Abstract
The two classes of leukotriene modifiers work by inhibiting different portions of the same pathway. We hypothesized that single nucleotide polymorphisms (SNPs) in genes associated with response to montelukast (a cys-leukotriene receptor antagonist) would also be associated with response to zileuton (a 5-lipoxygenase inhibitor). We genotyped 26 SNPs that had previously been interrogated for association with montelukast response in five candidate genes (ABCC1, ALOX5, CYSLTR1, LTA4H, LTC4S) in a population of 577 asthmatics who participated in a clinical trial comparing intermittent and continuous-release zileuton to placebo. After adjusting for age and sex, six SNPs in three genes were associated with longitudinal FEV1 in response to zileuton (p-values 0.005–0.05). The SNPs included the two (ALOX5 rs2115819 and ABCC1 rs119774) that we had previously reported to be associated with FEV1 response to montelukast. Thus, the lung function response to zileuton is modulated by several of the loci that also influence montelukast response.
Keywords: leukotriene, genetics, FEV1, polymorphism, lung function
Introduction
Two classes of leukotriene modifiers, CysLT1 receptor antagonists (e.g. montelukast)[1, 2] and 5-lipoxygenase inhibitors (e.g. zileuton)[3, 4], exist. Recently, we described the association of leukotriene pathway gene variants with montelukast response in asthma[5]. Zileuton, a 5-lipoxygenase (5-LO) inhibitor, directly influences production of leukotrienes, the substrate for montelukast antagonism. Therefore, we hypothesized that single nucleotide polymorphisms (SNPs) in genes associated with response to montelukast would also be associated with response to zileuton. We tested this hypothesis in a clinical trial comprised of 577 asthmatics.
Methods
Study Population
The zileuton clinical trial was a 12 week study investigating the efficacy of zileuton controlled-release vs. zileuton immediate-release vs. placebo in patients with moderate asthma. Details regarding enrollment, randomization, and primary clinical outcome have been published[4]. Clinical outcomes, including forced expiratory volume at one second (FEV1), were assessed at multiple visits. Our primary outcome was FEV1 over the trial duration in those subjects taking zileuton. This outcome measure was meant to approximate the lung function outcome from the montelukast study. To the best of our knowledge, there was no overlap in the montelukast and zileuton study populations. DNA was available in 577 of the 591 randomized subjects. Written informed consent for both clinical trial and genetic studies was obtained upon enrollment. This study was approved by the Brigham and Women’s Hospital Institutional Review Board.
Genotyping and Quality Control
We genotyped 26 SNPs in 5 candidate genes (ABCC1, ALOX5, CYSLTR1, LTA4H, LTC4S) in the 5-lipoxygenase pathway. Each SNP was one of 28 that had been genotyped in our montelukast study[5] (two SNPs, rs892691 and rs730012, were not successfully genotyped). The original montelukast study SNPs were selected to contain all known non-synonymous SNP variants and to provide coverage with validated SNPs approximately every 5–10 kb within the selected candidate genes. Genotyping was perfomed using the SEQUENOM MassARRAY MALDI-TOF mass spectrometry platform (Sequenom, San Diego, CA), with iPLEX chemistry. Protocol details, SNP flanking sequence, and primer data are available from the authors. Duplicate genotyping performed on ~10% of the samples demonstrated <1% discordance. Genotype completion rates were ≥95% for all loci; each locus was in Hardy-Weinberg equilibrium.
Statistical Analysis
Due to potential population stratification, our analyses were restricted to self-identified Caucasians. To allow for increased statistical power inherent in studies with repeated measures over time, mixed models were used. The relationship between time and FEV1 was assessed using an unstructured covariance matrix and log-likelihood testing, which permitted modeling time as a continuous trait. All SNPs were initially screened for genotypic association (non-model based, 2 degree of freedom) with FEV1, adjusting for age, sex, and time. We subsequently tested only those SNPs that met our screening criteria of p<0.10 using additive, dominant, or recessive models (as appropriate, based upon the genotypic means noted in the screeing step) in multivariable analyses adjusting for age, sex, and time. Analyses were performed utilizing SAS (Version 9, Cary, NC).
Results
The zileuton clinical trial results have been published[4]. Characteristics of genotyped trial participants are shown in Table 1. 239 self-designated Caucasian asthmatics treated with zileuton form the primary analytic strata. There were no significant differences between the zileuton and placebo groups with respect to age, sex, racial distribution, or baseline level of lung function. A significant improvement (p<0.01) in FEV1, our primary therapeutic outcome, was noted[4]. However, significant inter-individual variability in the FEV1 response to zileuton existed (data not shown). Moreover, the intra-individual repeatability between responses was high, with Pearson’s correlation coefficients of 0.88 and 0.87 for 1 month vs. two- and three-month FEV1 change from baseline, respectively. The combination of high intra-individual repeatability combined with substantial inter-individual variability strongly supports a potential genetic basis to the FEV1 response to zileuton[6].
Table 1.
Baseline Characteristics of Zileuton Trial*
| Zileuton | Placebo | P-value | |
|---|---|---|---|
| N | 293 | 284 | |
| Age, mean (sd) | 34.1 (12.3) | 34.5 (12.9) | 0.76 |
| range | 12 – 81 | 12 – 78 | |
| Sex, n(%) | 0.70 | ||
| male | 147 (50.2) | 138 (48.6) | |
| female | 146 (49.8) | 146 (51.4) | |
| Race, n(%) | 0.78 | ||
| Caucasian | 239 (81.6) | 239 (84.2) | |
| African American | 32 (10.9) | 25 (8.8) | |
| Other | 22 (7.5) | 20 (7.0) | |
| FEV1% of Predicted, mean (sd) | 57.9 (10.5) | 59.1 (10.8) | 0.18 |
For subjects genotyped
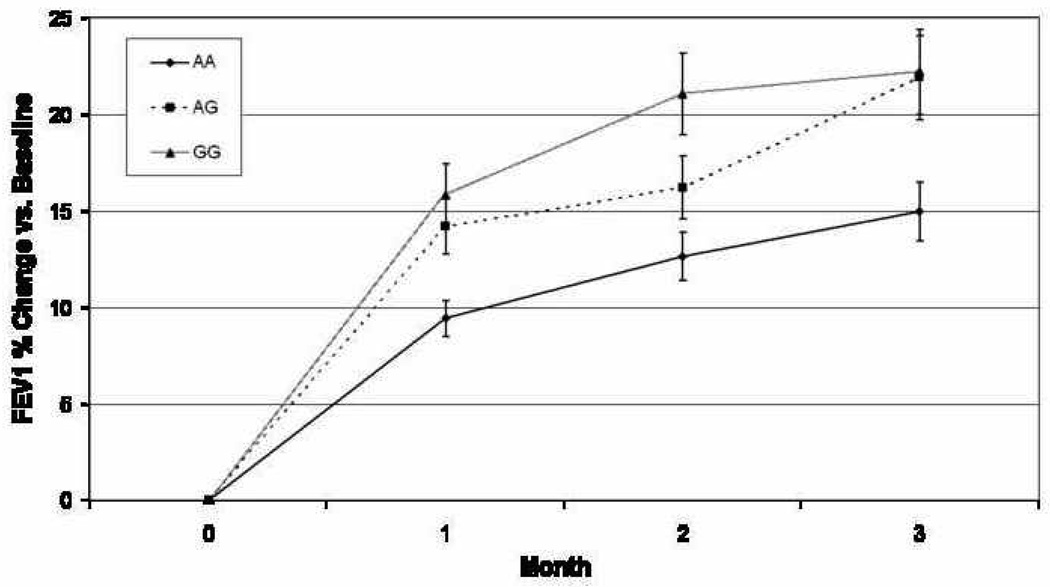
Of 26 SNPs genotyped in five candidate genes, seven met our screening criteria (p<0.10) in longitudinal genotypic models, with six SNPs associated with level of lung function in response to zileuton therapy in the final multivariable models (Table 2). Two of the six, ALOX5 rs2115819 and ABCC1 rs119774 were previously associated with lung function response to montelukast[5]. For rs2115819, the genotypic models associated with zileuton response corresponded well to those of montelukast, with homozygous “AA” subjects having the least improvement following drug therapy with both agents and heterozygous subjects having intermediate levels (Figure 1). For rs119774, there were no reported subjects homozygous for the recessive allele in the montelukast study, therefore direct genotypic comparisons are impossible to make. However, in both studies the recessive allele was associated with improvements in lung function while on anti-leukotriene therapy.
Table 2.
Genotypic and Final Models of Associated SNPs
| Gene | SNP | Genotype* | Mean FEV 1§ | SEM | P-value+ | Final Model | P-Value† |
|---|---|---|---|---|---|---|---|
| ALOX5 | RS892690 | 0 | 2.41 | 0.05 | 0.05 | Dominant | 0.01 |
| 1 | 2.27 | 0.04 | |||||
| 2 | 2.23 | 0.07 | |||||
| ALOX5 | RS2029253 | 0 | 2.41 | 0.05 | 0.1 | Additive | 0.03 |
| 1 | 2.29 | 0.05 | |||||
| 2 | 2.24 | 0.07 | |||||
| ALOX5 | RS2115819‡ | 0 | 2.41 | 0.05 | 0.04 | Additive | 0.01 |
| 1 | 2.32 | 0.05 | |||||
| 2 | 2.20 | 0.06 | |||||
| ABCC1 | RS215066 | 0 | 2.34 | 0.03 | 0.02 | Recessive | 0.05 |
| 1 | 2.13 | 0.10 | |||||
| 2 | 3.13 | 0.41 | |||||
| ABCC1 | RS119774‡ | 0 | 2.34 | 0.03 | 0.02 | Recessive | 0.05 |
| 1 | 2.14 | 0.09 | |||||
| 2 | 3.12 | 0.41 | |||||
| ABCC1 | RS3902401 | 0 | 2.30 | 0.03 | 0.07 | Recessive | 0.22 |
| 1 | 2.49 | 0.09 | |||||
| 2 | 2.03 | 0.24 | |||||
| LTC4S | RS272431 | 0 | 2.30 | 0.03 | 0.006 | Additive | 0.005 |
| 1 | 3.11 | 0.29 | |||||
Genotype values 0 - homozygous wild type, 1 - heterozygotes, 2 - homozygous mutant allele
Least-squares mean value over time, adjusted for age, sex, and time
P-value from genotypic longitudinal models comparing each genotype vs. the others, adjusted for age, sex, and time
P-value from final model selected (additive, dominant, or recesssive) based upon noted effects on genotypic means, adjusted for age, sex, and time
SNP previously associated with response to montelukast
Figure 1. Change in FEV1 vs. baseline while taking zileuton, stratified by ALOX5 rs2115819 genotype.
In general, each of the genotypic groups improved their lung function while on the medication. However, those subjects with a “G” allele in general, and “GG” homozygotes in particular, improved their lung function significantly more than “AA” homozygotes. This is consistent with findings previously reported for this SNP in association with response to the leukotriene antagonist, montelukast (reference 5). The maximal, time-specific percent of phenotypic variability explained by this SNP, 3.1%, occurred at the end of month 2.
Discussion
While leukotriene modifiers may not be as efficacious as inhaled corticosteroids in asthma therapy[2, 7], leukotriene modifiers are orally-administered and, thus, generally preferred among patients[8]. Moreover, treatment response to all asthma medications is highly heterogeneous[7], with some individuals responding to leukotriene modifiers but not to inhaled corticosteroids[9]. In this study, we demonstrate that the lung function response to zileuton in asthma has wide inter-individual variability and high intra-individual repeatability, thereby strongly supporting the potential for a genetic basis to this response[6]. We subsequently detail the association of six SNPs in three candidate genes with zileuton response. The two major classes of asthma medications targeting the leukotriene pathway are proximately related, in that 5-LO inhibitors block cysteinyl leukotriene production from arachadonic acid, while CysLT1 leukotriene receptor antagonists block cysteinyl leukotrienes from binding to their primary receptor. Importantly, we have now demonstrated that the two SNPs previously associated with lung function response to montelukast (a leukotriene receptor antagonist) in a prior candidate gene study[5] are also associated with level of lung function in association with zileuton (a 5-LO inhibitor) therapy. To our knowledge, multiple common loci influencing the genetic response to more than one class of medications within the same therapeutic pathway have not previously been demonstrated.
In our study, one SNP, rs2115819 was associated with relative decrements in response to both zileuton and montelukast, while the second SNP, rs119774 was associated with relative improvements. Leukotrienes have now been implicated in a wide variety of diseases, leading to a widely postulated therapeutic benefit to their blockade[10, 11]. Therefore, the presence of common genetic determinants of the therapeutic response to multiple anti-leukotriene pathway agents may have broad implications for a wide variety of diseases.
Pharmacogenetic studies, such as the present, may identify subsets of individuals who respond to leukotriene modifiers but not to inhaled corticosteroids[9], or to both therapies equally well[9]. In one of the earliest descriptions of asthma pharmacogenetics, promoter microsatellite variation in the ALOX5 gene (which encodes for 5-LO) was first demonstrated to diminish transcription factor binding[12] and subsequently associated with decreased response to treatment with 5-LO inhibition in asthma[13]. The same ALOX5 promoter polymorphism was later associated with decreased response to montelukast[14]. We now report two additional polymorphic variants that can influence both the response to zileuton and montelukast therapy in asthma.
Besides the two SNPs reported to be associated with response to both zileuton and montelukast, we report the association of four additional SNPs with lung function response to zileuton therapy, including variants in ALOX5 (rs892690, rs2029253), ABCC1 (rs215066), and LTC4S (rs272431). None of these SNPs was associated with response to montelukast therapy. Reasons that these SNPs were not associated in our original investigation include statistical power and therapeutic heterogeneity. From a power perspective, the zileuton study contains over three times as many subjects randomized to an anti-leukotriene agent as the montelukast study did. And while there are variants that influence response to both classes of medications, there are likely also loci that specifically alter response to one class or the other. Larger studies will be needed to test the generalizability of additional SNPs across multiple forms of leukotriene modifiers.
One potential limitation of our study is that the SNPs selected may not have comprehensively covered each of the candidate genes. Therefore, we cannot fully exclude any gene not associated with zileuton response from considerations for future studies. However, the focus of this manuscript was to evaluate similarities and differences in the pharmacogenetic response to zileuton and montelukast, thereby requiring that SNPs chosen overlap with those previously studied. While three montelukast study variants were not successfully genotyped in the zileuton DNA (including the SP-1 binding motif), none were associated with lung function response in the montelukast study. None of our reported associations met Bonferroni-adjusted criteria for significance. Moreover, it is possible that some of our associations within a candidate gene could be due to linkage disequilibrium with another genotyped variant. Of the significant SNPs (Table 2), only two, rs2029253 and rs2115819, were in high LD (r2 = 0.81), with no other pairs of SNPs having an r2 value of >0.60. Due to concerns over the potential for population stratification, our study was also confined to Caucasians, thereby potentially limiting generalizability of our findings to other racial groups.
In conclusion, we have presented evidence that pharmacogenetic loci influencing the response to leukotriene antagonists also influence the response to 5-LO inhibition in asthma. Thus, it is possible that, in the future, a single “pharmacogenetic profile” may be able to ascertain response to leukotriene modifiers as a whole rather than needing individual tests for each class of medication. This may also have broad implications for the pharamacogenetic study of other therapeutic pathways.
Acknowledgments
Support: This work was funded by NIH: HL65899, HL74755, and HG3983
References Cited
- 1.Knorr B, Matz J, Bernstein JA, et al. Montelukast for chronic asthma in 6-to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast Study Group. Jama. 1998;279:1181–1186. doi: 10.1001/jama.279.15.1181. [DOI] [PubMed] [Google Scholar]
- 2.The American Lung Association Asthma Clinical Research Centers. Randomized Comparison of Strategies for Reducing Treatment in Mild Persistent Asthma. N Engl J Med. 2007;356:2027–2039. doi: 10.1056/NEJMoa070013. [DOI] [PubMed] [Google Scholar]
- 3.Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zileuton Clinical Trial Group. Jama. 1996;275:931–936. [PubMed] [Google Scholar]
- 4.Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99:178–184. doi: 10.1016/S1081-1206(10)60642-4. [DOI] [PubMed] [Google Scholar]
- 5.Lima JJ, Zhang S, Grant A, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 7.Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130:487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bukstein DA, Bratton DL, Firriolo KM, et al. Evaluation of parental preference for the treatment of asthmatic children aged 6 to 11 years with oral montelukast or inhaled cromolyn: a randomized, open-label, crossover study. J Asthma. 2003;40:475–485. doi: 10.1081/jas-120018714. [DOI] [PubMed] [Google Scholar]
- 9.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Osher E, Weisinger G, Limor R, Tordjman K, Stern N. The 5 lipoxygenase system in the vasculature: emerging role in health and disease. Mol Cell Endocrinol. 2006;252:201–206. doi: 10.1016/j.mce.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Claria J, Romano M. Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr Pharm Des. 2005;11:3431–3447. doi: 10.2174/138161205774370753. [DOI] [PubMed] [Google Scholar]
- 12.In KH, Asano K, Beier D, et al. Naturally occurring mutations in the human 5-lipoxygenase gene promoter that modify transcription factor binding and reporter gene transcription. J Clin Invest. 1997;99:1130–1137. doi: 10.1172/JCI119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drazen JM, Yandava CN, Dube L, et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999;22:168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- 14.Klotsman M, York TP, Pillia S, et al. Pharmacogenetics of the 5-lipoxygenase biosynthetic pathway and variable clinical response to montelukast. Pharmacogenet Genomics. 2007;17:189–196. doi: 10.1097/FPC.0b013e3280120043. [DOI] [PubMed] [Google Scholar]