993; Nelson et al., 2000). Similar to voluntary response suppression, working memory is known to have a protracted developmental trajectory (Beveridge, Jarrold, & Pettit, 2002; Brocki & Bohlin, 2004; DeLuca et al., 2003; Demetriou, Christou, Spanoudis, & Platsidou, 2002; Gathercole, Pickering, Ambridge, & Wearing, 2004; Hitch, Halliday, Dodd, & Littler, 1989; Luciana, Conklin, Hooper, & Yarger, 2005; Luna et al., 2004; Swanson, 1999; Zald and Iacono, 1998).
Spatial working memory (SWM), as a model of WM, refers to those processes which support the on-line maintenance and manipulation (when required) of visual–spatial information. A typical SWM task requires encoding of the spatial location of a stimulus, maintenance of the representation of that location across a delay period, and, finally, a volitional response to the remembered location. Importantly, this response is guided solely by the internal, mnemonic representation of the stimulus location and not by external stimuli. Various SWM tasks require that representations be maintained across different delay periods, which sometimes have an interference stimulus or manipulation requirement (Kwon, Reiss, & Menon, 2002; Swanson, 1999). Common across SWM tasks is that the accuracy of the memory-guided response is used as an index of working-memory capacity/ability.
Spatial working memory is particularly amenable to measurement using oculomotor tasks given that eye-movement measures can provide the level of resolution needed to identify small improvements in precision. The memory-guided saccade (MGS) task, also known as the oculomotor delayed response task, has proven to be a sensitive measure of developmental change in SWM. The MGS task was initially designed for single-cell studies in non-human primates investigating different aspects of voluntary oculomotor control (Funahashi et al., 1989; Hikosaka, Sakamoto, & Usui, 1989). This task requires an eye-movement response guided solely by the representation in working memory of the remembered location of a previously presented visual cue. Specifically, while subjects fixate a central target, a peripheral target is briefly presented at an unpredictable location in the periphery. Subjects must retain fixation and simply remember the location of the probe. Trials where subjects gaze at the probe are not included and represent inhibitory failures. Subjects retain fixation for different delay durations. When the fixation cue is extinguished, subjects make a voluntary saccade in the absence of a visual stimulus to the remembered location. Responses in the MGS task typically involve at least two saccades. The initial large saccade brings gaze near the location of the remembered target location and is guided by the ability to voluntarily make a response in the absence of a visual stimulus as well as SWM processes. Subsequently, there are one or more additional smaller saccades that correct to a more precise location, which are guided more directly by SWM and error detection processes. The MGS task does not require manipulation of the representation in working memory and, as such, is an optimal measure of WM encoding and maintenance, which are the components that directly speak to the unique neural computations of keeping information on-line. Processes involved in manipulation during WM maintenance involve inhibitory control and as thus, do not allow the direct probing of mnemonic processes.
While many studies have investigated MGS performance in young populations with psychopathology (Fukushima, Tanaka, Williams, & Fukushima, 2005; Goto et al., 2005) few have looked at the development of MGS performance (Hikosaka, 1997; Luna et al., 2004). Hikosaka (1997) studied MGS performance in 5–76 year old subjects and found that young and elderly subjects showed increased inhibitory failures during the encoding phase of the task and overall longer latencies to initiate memory-guided saccades. These results, however, did not speak to the age-related changes in the fidelity of the remembered response.
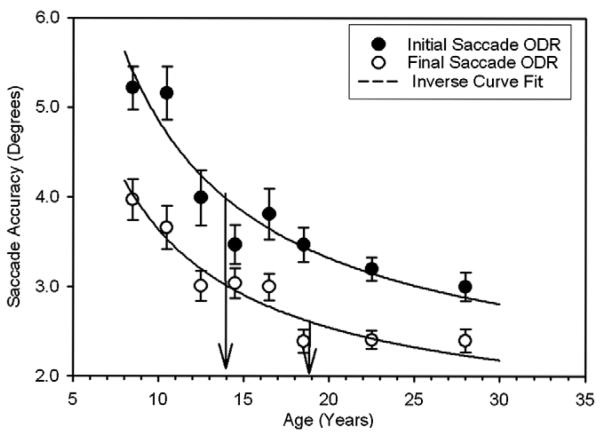
We performed a study on 245 8–30 year olds and characterized the nature of the memory-guided responses that did not have inhibitory errors (Luna et al., 2004). We found, similar to Hikosaka's (1997) results, that the latency to initiate a correct MGS decreased with age until 14–15 years of age (see Fig. 2). This result also confirms developmental changes in response latency for visually guided saccades, antisaccades, and a range of cognitive tasks indicating an independent trajectory regarding improvements in speed of processing which show a strikingly similar development in adolescence. We also found that with age there were less inhibitory errors of gazing towards the probe supporting developmental improvements in inhibitory control. Moreover, we studied the accuracy of the response by measuring the distance between the end point of the saccade and the exact location of the remembered stimulus. Results indicated that the accuracy of the first saccade was mature at approximately 15 years of age (see Fig. 6), similar to results on the antisaccade task and speed of processing. This suggests that the general processes supporting voluntary control are mature by adolescence. However, we found that the accuracy of the final corrective saccade continued to show improvements into the second decade of life, indicating that WM processes, as well as performance monitoring processes, are still immature in adolescence. The latter result provides further evidence for our findings regarding a protracted development of error processing in the antisaccade task. We also found that the age effects were present regardless of the duration of the delay period, which ranged from 1 to 8 s in duration, indicating that encoding as well as mnemonic processes underlie development of working memory. Interestingly, in a study of aging, we found that older subjects showed decreased accuracy of the initial response but the accuracy of the last saccade was comparable to that of young adults (Sweeney, Rosano, Berman, & Luna, 2001). These results suggest that in aging voluntary control is sluggish while performance monitoring and working-memory processes may be preserved.
Fig. 6.
Mean ± 1 standard error of the mean for the accuracy to initiate a memory-guided saccade (solid circles) and the accuracy of the final gaze location (open circles) in the ODR task for each age group. Thick lines indicate the inverse curve fit for these data across the age-range studied. Arrows depict the age at which change-point analyses indicate adult levels of performance were reached (from Luna et al. (2004). Child Development, 75, 1357–1372).
In sum, the ability to generate memory-guided saccades improves with age and is supported by improvements in speed of processing and response inhibition, as well as by processes more directly related to the ability to guide behavior based on a WM representation, encoding, and performance monitoring. These results could potentially be informative regarding psychopathology where performance on this task is typically impaired (Sweeney et al., 2004).
7.2.2. Development of brain function underlying working memory
A widely distributed brain circuitry underlies spatial working memory in the adult and includes dorsolateral prefrontal cortex (DLPFC), the cortical eye fields, anterior cingulate cortex, insula, basal ganglia, thalamus, and lateral cerebellum (Hikosaka & Wurtz, 1983; Sweeney et al., 1996). The circuitry has been well characterized using the memory-guided saccade task in adults using fMRI (Brown et al., 2004; Curtis, Rao, & D'Esposito, 2004; Geier, Garver, & Luna, 2007; Postle, Berger, Taich, & D'Esposito, 2000; Sweeney et al., 1995). However, only one study has used the MGS task to track developmental change (Scherf, Sweeney, & Luna, 2006) (discussed below). Neuroimaging studies on the development of working memory which use prototypical neuropsychological assessment tests have typically used memory-guided button press responses and have focused on the role of prefrontal cortex. These studies have generally found that with age there is decreased participation of prefrontal regions with development, which has been ascribed to a decrease in effort needed to perform the task with age (Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; Klingberg, Forssberg, & Westerberg, 2002; Olesen, Macoveanu, Tegner, & Klingberg, 2006; Scherf et al., 2006). While this decrease in activity could be supported by a diffuse to focal shift in specialization of prefrontal cortex (Durston et al., 2006), our findings below and recent findings on age-related changes in brain circuitries (Fair et al., 2007) indicate that the ability to establish a widely distributed circuitry supports cognitive control that lessens the dependence on prefrontal systems. As described above, using oculomotor tasks allows for a better fit to developmental questions and controls for the effects of strategy formation that may confound the results of studies that lend themselves to strategizing.
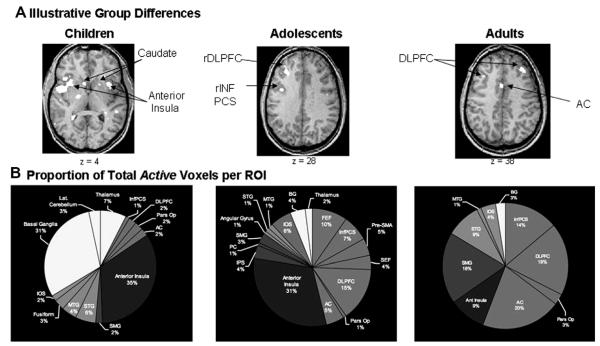
We performed a blocked design fMRI study on 30 8–47 year olds comparing activity during memory-guided saccades and visually guided saccades (Scherf et al., 2006). We found that recruitment of DLPFC increased from childhood to adolescence and subsequently decreased from adolescence to adulthood (see Fig. 7). Instead, children relied more on basal ganglia and insula, whereas adults recruited additional regions, including temporal regions. Importantly, we saw a transition to more distributed function (see Fig. 7) with age as different regions contributed in a similar fashion to support ODR performance. Oculomotor regions did not show changes with age. These results suggest that a transition to more distributed circuitry results in a more efficient system for working-memory processing, supporting adult-level performance. Additional regions recruited by adults may also support performance monitoring and encoding processes that contribute to improvements in the precision of working memory.
Fig. 7.
Imaging results from both magnitude and extent of activation analyses. (A) Proportion of total number of voxels in each region of interest submitted to extent of activation analyses in all groups. (B) Each group image represents illustrative differences in both the magnitude and extent of activation in the group-averaged percent signal change functional maps. Children showed stronger activation bilaterally in the caudate nucleus, the thalamus, and anterior insula. Adolescents showed the strongest right DLPFC activation, and adults showed concentrated activation in left prefrontal and posterior parietal regions. (C) Group differences in the extent of activation as measured by the proportion of total active voxels in each region of interest for each age group. Despite the fact that the proportion of total voxels in the extent of activation analyses was consistent across the age groups, the groups showed large differences in the proportion of total active voxels across the regions of interest (from Scherf et al. (2006). Journal of Cognitive Neuroscience, 18(7), 1045–1058).
In summary, the ability to perform memory-guided saccade tasks is present early in development. What continues to improve through adolescence is the precision of the working memory driven response which may be supported by enhanced encoding and performance monitoring processes which in turn are subserved by refined and functionally integrated brain circuitry including prefrontal and parietal systems.
8. Abnormal developmental trajectories
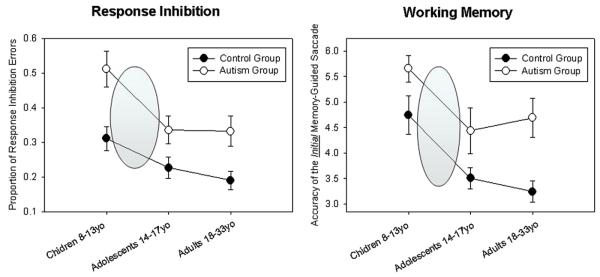
One major goal of characterizing typical development is to establish a template that can be used to discern the neurobiological basis of impaired development, as demonstrated in various psychopathologies. While there is a large literature delineating impaired oculomotor control across different psychiatric disorders during adulthood and in childhood (Sweeney et al., 2004; and see other contributions to this issue: O'Driscoll & Callahan, Gooding & Basso; Calkins; Levy), differences in developmental trajectories have not been adequately explored. Different developmental processes can be distinguished quantitatively providing mechanisms to better identify impaired trajectories. As an example of such an approach, we recently performed a cross-sectional developmental study on individuals with autism that have a normal IQ (Luna, Doll, Hegedus, Minshew, & Sweeney, 2006). While there is evidence for impaired oculomotor control in early development (Goldberg et al., 2002) and in adulthood (Minshew, Sweeney, & Luna, 2002) in autism, the shape of the developmental trajectory had not been well understood. That is, it was not clear if in autism there was a lack of developmental progression, delayed development, or deterioration with age. We studied children, adolescents, and adults with high functioning autism and IQ, gender, and age-matched typically developing individuals using the visually guided saccade, antisaccade, and ODR tasks. We found that while there was impairment at all ages in the autism group, performance in the tasks with higher cognitive control (antisaccades and ODR) by the autism group showed equivalent improvements from childhood to adolescence as the typically developing group (see Fig. 8). Two significant implications emerge from this work. First, evidence of a normative improvement from childhood to adolescence suggests that brain maturation underlying this stage of development (synaptic pruning and myelination) is preserved. Second, our results indicate that this stage of plasticity from childhood to adolescence, where behavior is changing at a fast pace, is also available in autism. This suggests a potential window of opportunity for interventions and treatment. This is especially relevant to autism where treatment has been focused on the first years of life.
Fig. 8.
Mean (±1 SEM) of the proportion of trials with response inhibition failures in the antisaccade task (left graph) and absolute error of the initial memory-guided saccade in the ODR task (right) for the autism group (open circles) and the control group (solid circles). Shaded circles indicate the similarities in the rates of improvements between groups (from Luna et al. (2007). Biological Psychiatry, 61(4), 474–481).
9. Summary and conclusions
The transition from adolescence to adulthood is of particular relevance to understanding psychopathology due to its emergence during this stage of development. Oculomotor studies provide an ideal neuroscience model to investigate the association between brain mechanisms and behavior that could potentially inform us of the neurobiological basis of the developmental aspect of psychopathology. We have reviewed the literature regarding the development of oculomotor control with a special emphasis on this transition from adolescence to adulthood. The literature indicates that basic aspects of sensorimotor control are, for the most part, mature by childhood. However, processes that support the cognitive control of eye movements have a protracted development into adolescence, which makes executive systems particularly vulnerable to psychopathology. The speed of information processing, as well as the ability to generate a voluntary eye movement, begins to show maturity in mid-adolescence as evident by developmental improvements in the performance of antisaccades and memory-guided saccades. Executive abilities are present early in development; however, the ability to use executive systems in a consistent and flexible manner continues to mature even past adolescence. The abilities to retain a task set and to monitor performance continue to show improvements beyond adolescence and may underlie improvements in the cognitive control of behavior. Motivational processes may also influence the ability to cognitively guide eye movements especially in adolescents who may be particularly sensitive to incentives.
The distinct developmental trajectories of different types of saccadic responses reflect the maturational schedules of unique brain systems. Namely, the adult-level appearance of more reflexive eye-movement responses in childhood indicate the integrity of subcortical and basic cortical systems that support basic sensorimotor function early in life. The more protracted development of the voluntary control of eye movements parallels the continued maturation into adolescence of synaptic pruning and myelination, which support the functional integration of prefrontal systems with the rest of the brain. While a casual link between maturational changes in brain structure and cognitive development is not yet firmly established, processes such as synaptic pruning and myelination undoubtedly play a substantial role. Increased efficiency of brain regional processes afforded by synaptic pruning, which reaches adult levels in adolescence, would support the complicated computations necessary to perform voluntary saccades. Myelination, which continues through adolescence and enhances functional connectivity, would support the functional integration of widely distributed circuitry also crucial for the processes that underlie voluntary control of eye movements and, importantly, speed of responses. Hence, the transition to adult-level performance may be supported by the coming on-line of a more widely distributed circuitry that becomes less reliant on prefrontal systems as brain processes become better specialized and efficient. This transition in the operation of brain systems may be particularly vulnerable to impairment and may underlie the emergence of psychopathology.
10. Future directions
Given the sensitivity of oculomotor tasks to detect changes in behavior during the transition from adolescence to adulthood, its ability to probe the integrity of executive circuitry, and its reliable use in neuroimaging studies, future studies should aim to further use these methods to characterize the developmental profile of neuropsychiatric disorders. While some studies have begun to take such an approach, the consistent characterization of specific psychiatric illnesses using interdisciplinary approaches are just beginning. Future studies would benefit from identifying the presence of oculomotor impairment behaviorally, characterizing differences in brain function using fMRI, and, importantly, using structural methodologies such as DTI in the context of development to further our understanding of the brain structural basis of psychiatric disorders. These studies should take into consideration that there is generalizability in impairments observed across different disorders and comparative studies would allow specific functional processes to be better understood. While there is evidence for impairments in cognitively driven saccades in schizophrenia, autism, and depression, these are qualitatively different disorders that may present with similar behavioral impairments but are supported by distinct brain functional and structural profiles. Characterizing developmental trajectories would allow us to identify other aspects that are specific to different disorders. As described above, in our study of the developmental progression of eye-movement control in autism, there exists potential to uncover windows of plasticity as well as what stages of brain maturation may be particularly vulnerable in different disorders. Another area of future studies is to use oculomotor tasks to probe the nature of within-subject variability inherent in psychiatric illness. That is, psychiatric patients have a higher proportion of trials showing impairments in the executive control of eye movements indicating that some trials can be performed correctly. This suggests that a basic circuitry is intact but is limited in its flexible use. Investigating variables that influence intra-subject variability, especially in a developmental context where we observe relatively greater variability, could also be informative regarding the nature of the neurobiological basis of different psychiatric disorders.
References
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. Journal of Neurophysiology. 2000;84:2166–2170. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. Journal of Neurophysiology. 2004;91:1672–1689. doi: 10.1152/jn.00138.2003. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends in Pharmacological Sciences. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Aring E, Grönlund MA, Hellström A, Ygge J. Visual fixation development in children. Graefes Archives of Clinical and Experimental Opthalmology. 2007;245:1659–1665. doi: 10.1007/s00417-007-0585-6. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cerebral Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44:2259–2269. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Salapatek P. Saccadic localization of visual targets by the very young human infant. Perception & Psychophysics. 1975;7:293–302. [Google Scholar]
- Baddeley A. Working memory. Oxford University Press; New York: 1986. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Exploring the central executive. Quarterly Journal of Experimental Psychology. 1996;494:5–28. [Google Scholar]
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. Guilford Press; New York: 1988. [Google Scholar]
- Basso MA. Cognitive set and oculomotor control review. Neuron. 1998;21:665–668. doi: 10.1016/s0896-6273(00)80583-8. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, et al. Cortical networks subserving pursuit and saccadic eye movements in humans: An fMRI study. Human Brain Mapping. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge M, Jarrold C, Pettit E. An experimental approach to executive fingerprinting in young children. Infant and Child Development. 2002;11:107–123. [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The resources construct in cognitive development: Diverse sources of evidence and a theory of inefficient inhibition. Developmental Review. 1990;10:48–71. [Google Scholar]
- Bon L, Lucchetti C. Neurons signalling the maintenance of attentive fixation in frontal area 6a beta of macaque monkey. Experimental Brain Research. 1990;82:231–233. doi: 10.1007/BF00230859. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Bohlin G. Executive functions in children aged 6 to 13: A dimensional and developmental study. Developmental Neuropsychology. 2004;26:571–593. doi: 10.1207/s15326942dn2602_3. [DOI] [PubMed] [Google Scholar]
- Brown MR, Desouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, et al. Comparison of memory- and visually guided saccades using event-related fMRI. Journal of Neurophysiology. 2004;91:873–889. doi: 10.1152/jn.00382.2003. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: Rapid Event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. Journal of Neurophysiology. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W. Graded functional activation in the visuospatial system with the amount of task demand. Journal of Cognitive Neuroscience. 1999;11:9–24. doi: 10.1162/089892999563210. [DOI] [PubMed] [Google Scholar]
- Case R. The role of the frontal lobes in the regulation of cognitive development. Brain and Cognition. 1992;20:51–73. doi: 10.1016/0278-2626(92)90061-p. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Bates JF, Makris N. The developing human brain: A morphometric profile. In: Thatcher RW, Reid Lyon G, Rumsey J, Krasnegor NA, editors. Developmental neuroimaging: mapping the development of brain and behavior. Academic Press; New York: 1996. pp. 3–14. [Google Scholar]
- Cepeda NJ, Kramer AF. Changes in executive control across the life span: Examinations of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Petenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT. A critical period of brain development: Studies of cerebral glucose utilization with PET. Preventive Medicine. 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Cohen ME, Ross LE. Latency and accuracy characteristics of saccades and corrective saccades in children and adults. Journal of Experimental Child Psychology. 1978;26:517–527. doi: 10.1016/0022-0965(78)90130-3. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, DeSouza JFX, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. Journal of Neurophysiology. 2000;84:1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Conturo TE, McKinstry RC, Akbudak E, Robinson BH. Encoding of anisotropic diffusion with tetrahedral gradients: A general mathematical diffusion formalism and experimental results. Magnetic Resonance in Medicine. 1996;35:399–412. doi: 10.1002/mrm.1910350319. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. Journal of Neuroscience. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel BM, Lee DN. Development of looking with head and eyes. Journal of Experimental Child Psychology. 1990;50:200–216. doi: 10.1016/0022-0965(90)90039-b. [DOI] [PubMed] [Google Scholar]
- DeLuca CR, Wood SJ, Anderson V, Bucanan J, Proffitt TM, Mahony K. Normative data from the Cantab: I. Development of executive function over the lifespan. Journal of Clinical & Experimental Neuropsychology. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Demetriou A, Christou C, Spanoudis G, Platsidou M. The development of mental processing: Efficiency, working memory, and thinking. Monographs of the Society for Research in Child Development. 2002;67:1–155. discussion 156. [PubMed] [Google Scholar]
- Dempster FN. Resistance to interference: Developmental changes in a basic processing mechanism. In: Howe ML, Pasnak R, editors. Emerging themes in cognitive development, volumeI: Foundations. Springer-Verlag; New York: 1993. pp. 3–27. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Reviews in Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Martin P, Munoz DP. Neuronal activity in money superior colliculus related to the initiation of saccadic eye movements. Journal of Neuroscience. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. Journal of Neurophysiology. 1995;73:2558–2562. doi: 10.1152/jn.1995.73.6.2558. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Meng F, Li L, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. Journal of Neuroscience. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eenshuistra RM, Ridderinkhof KR, Weidema MA, van der Molen MW. Developmental changes in oculomotor control and working-memory efficiency. Acta Psychologica (Amsterdam) 2007;124:139–158. doi: 10.1016/j.actpsy.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Engbert R. Microsaccades: A microcosm for research on oculomotor control, attention, and visual perception. Progress in Brain Research. 2006;154:177–192. doi: 10.1016/S0079-6123(06)54009-9. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Shinoda Y, Wise SP. Neurophysiological approaches to higher brain functions. Wiley; New York, NY: 1984. [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: A review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti F, Inchingolo P, Pensiero S, Spanio M. Saccadic eye movement conjugation in children. Vision Research. 1995;35:3217–3228. doi: 10.1016/0042-6989(95)00152-5. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Gezeck S, Hartnegg K. The analysis of saccadic eye movements from gap and overlap paradigms. Brain Research Protocols. 1997;2:47–52. doi: 10.1016/s1385-299x(97)00027-5. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Kurkin S, Kaneko CR, Fukushima K. The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions. Journal of Vestibular Research. 2006;16:1–22. [PMC free article] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Takeichi N, Kaneko CRS, Fukushima K. Involvement of the frontal oculomotor areas in developmental compensation for the directional asymmetry in smooth-pursuit eye movements in young primates. Annals of the New York Academy of Sciences. 2003;1004:451–456. [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain & Development. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Tanaka S, Williams JD, Fukushima K. Voluntary control of saccadic and smooth-pursuit eye movements in children with learning disorders. Brain and Development. 2005;27:579–588. doi: 10.1016/j.braindev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. Journal of Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funk CJ, Anderson ME. Saccadic eye movements and eye-head coordination in children. Perceptual and Motor Skills. 1977;44:599–610. doi: 10.2466/pms.1977.44.2.599. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 3rd ed. Raven Press; New York: 1997. [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuroimage. 2007;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. The Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Landa RJ, Tien A, et al. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. Journal of Neurophysiology. 1990;64:489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bushnell MC, Bruce CJ. The effect of attentive fixation on eye movements evoked by electrical stimulation of the frontal eye fields. Experimental Brain Research. 1986;61:579–584. doi: 10.1007/BF00237584. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Chafee M, Friedman H. Allocation of function in distributed circuits. In: Ono T, Squire LR, Raichle ME, Perrett DI, Fukuda M, editors. Brain mechanisms of perception and memory: From neuron to behavior. Oxford University Press; New York: 1993. pp. 445–456. [Google Scholar]
- Goto Y, Aihara M, Hatakeyama K, Kitama T, Sato Y, Nakazawa S. Study of saccadic eye movement in children with attention deficit/hyperactivity disorder. No To Hattatsu. 2005;37:10–14. [PubMed] [Google Scholar]
- Gredebäck G, von Hofsten C, Karlsson J, Aus K. The development of two-dimensional tracking: A longitudinal study of circular pursuit. Experimental Brain Research. 2005;163:204–213. doi: 10.1007/s00221-004-2162-0. [DOI] [PubMed] [Google Scholar]
- Grönqvist H, Gredebäck G, Hofsten C. Developmental asymmetries between horizontal and vertical tracking. Vision Research. 2006;46:1754–1761. doi: 10.1016/j.visres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Experimental Brain Research. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hainline L, Turkel J, Abramov I, Lemerise E, Harris CM. Characteristics of saccades in human infants. Vision Research. 1984;24:1771–1780. doi: 10.1016/0042-6989(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Haishi K, Kokubun M. Developmental trends in pursuit eye movements among preschool children. Perceptual and Motor Skills. 1995;81:1131–1137. doi: 10.2466/pms.1995.81.3f.1131. [DOI] [PubMed] [Google Scholar]
- Haishi K, Kokubun M. Development of psychological aspect in pursuit eye movements among preschoolers. Perceptual and Motor Skills. 1998;86:146. doi: 10.2466/pms.1998.86.1.146. [DOI] [PubMed] [Google Scholar]
- Hale S. A global developmental trend in cognitive processing speed. Child Development. 1990;61:653–663. [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: Development and psychopathology related differences. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Jacobs M, Shawkat F, Taylor D. The development of saccadic accuracy in the first seven months. Clinical Vision Sciences. 1993;8:85–96. [Google Scholar]
- Henik A, Rafal R, Rhodes D. Endogenously generated and visually guided saccades after lesions of the human frontal eye fields. Journal of Cognitive Neuroscience. 1994;6:400–411. doi: 10.1162/jocn.1994.6.4.400. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Changes and disorders in voluntary saccades during development and aging. No To Hattatsu. 1997;29:213–219. [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movement. Journal of Neurophysiology. 1989;61:780–798. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. Journal of Neurophysiology. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Hitch GJ, Halliday MS, Dodd A, Littler JE. Development of rehearsal in short-term memory: Differences between pictorial and spoken stimuli. British Journal of Developmental Psychology. 1989;7:347–362. [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Investigative Ophthalmology & Visual Science. 2006;47:2478–2484. doi: 10.1167/iovs.05-1311. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Harris CM, Shawkat F, Taylor D. Smooth pursuit development in infants. Australian & New Zealand Journal of Ophthalmology. 1997;25:199–206. doi: 10.1111/j.1442-9071.1997.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biological Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Johnson MH. The inhibition of automatic saccades in early infancy. Developmental Psychobiology. 1995;28:281–291. doi: 10.1002/dev.420280504. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade–antisaccade tasks. Journal of Cognitive Neuroscience. 2006;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Kail R, Park Y. Global developmental change in processing time. Merrill-Palmer Quarterly. 1992;38:525–541. [Google Scholar]
- Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology. General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Development of attentional allocation in the dual task paradigm. International Journal of Psychophysiology. 2004;52:7–21. doi: 10.1016/j.ijpsycho.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, Harris M. Development of oculomotor functioning in preadolescence, adolescence, and adulthood. Psychophysiology. 1998;35:64–72. [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Keating EG, Gooley SG. Disconnection of parietal and occipital access to the saccadic oculomotor system. Experimental Brain Research. 1988;70:385–398. doi: 10.1007/BF00248363. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Klein C. Developmental functions for saccadic eye movement parameters derived from pro- and antisaccade tasks. Experimental Brain Research. 2001;139:1–17. doi: 10.1007/s002210100711. [DOI] [PubMed] [Google Scholar]
- Klein C, Feige B. An independent components analysis (ICA) approach to the study of developmental differences in the saccadic contingent negative variation. Biological Psychology. 2005;70:105–114. doi: 10.1016/j.biopsycho.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Klein C, Foerster F, Hartnegg K. Regression-based developmental models exemplified for Wisconsin Card Sorting Test parameters: Statistics and software for individual predictions. Journal of Clinical and Expermental Neuropsychology. 2007;29:25–35. doi: 10.1080/13803390500276859. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F, Hartnegg K, Fischer B. Lifespan development of pro-and anti-saccades: Multiple regression models for point estimates. Brain Research and Developmental Brain Research. 2005;160:113–123. doi: 10.1016/j.devbrainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss RL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer B, Renshaw PF, Sachs GS. Major depression and the basal ganglia. The Psychiatric clinics of North America. 1997;20:885–896. doi: 10.1016/s0193-953x(05)70350-6. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. Journal of Neuroscience. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF, Furneaux S. The knowledge base of the oculomotor system. [Review] Philosophical Transactions of the Royal Society of London – Series B: Biological Sciences. 1997;352:1231–1239. doi: 10.1098/rstb.1997.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movement. 2nd ed. F.A. Davis; Philadelphia: 1991. [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 3rd ed. Oxford University Press; New York: 1999. [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proceedings of the Association of American Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon SE. Executive control of visual attention in dual-task situations. Psychological Review. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2006;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Cognitive functional magnetic resonance imaging at very-high-field: Eye movement control. Topics in Magnetic Resonance Imaging. 1999;10:3–15. doi: 10.1097/00002142-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: A strategy for testing neurodevelopmental hypotheses. Schizophrenia Bulletin. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, et al. Dorsal cortical regions subserving visually-guided saccades in humans: An fMRI study. Cerebral Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: Cortical and subcortical networks. Psychiatry Research. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Mobashery M, Kimmig H, Fischer B. The role of fixation and visual attention in the occurrence of express saccades in man. European Archives of Psychiatry and Neurological Sciences. 1986;235:269–275. doi: 10.1007/BF00515913. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews in Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller LM, Sun FT, Curtis CE, D'Esposito M. Human Brain Mapping. Functional interactions between oculomotor regions during prosaccades and antisaccades. 2005 doi: 10.1002/hbm.20146. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sweeney J, Luna B. Autism as a selective disorder of complex information processing and underdevelopment of neocortical systems. Molecular Psychiatry. 2002;7:S14–S15. doi: 10.1038/sj.mp.4001166. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Control of mental processes. In: Bruce V, editor. Unsolved mysteries of the mind: Tutorial essays in cognition. Taylor & Francis; 1996. pp. 93–148. [Google Scholar]
- Monsell S, Mizon GA. Can the task-cuing paradigm measure an endogenous task-set reconfiguration process? Journal of Experimental Psychology. Human Perception and Performance. 2006;32:493–516. doi: 10.1037/0096-1523.32.3.493. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Andersen RA, Motter BC. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. Journal of Neuroscience. 1981;1:1218–1225. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Review. Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. Journal of Neurophysiology. 1992;67:1000–1002. doi: 10.1152/jn.1992.67.4.1000. [DOI] [PubMed] [Google Scholar]
- Muri RM, Nirkko AC, Ozdoba C, Felblinger J, Heid O, Schroth G, et al. Functional organization of cortical control of prosaccades and antisaccades in the frontal lobe. A functional magnetic resonance imaging (fMRI) study. Neuroimage. 1996;3:352. doi: 10.1136/jnnp.65.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Hale S, Wagstaff D, Poon LW, Smith GA. The information-loss model: A mathematical theory of age-related cognitive slowing. Psychological Review. 1990;97:475–487. doi: 10.1037/0033-295x.97.4.475. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwitt CL. Functional neuroanatomy of spatial working memory in children. Developmental Psychology. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movement. II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: Will and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro E, editors. Consciousness and self-regulation. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cerebral Cortex. 2006 doi: 10.1093/cercor/bhl014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Youngd DA, Freedman R. Age diminishes performance on an antisaccade eye movement task. Neurobiology of Aging. 1997;18:483–489. doi: 10.1016/s0197-4580(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Paus T. The development of sustained attention in children might be related to the maturation of frontal cortical functions. Acta Neurobiologiae Experimentalis. 1989;49:51–55. [PubMed] [Google Scholar]
- Paus T, Babenko V, Radil T. Development of an ability to maintain verbally instructed central gaze fixation studied in 8- to 10-year-old children. International Journal of Psychophysiology. 1990;10:53–61. doi: 10.1016/0167-8760(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Paus T, Kalina M, Patockova L, Angerova Y, Cerny R, Mecir P, et al. Medial vs lateral frontal lobe lesions and differential impairment of central-gaze fixation maintenance in man. Brain. 1991;114:2051–2067. doi: 10.1093/brain/114.5.2051. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. Journal of Neurophysiology. 1997;77:3386–3390. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI. Cortical control of saccades. Annals of Neurology. 1995;37:557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D'Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. Journal of Cognitive Neuroscience. 2000;12:2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Marler P. What signals are responsible for synaptic changes in visual cortical plasticity? In: Rauschecker JP, Marler P, editors. Imprinting and cortical plasticity. Wiley; New York: 1987. pp. 193–200. [Google Scholar]
- Regal D, Ashmead D, Salapatek P. The coordination of eye and head movements during early infancy: A selective review. Behavioural Brain Research. 1983;10:125–132. doi: 10.1016/0166-4328(83)90158-4. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Goldberg ME. Sensory and behavioral properties of neurons in posterior parietal cortex of the awake, trained monkey. Fed Proc. 1978;37:2258–2261. [PubMed] [Google Scholar]
- Robinson DL, Goldberg ME, Stanton GB. Parietal association cortex in the primate: Sensory mechanisms and behavioral modulations. Journal of Neurophysiology. 1978;41:910–932. doi: 10.1152/jn.1978.41.4.910. [DOI] [PubMed] [Google Scholar]
- Rosander K. Visual tracking and its relationship to cortical development. Progress in Brain Research. 2007;164:105–122. doi: 10.1016/S0079-6123(07)64006-0. [DOI] [PubMed] [Google Scholar]
- Rosander K, von Hofsten C. Development of gaze tracking of small and large objects. Experimental Brain Research. 2002;146:257–264. doi: 10.1007/s00221-002-1161-2. [DOI] [PubMed] [Google Scholar]
- Ross RG, Harris JG, Olincy A, Radant A. Eye movement task measures inhibition and spatial working memory in adults with schizophrenia, ADHD, and a normal comparison group. Psychiatry Research. 2000;95:35–42. doi: 10.1016/s0165-1781(00)00153-0. [DOI] [PubMed] [Google Scholar]
- Ross RG, Hommer D, Breiger D, Varley C, Radant A. Eye movement task related to frontal lobe functioning in children with attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:869–874. doi: 10.1097/00004583-199407000-00013. [DOI] [PubMed] [Google Scholar]
- Ross RG, Radant AD, Hommer DW. A developmental study of smooth pursuit eye movements in normal children from 7 to 15 years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:783–791. doi: 10.1097/00004583-199307000-00012. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Culee C, Roucoux M. Development of fixation and pursuit eye movements in human infants. Behavioural Brain Research. 1983;10:133–139. doi: 10.1016/0166-4328(83)90159-6. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütsche A, Baumann A, Jiang X, Mojon DS. Development of visual pursuit in the first 6 years of life. Graefes Archives of Clinical and Experimental Opthalmology. 2006;244:1406–1411. doi: 10.1007/s00417-005-0248-4. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Lillakas L, Dennis M, Steinbach MJ. Smooth pursuit eye movements in children. Experimental Brain Research. 2006;169:139–143. doi: 10.1007/s00221-005-0292-7. [DOI] [PubMed] [Google Scholar]
- Sano M, Kaga K, Kuan CC, Ino K, Mima K. Early myelination patterns in the brainstem auditory nuclei and pathway: MRI evaluation study. International Journal of Pediatric Otorhinolaryngology. 2007;71:1105–1115. doi: 10.1016/j.ijporl.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. Journal of Neurophysiology. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schneider DW, Logan GD. Hierarchical control of cognitive processes: Switching tasks in sequences. Journal of Experimental Psychology. General. 2006;135:623–640. doi: 10.1037/0096-3445.135.4.623. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84:1–53. [Google Scholar]
- Shea SL, Aslin RN. Oculomotor responses to step-ramp targets by young human infants. Vision Research. 1990;30:1077–1092. doi: 10.1016/0042-6989(90)90116-3. [DOI] [PubMed] [Google Scholar]
- Shibutani H, Sakata H, Hyvarinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Experimental Brain Research. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and general theory. Psychological Review. 1977;84 [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Swanson HL. What develops in working memory? A life span perspective. Developmental Psychology. 1999;35:986–1000. doi: 10.1037//0012-1649.35.4.986. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, et al. Eye tracking abnormalities in schizophrenia: Evidence for dysfunction in the frontal eye fields. Biological Psychiatry. 1998;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. PET studies of voluntary saccadic eye movements and spatial working memory. Schizophrenia Research. 1995;15:100. doi: 10.1152/jn.1996.75.1.454. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Rosano C, Berman RA, Luna B. Inhibitory control of attention declines more than working memory during normal aging. Neurobiology of Aging. 2001;22:39–47. doi: 10.1016/s0197-4580(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Takarae Y, Macmillan C, Luna B, Minshew NJ. Eye movements in neurodevelopmental disorders. Current Opinion in Neurology. 2004;17:37–42. doi: 10.1097/00019052-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Takeichi N, Fukushima J, Kurkin S, Yamanobe T, Shinmei Y, Fukushima K. Directional asymmetry in smooth ocular tracking in the presence of visual background in young and adult primates. Experimental Brain Research. 2003;149:380–390. doi: 10.1007/s00221-002-1367-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Matsushita H, Sakai H, Kawano H, Yoshimoto K, Sawada T. Developmental changes in cerebrospinal fluid concentrations of monoamine-related substances revealed with a Coulochem electrode array system. Journal of Child Neurology. 2000;15:267–270. doi: 10.1177/088307380001500415. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Human cerebral hemispheres develop at different rates and ages. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stigchel S, Merten H, Meeter M, Theeuwes J. The effects of a task-irrelevant visual event on spatial working memory. Psychonomic Bulletin & Review. 2007;14:1066–1071. doi: 10.3758/bf03193092. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. The Journal of Neuroscience. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. doi: 10.1093/cercor/bhn012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hofsten C, Rosander K. Development of smooth pursuit tracking in young infants. Vision Research. 1997;37:1799–1810. doi: 10.1016/s0042-6989(96)00332-x. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: Control, perceived oldness, and content. Journal of Neuroscience. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Shulman GL, Buckner RL, Miezin FM, Velanova K, Petersen SE. Evidence for separate perceptual reactivation and search processes during remembering. Cerebral Cortex. 2005 doi: 10.1093/cercor/bhj037. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Blackwell Scientific; Oxford: 1967. pp. 3–70. [Google Scholar]
- Ygge J, Aring E, Han Y, Bolzani R, Hellström A. Fixation stability in normal children. Annals New York Academy of Sciences. 2005;1039:480–483. doi: 10.1196/annals.1325.049. [DOI] [PubMed] [Google Scholar]
- Zald DH, Iacono WG. The development of spatial working memory abilities. Developmental Neuropsychology. 1998;14:563–578. [Google Scholar]