Abstract
Aging is the most important risk factor for common neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases. Aging in the central nervous system has been associated with elevated mutation load in mitochondrial DNA, defects in mitochondrial respiration and increased oxidative damage. These observations support a ‘vicious cycle’ theory which states that there is a feedback mechanism connecting these events in aging and age-associated neurodegeneration. Despite being an extremely attractive hypothesis, the bulk of the evidence supporting the mitochondrial vicious cycle model comes from pharmacological experiments in which the modes of mitochondrial enzyme inhibition are far from those observed in real life. Furthermore, recent in vivo evidence does not support this model. In this review, we focus on the relationship among the components of the putative vicious cycle, with particular emphasis on the role of mitochondrial defects on oxidative stress.
Mitochondrial respiratory chain and reactive oxygen species production
Mitochondria, being the key players in ATP production and diverse cell signaling events, are essential organelles for the survival of eukaryotic cells. Unlike all other organelles in animals, the mitochondria have their own genome (mitochondrial DNA; mtDNA) that encodes components of the oxidative phosphorylation (OXPHOS) system. The mitochondrial OXPHOS machinery is composed of five multisubunit complexes (complex I–V). From Krebs cycle intermediates (NADH and FADH2), electrons feed into complex I or II, and are transferred to complex III, then to complex IV, and finally to O2. The redox energy released during the electron transfer process in complexes I, III and IV is utilized to actively pump out H+ from the mitochondrial matrix to the intermembrane space, generating the electrochemical gradient of H+ across the inner membrane which is ultimately utilized by complex V to produce ATP [1].
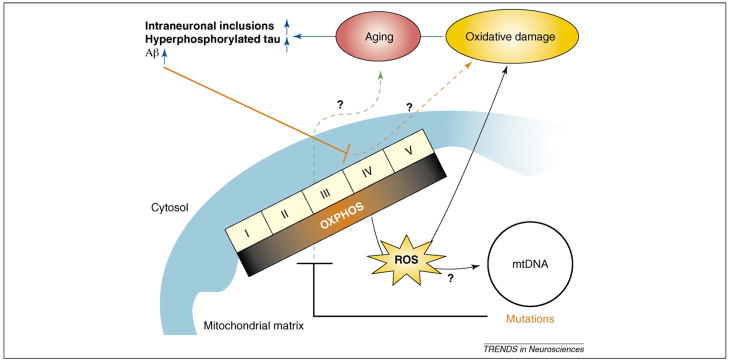
This elegant system for energy production, however, is not perfect. A small portion (up to 2%) of electrons passing through the electron transport chain, mostly at complex I and complex III, react with molecular oxygen and yield superoxide anion, which can be converted into other reactive oxygen species (ROS) such as hydrogen peroxide and the highly reactive hydroxyl radical through enzymatic and nonenzymatic reactions [2]. Cells are endowed with robust endogenous antioxidant systems to counteract excessive ROS. It is believed that ROS, in particular hydrogen peroxide, have physiological roles as signaling molecules [3,4]. However, when ROS production overwhelms the endogenous antioxidant systems, they can potentially damage various types of macromolecules, including proteins, lipids and nucleic acids. These damages are collectively referred to as ‘oxidative stress,’ and have been implicated in aging and various pathological processes. The mitochondrial ‘vicious cycle’ theory of aging states that ROS, generated from OXPHOS, induces mutations in the mtDNA, which in turn leads to OXPHOS dysfunction (Figure 1) [2,5]. The impaired OXPHOS function would lead to further production of ROS, which further exacerbates mtDNA mutations.
Figure 1.
OXPHOS–oxidative stress–neurodegeneration connections. OXPHOS activity produces ROS, which could induce mutations in the mtDNA. In turn, mtDNA mutations can lead to the inhibition of OXPHOS, which can increase the production of ROS. The ‘vicious cycle’ theory of aging suggests that an exponential increase in ROS production and oxidative damage mediated by these interactions could be a strong contributor to age-associated neurodegenerative diseases. However, in vivo evidence for a causative relationship between these players has not been provided. Defects in OXPHOS can contribute to aging in an oxidative stress-independent manner, and the latter could be a marker of senescence. In addition, misfolded proteins, such as Aβ, were also shown to impair OXPHOS and possibly other metabolic systems.
In this review, we discuss recent progress and some surprising new data that raise important questions regarding the mitochondrial vicious cycle and its contribution to aging and major neurodegenerative conditions, with emphasis on Alzheimer’s disease (AD) and Parkinson’s disease (PD). The main question we address here is whether OXPHOS defects are responsible for an increased oxidative stress in vivo.
Mitochondrial DNA mutations and oxidative stress in the aging brain and in neurodegenerative diseases
ROS-mediated formation of mutated mtDNA (large-scale deletions and point mutations) has been implicated in physiological senescence and age-related disorders such as sporadic neurodegenerative disorders, type II diabetes, cancer and cardiac diseases (reviewed in Refs [2,6]). Indeed, mtDNA with large deletions has been reported to accumulate with aging in various tissues of various mammalian species (reviewed by Kujoth et al. [7]). Although these observations do not address whether the accumulation of mutated mtDNA has a causal role in aging, they suggest that mutated mtDNA serves as a useful biomarker of aging regardless of the lifespan of specific organisms. The mammalian central nervous system (CNS) is not an exception. Several reports have presented clear age-dependent increases in the amount of deleted mtDNA in the brains of rodents and human [7]. Although in these reports the fraction of deleted mtDNA was estimated to be very low (less than 1%), recent studies that employed single-cell dissection in combination with quantitative real-time PCR showed that, in the human substantia nigra neurons of elderly subjects, deleted mutant mtDNA species accumulate to up to ~45% of total mtDNA [8,9]. This mutated:wild-type mtDNA ratio tended to be higher in PD patients [9], raising the intriguing possibility that stochastic age-associated elevation of mtDNA mutations could be responsible for sporadic neurodegeneration in nigral neurons. It appears that the extensive accumulation of deleted mtDNA is unique to the nigral neurons and not as pronounced in other regions or even in other cell types in the aging CNS. Kraytsberg et al. observed a heterogeneous staining pattern in aged substantia nigra neurons after in situ cytochrome oxidase (COX) activity staining, and noticed that COX-negative neurons contain a higher proportion of deleted mtDNA than COX-positive neurons [8]. The nature of mutations was apparently somatic (not inherited), because individual neurons contained unique types of deleted mtDNA. Although earlier studies were unable to demonstrate the correlation between the accumulation of deleted mtDNA and AD by analyzing human cortical tissues [10,11], there could be other ‘regional hotspots’ where deleted mtDNA preferentially accumulates and contributes to neuronal death in AD. It remains to be determined whether the COX deficiency correlates to oxidative stress in individual brain cells in any of these conditions.
Contribution of mutated mitochondrial DNA to aging and oxidative stress in mice
The ‘mutator mice,’ expressing a proofreading-deficient mitochondrial DNA polymerase (POLG), accumulate mtDNA mutations in an age-dependent manner and develop many features of premature aging [12–14]. Murine embryonic fibroblasts prepared from the mutator mice exhibited defects in OXPHOS function but, surprisingly, those cells as well as tissues from adult mice did not exhibit increased ROS production or oxidative damage. Although the generation of ROS depends on the specific defect in OXPHOS components and residual activities, the random nature of mutations in these mice would be expected to create such defects. The lack of oxidative stress raises questions of whether naturally accumulating mtDNA mutations are responsible for increased oxidative damage [15]. These results imply that mtDNA mutations could accelerate aging, even without the involvement of oxidative stress.
Contribution of ROS to mutations in mitochondrial DNA
Mounting evidence suggests that oxidative damage increases in the mammalian brain during aging [16]. It is generally assumed that mitochondria-driven ROS induces mutations in mtDNA. Recent evidence suggests that double-strand breaks might be the main mediators of mtDNA deletions. Work from our laboratory showed that double-strand breaks in mtDNA induce the formation of large deletions in mouse muscle [17] and brain (H.F. and C.T.M., unpublished), suggesting that this could be the main mechanism accounting for the generation of age-associated deletions in mtDNA. This mechanism has also been proposed to explain the occurrence of multiple mtDNA deletions in patients with mitochondrial disorders [18]. Double-strand breaks can be caused by ROS [19], but it can also be caused by stalling of the DNA replication fork [18]. Elucidating the mechanisms of formation of mtDNA deletions could give us new insights into whether ROS play a major role in the generation of age-associated mtDNA rearrangements and in the vicious cycle theory of aging.
Complex I defects, oxidative stress and Parkinson’s disease
Mitochondrial complex I (NADH:ubiquinone oxidoreductase), a 45-subunit enzyme complex, catalyzes the first step of the electron transport chain utilizing noncovalently bound prosthetic groups (flavin mononucleotide or FMN, and ion–sulfur clusters) [20,21]. Seven subunits (ND1, 2, 3, 4L, 4, 5, 6) are encoded by mtDNA, whereas the others are encoded by nuclear DNA. The mtDNA-encoded proteins comprise half of the ‘catalytic core,’ whereas seven subunits encoded by nuclear DNA (NDUF-S1, S2, S3, S7, S8, V1, V2) comprise the other half.
Contribution of complex I defects to oxidative stress and neurodegeneration in PD
Several genes associated with familial PD have been identified in the last few years [22]. Although some of these gene products localize to mitochondria, a direct link with OXPHOS or oxidative stress has not been established yet.
A complex I defect and increased oxidative damage are consistent features of PD brains, in which the dopaminergic neurons in the substantia nigra pars compacta are preferentially affected, even though the defect is also observed in other cell types (reviewed by Dawson and Dawson [23]). The notion that complex I defects are central to the development of sporadic PD originates from the observation that complex I inhibition induced by rotenone or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine promotes PD-like symptoms and the formation of cytoplasmic inclusions. The formation of these protein aggregates, containing α-synuclein (Lewy bodies), has been accompanied by selective degeneration of nigral neurons in rodents and primates [23,24]. Oxidative stress has been suggested to play an important role in neuronal death in these pharmacological models of PD [25,26]. However, a low concentration of rotenone (5 nM), which is sufficient to induce production of ROS, still exerted neurotoxic effects in cultured cells even in the presence of an antioxidant [27]. Therefore, both oxidative stress-dependent and -independent mechanisms are likely to play a pathogenic role in models of PD induced by complex I inhibitors.
Bioenergetic defects rather than oxidative stress might be a more critical player in the development of PD, as antioxidant treatments, including coenzyme Q10, have not shown clear benefits in clinical trials [28,29]. The notion that a complex I defect leads to a major increase in ROS production derives in part from the fact that rotenone, the most commonly used complex I inhibitor, increases ROS production in isolated mitochondria in the presence of excess NADH-linked substrates [30–32]. However, not all mutations in complex I subunits lead to an increase in ROS production [33]. Future detailed studies unraveling the molecular mechanisms whereby complex I is inhibited in sporadic PD patients will help us understand the contribution of complex I defects to oxidative stress and neurodegeneration in PD.
Origins of complex I defects in PD
Although it is plausible that chronic exposure to complex I inhibitors such as pesticides could contribute to the development of idiopathic PD, it is unlikely that toxin exposure fully accounts for a selective complex I defect in the general population. Furthermore, PD patients without any history of toxin exposure also exhibited a complex I defect in platelets [34].
The hypothesis that mutations in mtDNA account for complex I defects in PD has been a matter of debate for a decade. To examine the direct contribution of mtDNA to complex I defects, several groups have generated and characterized cybrid cell lines, in which platelet mitochondria from PD patients or age-matched controls were transferred to mtDNA-less human cell lines (rho0 cell lines). Gu et al. [34] and Swerdlow et al. [35] independently studied PD cybrid lines generated on nuclear backgrounds of lung carcinoma and neuroblastoma, respectively, and consistently observed an ~20% decrease in complex I activity in PD-derived cybrids. Later studies further demonstrated that PD cybrids produce more ROS, suggesting an important role of mtDNA mutations in oxidative stress and PD. However, no unequivocal mtDNA mutation accounting for the complex I defect has been reported to date in PD cybrids, and a similar cybrid study failed to demonstrate the transfer of complex I defects from PD platelets to HeLa rho0 cells [36]. A comparison of the entire mtDNA sequence from PD and control substantia nigra did not identify clear pathogenic mutations in PD mtDNA, and questioned the involvement of mtDNA in PD pathogenesis [37]. One drawback of this study, however, is that sequencing analysis might miss heteroplasmic mtDNA species that could be concentrated in disease-relevant cells. A more recent study employed a cloning-based sequence analysis to study the mutation load in the gene encoding ND4 in the cerebral cortex and substantia nigra of PD patients [38]. Although this study revealed a clear age-dependent increase in point mutations in control subjects, there was no significant difference in mutation load between PD and control groups. By contrast, more comprehensive studies identified dozens of amino acid-changing mutations that predominantly occur in the gene encoding ND5 of PD frontal lobe samples [39,40]. The mutations identified in these studies occurred at low frequencies of 0.9–1.2%, and their functional significance is unclear. One study identified a particular single-nucleotide polymorphism (10398G) within the gene encoding ND3 (part of haplogroups J and K) as a factor that significantly reduces the risk of PD [41]. Yet another study implicated a polymorphism in a nuclear-encoded complex I subunit, NDUFV2, as a determinant of susceptibility to PD [42]. The functional impact of such mutations and polymorphisms on complex I activity and ROS production is still undetermined.
If mtDNA mutations cause systemic complex I defects in PD patients, are those mutations inherited or acquired? In idiopathic PD, the evidence of maternal inheritance is at best scarce [43]. In the context of age dependency of PD onset, stochastic age-dependent somatic (acquired) mutations would fit better. Although it is difficult to envisage that somatic mutations would preferentially affect genes that encode complex I subunits, such subunits are responsible for a relatively high percentage of mtDNA coding capacity.
Complex IV defects, oxidative stress and Alzheimer’s disease
Complex IV or COX is the terminal enzyme of the mitochondrial electron transport chain. The detailed structural aspects of this enzyme complex and highly coordinated assembly processes have been reviewed recently [44,45]. In brief, the catalytic core of complex IV is composed of three subunits (COX I, II and III) encoded by the mtDNA. The remaining ten accessory subunits are encoded by nuclear DNA and provide structural support and regulatory functions for the catalytic core. Redox centers situated in COX I (heme a, heme a3 and CuB) and COX II (CuA) coordinately mediate the electron transfer from cytochrome c to molecular oxygen. In addition to the structural components of complex IV, several assembly factors are required to complete the maturation of this complex. For example, COX10 catalyzes the farnesylation of protoheme (heme b) during the biosynthesis of heme a, which subsequently becomes incorporated into COX I and mediates electron transfer. Our targeted disruption of COX10 gene confirmed that this enzyme is essential for the synthesis of functional COX I and assembly of complex IV in mammals [46]. Unlike other respiratory complexes, complex IV has the capacity to retain partially reduced, unstable intermediates during electron transport, and therefore it is not considered a site of ROS production [47]. However, COX inhibition could lead to a backup of reduced complexes upstream in the respiratory chain, which has been proposed to increase ROS production [48–50].
Contribution of complex IV defects to oxidative stress and neurodegeneration in AD
Reduced complex IV activity and increased oxidative damage are consistent features in AD brain. The major histopathological features of AD brain include the accumulation of diffuse/neuritic plaques composed of β-amyloid (Aβ) and the appearance of neurofibrillary tangles composed of phosphorylated tau [51]. Although the detailed interactions between Aβ and tau remain unknown, recent findings in triple transgenic mice harboring mutant APP, mutant presenilin 1 and mutant tau provided support for Aβ positively regulating tau pathology [52].
To address the contribution of oxidative stress to the development of pathological features of AD, different groups employed various pharmacological and genetic approaches to modulate oxidative stress in mouse models of AD expressing mutant forms of human APP [53] (and references therein). These studies consistently demonstrated a strong correlation between oxidative stress and accumulation of Aβ with the possible involvement of an APP-processing enzyme, β-secretase [53–57]. A recent report using heterozygous Mn-SOD knockout mice also showed that oxidative stress modulates hyperphosphorylation of tau, which leads to the formation of neurofibrillary tangles[58]. Although it is not clear whether oxidative stress affects phosphorylation of tau independently or through the increased accumulation of Aβ, mounting evidence is suggestive of the causal role of oxidative stress in the development of AD.
To assess whether a genetically induced complex IV deficiency affects oxidative stress and production/accumulation of Aβ, we have first generated neuron-specific conditional COX10 knockout mice and then crossed them with a mouse model of AD to generate complex IV-deficient AD mice (COXd/AD mice) [53]. Contrary to our expectation, brains of COXd/AD mice exhibited less amyloid plaque and reduced Aβ deposition, as compared to AD mice. In addition, we were unable to demonstrate increased ROS production from mitochondria or cells lacking complex IV activity. In fact, the reduction in Aβ deposition was accompanied by reduced oxidative damage. We concluded that an isolated complex IV defect does not contribute to oxidative stress and AD pathology, and that the increased oxidative damage found in patients and animal models of AD is a consequence of Aβ accumulation. Furthermore, we showed that partial inhibition of complex IV in fibroblasts does not affect ROS production [53]. Nevertheless, the effect of partial complex IV inhibition on ROS production in post-mitotic neurons or adult CNS in vivo and its potential contribution to AD remain to be investigated. Of note, a partial impairment of complex IV activity induced by the targeted disruption of the complex IV assembly factor SURF1 prolongs the lifespan of mice and protects neurons from kainic acid-induced excitotoxicity [59]. In general, prolonged lifespan and resistance against excitotoxicity are associated with reduced oxidative stress or increased resistance against oxidative stress [60–63]. Therefore, it would be of particular interest to study whether the partial impairment of complex IV mediated by SURF1 knockout reduces oxidative damages in the CNS as we found in COX10 conditional knockout.
Origins of complex IV defects in AD
According to the vicious cycle theory of aging, age-related increases in mutations of mtDNA could affect mitochondrial OXPHOS, which in turn could induce further production of ROS. Although this model is extremely attractive, the relatively specific impairment of complex IV activity found in AD brains and platelets is not easily explainable from the stochastic accumulation of somatic mtDNA mutations. Furthermore, the evidence supporting the correlation between mtDNA mutations and AD is lacking [11,64,65].
Recent evidence suggests that Aβ and APP are imported or targeted to the mitochondria and impair complex IV activity. The proposed mechanisms of complex IV inhibition by Aβ include (i) blockage of mitochondrial import channels by APP to prevent the import of nuclear-encoded complex IV subunits [66], (ii) sequestration of heme by Aβ [67,68] and (iii) interaction between Aβ and Aβ-binding alcohol dehydrogenase (ABAD) [50,69]. The latter two mechanisms have been suggested to promote ROS production. Thus, an mtDNA-independent vicious cycle connecting Aβ, heme/ABAD, complex IV and ROS might participate in age-dependent exacerbation of AD pathology and complex IV defects. However, Aβ binds not only to heme a but also to protoheme (heme b). The interaction of Aβ with heme alone is sufficient to generate a peroxidase activity in vitro without the involvement of complex IV. The mechanism whereby ABAD–Aβ interaction leads to the reduction of complex IV activity is largely unknown. In addition, the effect of this interaction on other components of OXPHOS has never been studied. Although further studies are required to understand the mechanism by which complex IV is inhibited in AD patients and to clarify the origin of oxidative stress, it appears that the defect is a consequence, rather than the cause, of Aβ accumulation.
Conclusions
Pharmacological and genetic evaluations of OXPHOS inhibition on ROS production have improved our understanding of mechanisms and sources of ROS production. At the same time, however, it is becoming clear that genetic defects in OXPHOS do not necessarily lead to similar increased production of ROS or elevation of oxidative stress. This is probably related to the fact that most genetic defects result in a decrease in the levels of a functional OXPHOS complex, rather than in OXPHOS with altered chemistry. Although numerous studies support the importance of oxidative stress in aging and age-associated diseases including neurodegenerative ones, the validity of the ‘vicious cycle’ theory of aging bridging mtDNA, OXPHOS and ROS has not been substantiated by in vivo experiments. In fact, most of the emerging evidence in vivo does not support this theory. The jury is still out, and the burden of proof lies in future, more detailed studies in true aging models. To convincingly assert that the vicious cycle hypothesis plays an important role in aging and neurodegeneration, its proponents will have to clarify (i) how mtDNA mutations are induced in aging, (ii) the extent to which age-associated somatic mtDNA mutations affect cellular OXPHOS function and (iii) whether age-associated OXPHOS impairment affects ROS production and oxidative stress.
Acknowledgments
We are grateful to Giovanni Manfredi (Cornell University) for suggestions. Our work was supported by U.S. Public Health Service grants NS41777, EY10804 and CA085700, the Muscular Dystrophy Association and by the University of Miami Neuroscience Center. H.F. was supported by a Lois Pope LIFE Fellowship.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaban RS, et al. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Veal EA, et al. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Giorgio M, et al. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 5.Bandy B, Davison AJ. Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic Biol Med. 1990;8:523–539. doi: 10.1016/0891-5849(90)90152-9. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kujoth GC, et al. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraytsberg Y, et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 9.Bender A, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 10.Lezza AM, et al. Mitochondrial DNA 4977 bp deletion and OH8dG levels correlate in the brain of aged subjects but not Alzheimer’s disease patients. FASEB J. 1999;13:1083–1088. doi: 10.1096/fasebj.13.9.1083. [DOI] [PubMed] [Google Scholar]
- 11.Mawrin C, et al. Region-specific analysis of mitochondrial DNA deletions in neurodegenerative disorders in humans. Neurosci Lett. 2004;357:111–114. doi: 10.1016/j.neulet.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 12.Kujoth GC, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 13.Trifunovic A, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 14.Trifunovic A, et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava S, Moraes CT. Double-strand breaks of mouse muscle mtDNA promote large deletions similar to multiple mtDNA deletions in humans. Hum Mol Genet. 2005;14:893–902. doi: 10.1093/hmg/ddi082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanrooij S, et al. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 2007;35:3238–3251. doi: 10.1093/nar/gkm215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieber MR, Karanjawala ZE. Ageing, repetitive genomes and DNA damage. Nat Rev Mol Cell Biol. 2004;5:69–75. doi: 10.1038/nrm1281. [DOI] [PubMed] [Google Scholar]
- 20.Sazanov LA. Respiratory complex I: mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry. 2007;46:2275–2288. doi: 10.1021/bi602508x. [DOI] [PubMed] [Google Scholar]
- 21.Lenaz G, et al. Mitochondrial complex I: structural and functional aspects. Biochim Biophys Acta. 2006;1757:1406–1420. doi: 10.1016/j.bbabio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16 (R2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 23.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 24.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 25.Sherer TB, et al. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang LP, et al. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27:4326–4333. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung AY, Schwarzschild MA. Clinical trials for neuroprotection in Parkinson’s disease: overcoming angst and futility? Curr Opin Neurol. 2007;20:477–483. doi: 10.1097/WCO.0b013e32826388d6. [DOI] [PubMed] [Google Scholar]
- 29.Storch A, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- 30.St-Pierre J, et al. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 31.Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem. 2004;90:405–421. doi: 10.1111/j.1471-4159.2004.02489.x. [DOI] [PubMed] [Google Scholar]
- 32.Kushnareva Y, et al. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iuso A, et al. Dysfunctions of cellular oxidative metabolism in patients with mutations in the NDUFS1 and NDUFS4 genes of complex I. J Biol Chem. 2006;281:10374–10380. doi: 10.1074/jbc.M513387200. [DOI] [PubMed] [Google Scholar]
- 34.Gu M, et al. Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol. 1998;44:177–186. doi: 10.1002/ana.410440207. [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow RH, et al. Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 36.Aomi Y, et al. Cytoplasmic transfer of platelet mtDNA from elderly patients with Parkinson’s disease to mtDNA-less HeLa cells restores complete mitochondrial respiratory function. Biochem Biophys Res Commun. 2001;280:265–273. doi: 10.1006/bbrc.2000.4113. [DOI] [PubMed] [Google Scholar]
- 37.Vives-Bauza C, et al. Sequence analysis of the entire mitochondrial genome in Parkinson’s disease. Biochem Biophys Res Commun. 2002;290:1593–1601. doi: 10.1006/bbrc.2002.6388. [DOI] [PubMed] [Google Scholar]
- 38.Simon DK, et al. Somatic mitochondrial DNA mutations in cortex and substantia nigra in aging and Parkinson’s disease. Neurobiol Aging. 2004;25:71–81. doi: 10.1016/s0197-4580(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 39.Parker WD, Jr, Parks JK. Mitochondrial ND5 mutations in idiopathic Parkinson’s disease. Biochem Biophys Res Commun. 2005;326:667–669. doi: 10.1016/j.bbrc.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 40.Smigrodzki R, et al. High frequency of mitochondrial complex I mutations in Parkinson’s disease and aging. Neurobiol Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 41.van der Walt JM, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003;72:804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hattori N, et al. Genotype in the 24-kDa subunit gene (NDUFV2) of mitochondrial complex I and susceptibility to Parkinson disease. Genomics. 1998;49:52–58. doi: 10.1006/geno.1997.5192. [DOI] [PubMed] [Google Scholar]
- 43.Zweig RM, et al. The familial occurrence of Parkinson’s disease. Lack of evidence for maternal inheritance. Arch Neurol. 1992;49:1205–1207. doi: 10.1001/archneur.1992.00530350127029. [DOI] [PubMed] [Google Scholar]
- 44.Fontanesi F, et al. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am J Physiol Cell Physiol. 2006;291:C1129–C1147. doi: 10.1152/ajpcell.00233.2006. [DOI] [PubMed] [Google Scholar]
- 45.Moraes CT, et al. Defects in the biosynthesis of mitochondrial heme c and heme a in yeast and mammals. Biochim Biophys Acta. 2004;1659:153–159. doi: 10.1016/j.bbabio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Diaz F, et al. Cytochrome c oxidase is required for the assembly/ stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol. 2006;26:4872–4881. doi: 10.1128/MCB.01767-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prabhakaran K, et al. Cyanide induces different modes of death in cortical and mesencephalon cells. J Pharmacol Exp Ther. 2002;303:510–519. doi: 10.1124/jpet.102.039453. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson J, et al. Induction of mitochondrial oxidative stress in astrocytes by nitric oxide precedes disruption of energy metabolism. J Neurochem. 2005;95:388–395. doi: 10.1111/j.1471-4159.2005.03374.x. [DOI] [PubMed] [Google Scholar]
- 50.Takuma K, et al. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 51.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 52.Oddo S, et al. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease. A link between Aβ and tau pathology. J Biol Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 53.Fukui H, et al. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamagno E, et al. Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the β-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamagno E, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- 56.Fukumoto H, et al. β-Secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong Y, et al. Oxidative stress potentiates BACE1 gene expression and Aβ generation. J Neural Transm. 2005;112:455–469. doi: 10.1007/s00702-004-0255-3. [DOI] [PubMed] [Google Scholar]
- 58.Melov S, et al. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dell’agnello C, et al. Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet. 2007;16:431–444. doi: 10.1093/hmg/ddl477. [DOI] [PubMed] [Google Scholar]
- 60.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Schriner SE, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 62.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, et al. Over-expression of antioxidant enzymes protects cultured hippocampal and cortical neurons from necrotic insults. J Neurochem. 2003;87:1527–1534. doi: 10.1046/j.1471-4159.2003.02123.x. [DOI] [PubMed] [Google Scholar]
- 64.Elson JL, et al. Does the mitochondrial genome play a role in the etiology of Alzheimer’s disease? Hum Genet. 2006;119:241–254. doi: 10.1007/s00439-005-0123-8. [DOI] [PubMed] [Google Scholar]
- 65.Lin MT, et al. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- 66.Devi L, et al. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atamna H. Heme binding to amyloid-β peptide: mechanistic role in Alzheimer’s disease. J Alzheimers Dis. 2006;10:255–266. doi: 10.3233/jad-2006-102-310. [DOI] [PubMed] [Google Scholar]
- 68.Atamna H, Frey WH., II A role for heme in Alzheimer’s disease: heme binds amyloid β and has altered metabolism. Proc Natl Acad Sci U S A. 2004;101:11153–11158. doi: 10.1073/pnas.0404349101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lustbader JW, et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]