Abstract
Background/Aims
The endopin serpin protease inhibitors have been identified by molecular studies as components of secretory vesicles that produce neuropeptides. Endopin 1 inhibits trypsin-like serine proteases, and endopin 2 inhibits cathepsin L that produces neuropeptides in secretory vesicles. To assess the secretory vesicle and neuroendocrine tissue distribution of these endopins, the goal of this study was to define specific antisera for each endopin isoform and to examine their localization with neuropeptides and in neuroendocrine tissues.
Methods
These studies utilizes methods consisting of western blots, immunoelectron microscopy, and immunofluorescence microscopy for evaluation of the localization of endopin protease inhibitors in neuroendocrine tissues.
Results
Immunoelectron microscopy with these selective antisera demonstrated the localization of endopins 1 and 2 within secretory vesicles of adrenal medulla (bovine). Cellular immunofluorescence confocal microscopy illustrated the high level of colocalization of endopins 1 and 2 with enkephalin and NPY neuropeptides that are present in secretory vesicles of adrenal medullary chromaffin cells in primary culture. Tissue distribution studies (by western blots) showed the expression of endopins 1 and 2 in bovine brain, pituitary, adrenal medulla, and other neuroendocrine tissues.
Conclusions
These results implicate endopins 1 and 2 as endogenous protease inhibitors in neuropeptide-containing secretory vesicles and neuroendocrine tissues.
Keywords: serpin, endopins, protease inhibitor, neuropeptides, neuroendocrine
Introduction
Investigation of endogenous protease inhibitors in neuropeptide-secretory vesicles of bovine adrenal medullary chromaffin granules indicated the presence of serpins detected by antisera to human α1-antichymotrypsin (ACT). Molecular cloning of these endogenous ACT-like serpins, however, revealed two distinct isoforms of endogenous serpin protease inhibitors, termed endopin 1 and endopin 2 (endocrine serpins) [1-4]. Endopin 1 and 2 possess high homology with ACT, indicating their ACT-like serpin nature [5,6]. Each of these endopins possess distinct reactive site loop (RSL) domains that participate in determining the target protease specificity(ies) of serpins. Endopin 1 inhibits trypsin-like serine proteases, and does not inhibit chymotrypsin. In contrast, endopin 2 inhibits papain-like cysteine proteases, including cathepsin L, as well as the serine protease elastase. These endopins possess distinct target protease specificities that differ from human α1-antichymotrypsin which inhibits chymotrypsin [7].
Investigation of the subcellular and tissue distribution of the endopins is important to gain knowledge of their biological roles in different cell types. For this reason, this study examined the secretory vesicle localization of the endopins with neuropeptides by high resolution immunoelectron microscopy, which showed the presence of these endopins in neuropeptide-containing secretory vesicles. Furthermore, confocal immunofluorescence studies demonstrated the colocalization of endopins 1 and 2 with neuropeptides, enkephalin and NPY, in secretory vesicles of adrenal medullary chromaffin cells. Tissue distribution studies indicated the presence of endopins in brain, pituitary, adrenal medulla, and other neuroendocrine tissues (bovine). These findings implicate the possible roles of endopin 1 and 2 in the regulation of target proteases in secretory vesicles that produce neuropeptides for neurotransmitter and endocrine functions.
Experimental Procedures
Recombinant endopin 1 and endopin 2 for evaluation of specific antisera for endopins
The serpins endopin 1 and endopin 2 (bovine) were expressed in E. Coli as recombinant proteins with N-His tags, as described previously [1-3]. After IPTG-induced expression, each endopin was affinity purified by Ni++-affinity columns that bind the N-His tag of recombinant endopins. Affinity purification provides purified bovine endopin 1 and endopin 2, as described previously [2,3]. The purified recombinant endopins were utilized to assess the selectivity of antisera (rabbit) raised against a unique peptide region of endopin 1 (ENVVVKDQHRRVDGHT), and antisera (rabbit) raised against recombinant endopin 2 protein. Western blots included evaluation of the specificity of anti-endopin 1 and anti-endopin 2 to thse endopin isoforms, compared to the related human α1-antichymotrypsin (ACT) (from Athens Research and Technology, GA).
The specificities of the antibodies to endopin 1 and endopin 2 were demonstrated with respective preimmune serum. Preimmune serum was obtained from rabbits prior to injection with each endopin for antisera production, illustrating the specificities of the antisera to each endopin. Control western blots with preimmune serum to endopin 1, and preimmune serum to endopin 2, showed lack of immunostaining of each endopin.
Immunoelectron microscopy of endopin serpins in secretory vesicles of adrenal medulla chromaffin cells (bovine)
Secretory vesicles were isolated from bovine adrenal medulla by differential sucrose density centrifugation (PNAS-10), and were fixed in 0.2% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. Samples were osmicated in 2% osmium tetroxide in 0.1 M cacodylate and embedded in Epon 812. Ultrathin sections were partially deosmicated through 1% periodic acid/9% sodium periodate, and incubated in 3% normal goat serum in 1X TBS. Sections were incubated with mouse anti-(Met)enkephalin (Chemicon, CA) and detected with secondary goat anti-mouse IgG conjugated to 6 nm colloidal gold (Aurion, Netherlands). Sections were postfixed and immunostained with rabbit anti-endopin 1 or rabbit-anti-endopin 2 detected by goat anti-rabbit IgG conjugated to 15 nm colloidal gold. Sections were examined in a Tecnai-12 transmission electron microscope using a CCD camera and Digital Micrograph Software (Gatan Inc., CA). Controls included immunostaining with preimmune antisera, and incubation of sections with only secondary immunogold-labeled antisera; these controls showed no immunogold staining which demonstrated the specific detection of endopins with anti-endopin sera.
Confocal immunofluorescence microscopy of endopin colocalization with neuropeptides
Immunofluorescence confocal microscopy of chromaffin cells, performed as described [8], utilized rabbit anti-endopin 1 or rabbit anti-endopin 2, with antisera directed to the neuropeptide (Met)enkephalin (ME) using mouse anti-ME (Chemicon, CA) as well as NPY using sheep anti-NPY (Chemicon, CA). Endopins 1 or 2 were detected with anti-rabbit IgG Alexa Fluor 488 (green fluorescence, Molecular Probes, Oregon). ME was detected with anti-mouse IgG-Alexa Fluor 594 (red fluorescence), and NPY was detected with anti-sheep IgG Alexa Fluor 594. In addition, immunofluorescence staining of chromogranin A (CgA), a marker for dense core secretory vesicles, was conducted with anti-CgA (Chemicon) that was visualized with anti-mouse IgG-Alexa Fluor 594. Colocalization of endopins with neuropeptides (ME or NPY), as well as with CgA, was observed by merged images, utilizing the Nikon Eclipse 800 microscope coupled to a BCM confocal system and analyses with SIMPLE PCI software. Confocal images were deconvoluted as described previously [9]. Nuclei were observed by DAPI staining.
Controls for immunofluorescence staining included immunostaining with preimmune antisera, which did not show immunofluorescence; incubation of fixed cells with secondary Alexa-labeled secondary antibodies also showed absence of immunostaining. These controls support the specificity of antisera for endopins and neuropeptides that were found to be colocalized in neuroendocrine chromffin cells.
Distribution of endopins by western blots in neuroendcorine tissues
Western blots with anti-endopin 1 and anti-endopin 2 were performed to assess their distribution in endocrine and nervous system tissues. Bovine tissues analyzed consisted of adrenal medulla, pituitary, brain, intestine, thymus, lung, and liver (tissues obtained from Rockland Immunochemicals, PA). Tissue homogenates were prepared in neutral pH buffer (pH 7.4) with a cocktail of protease inhibitors, as described previously [10]. Protein concentration of homogenate samples were determined with the Biorad DC protein assay kit. Controls for western blots included assessment with preimmune serum and preadorption of antisera with endopin 1 and endopin 2, for evaluating specificities of the antisera to endopins.
Results
Specific antisera for endopin 1 and endopin 2
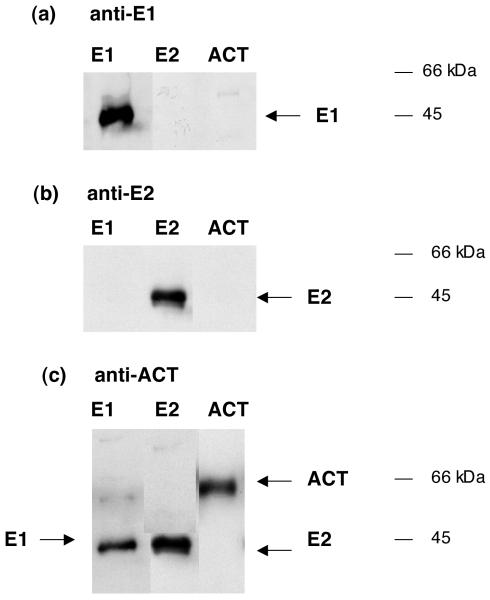
Antisera raised to endopin 1 and endopin 2 were evaluated for their specificity to selectively recognize each of these endopins, without crossreactivity to the related α1-antichymotrypsin (ACT) (figure 1). Results showed that anti-endopin 1 recognized recombinant endopin 1, and did not detect endopin 2 or ACT at similar levels in western blots. Also, the anti-endopin 2 serum detected low levels of recombinant endopin 2, but it did not detect endopin 1 or ACT at similar amounts. The homology of endopins 1 and 2 to ACT was demonstrated by detection of the endopins by anti-ACT. Thus, the antisera to endopin 1 and endopin 2 are selective for each of these serpins. Thus, the antisera developed to endopins 1 and 2 allow specific detection of each of these serpins.
Figure 1. Selective antibodies for endopin 1 and endopin 2.
a. Anti-endopin 1 (E1). The specificity of anti-endopin 1 was assessed with western blots for recombinant endopin 1 (E1, 10 ng, lane 1), recombinant endopin 2 (E2, 10 ng, lane 2), and purified human α1-antichymotrypsin (ACT, 10 ng, lane 3). Western blots were conducted with anti-endopin 1 at 1:500 dilution. Results indicate specific detection of E1, but not E2 or ACT, by the antibody directed to E1.
b. Anti-endopin 2 (E2). The specificity of anti-endopin 2 was assessed with western blots for endopin 1 (E1, 10 ng, lane 1), endopin 2 (E2, 10 ng, lane 2), and α1-antichymotrypsin (ACT, 10 ng, lane 3). Western blots utilized anti-endopin 2 at 1:500 dilution, and developed with secondary anti-rabbit as described previously (Hwang et al., 2007). Results indicate specific detection of E2, but not E1 or ACT, by the antibody directed to E2.
c. Anti-α1-antychymotrypsin (ACT). The specificity of anti-ACT was assessed with western blots for E1 (10 ng, lane 1), E2, (10 ng, lane 2), and ACT (10 ng, lane 3). Western blots utilized anti-ACT at 1:500 dilution. Results indicate that the anti-ACT detects the related serpins ACT, E1, and E2.
The electrophoretic mobility of recombinant bovine endopin 1 on SDS-PAGE gels shows its apparent molecular weight of ∼44-46 kDa, which is consistent with its theoretical molecular weight (MW) of 43.7 kDa [1]. Also, recombinant bovine endopin 2 on SDS-PAGE gels has an apparent MW of ∼44-46 kDa that is consistent with its theoretical MW of 47.2 kDa [2]. Alpha1-anti-chymotrypsin on SDS-PAGE is a 65-66 kDa band, representing native alpha1-antichymotrypsin (ACT) [11]. ACT is a glycoprotein as shown by its deglycosylation (by N-glycosidase F) to ∼45 kDa. The recombinant forms of bovine endopin 1 and endopin 2 appear to correspond to the apparent MW of deglycosylated human ACT. Recombinant endopins expressed in E. coli likely represent non-glycosylated proteins since E. coli lack glycosylation machinery [12] that is present in eukaryotic cells.
Localization of endopin 1 and endopin 2 within neuropeptide-containing secretory vesicles by immunoelectron and confocal immunofluorescence microscopy
Immunoelectron microscopy of secretory vesicles purified from adrenal medullary chromaffin cells (bovine) demonstrated the localization of endopin 1 and endopin 2 within this subcellular organelle (figure 2a, b). Immunogold detection of endopin 1 indicated its presence within secretory vesicles (fig. 2a) that contain the neuropeptide enkephalin (fig. 2c). Furthermore, the endopin 2 isoform was also present with these secretory vesicles (fig. 2b). Nearly all the secretory granules contained endopin 1, endopin 2, and (Met)enkephalin.
Figure 2. Immunoelectron microscopy demonstrates localization of endopin 1 and 2 in secretory vesicles.
a. Endopin 1. The presence of endopin 1 in secretory vesicles (purified from bovine adrenal medulla) was indicated by anti-endopin 1 (rabbit) detected with 15 nm colloidal gold conjugated to anti-rabbit IgG. Immunogold-labeled endopin 1 is present within the secretory vesicle organelle.
b. Endopin 2. The presence of endopin 2 in secretory vesicles (purified from bovine adrenal medulla) was indicated by anti-endopin 2 (rabbit) detected with 15 nm colloidal gold conjugated to anti-rabbit IgG. Immunogold-labeled endopin 2 is present within the secretory vesicle organelle.
c. Enkephalin. (Met)enkephalin in chromaffin secretory vesicles was detected with anti-(Met)-enkephalin (rabbit) visualized with 15 nm colloidal gold conjugated to anti-rabbit IgG. Results indicate the presence of (Met)enkephalin with these secretory vesicles.
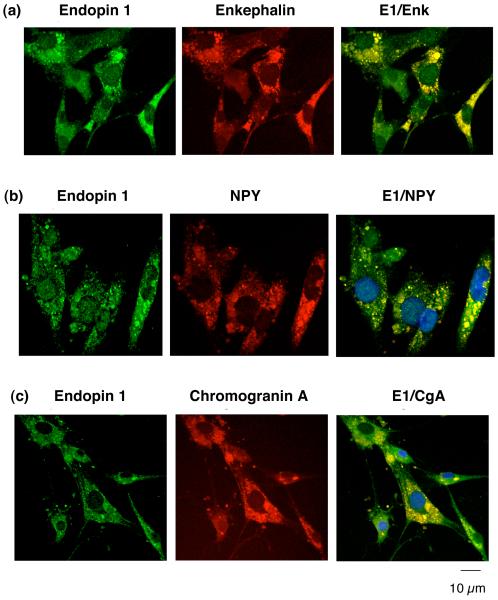
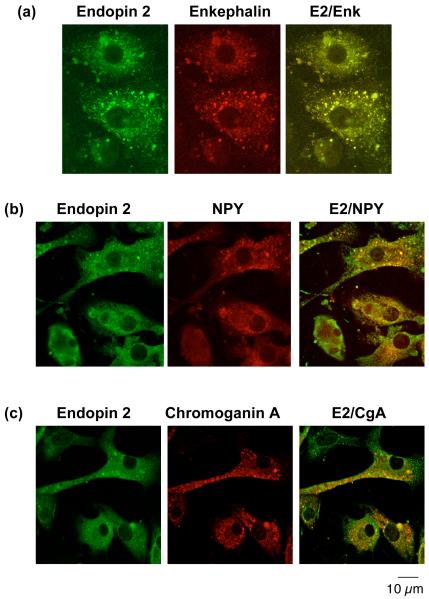
To evaluate the cellular localization of endopin 1 and 2 with neuropeptides in secretory vesicles, colocalization of the endopins with enkephalin and NPY in chromaffin cells was evaluated by confocal immunofluorescence microscopy. Endopin 1 showed a high degree of colocalization with enkephalin and NPY, as well as with the secretory vesicle marker chromogranin A (fig. 3). Endopin 2 also showed colocalization with the enkephalin and NPY neuropeptides, and with chromogranin A that represents an abundant secretory vesicle protein (fig. 4). Like the neuropeptides, endopin 1 and endopin 2 show a punctate pattern of subcellular distribution that is consistent with their colocalization with neuropeptide-containing secretory vesicles. These data clearly demonstrate the localization of endopin 1 and endopin 2 to secretory vesicles.
Figure 3. Endopin 1 colocalization with neuropeptides in secretory vesicles of chromaffin cells demonstrated by immunofluorescence confocal microscopy.
a. Endopin 1 and enkephalin. Immunofluorescence colocalization of endopin 1 (E1) with the enkephalin neuropeptide in chromaffin cells (bovine) was assessed by rabbit anti-endopin 1 detected with anti-rabbit IgG-Alexa Fluor 488 (green fluorescence), and detection of (Met)enkephalin (Enk) with mouse anti-Enk detected with anti-mouse IgG Alexa 594 (red fluorescence). The colocalization of endopin 1 and (Met)enkephalin is illustrated by overlay of the images, illustrated by yellow fluorescence.
b. Endopin 1 and NPY. Immunofluorescence colocalization of endopin 1 (E1) with the NPY neuropeptide in chromaffin cells (bovine) was assessed by rabbit anti-endopin 1 detected with anti-rabbit IgG Alexa Fluor 488 (green fluorescence), and detection of NPY with sheep anti-NPY detected with anti-sheep IgG Alexa 594 (red fluorescence). The colocalization of endopin 1 and NPY is illustrated by overlay of the images, illustrated by yellow fluorescence.
c. Endopin 1 and chromogranin A. Immunofluorescence colocalization of endopin 1 (E1) with the secretory vesicle marker chromogranin A (CgA) in chromaffin cells was assessed by rabbit anti-endopin 1 detected with anti-rabbit IgG Alexa Fluor 488 (green fluorescence), and detection of CgA with mouse anti-CgA detected with anti-mouse IgG Alexa 594 (red fluorescence). The colocalization of endopin 1 and CgA is illustrated by the overlay of the images, illustrated by yellow fluorescence.
Figure 4. Endopin 2 colocalization with neuropeptides in secretory vesicles of chromaffin cells demonstrated by immunofluorescence confocal microscopy.
a. Endopin 2 and enkephalin. Immunofluorescence colocalization of endopin 2 (E2) with the enkephalin neuropeptide in chromaffin cells (bovine) was assessed by rabbit anti-endopin 2 detected with anti-rabbit IgG-Alexa Fluor 488 (green fluorescence), and detection of (Met)enkephalin (Enk) with mouse anti-Enk detected with anti-mouse IgG Alexa 594 (red fluorescence). The colocalization of endopin 2 and (Met)enkephalin is illustrated by overlay of the images, illustrated by yellow fluorescence.
b. Endopin 2 and NPY. Immunofluorescence colocalization of endopin 2 (E2) with the NPY neuropeptide in chromaffin cells (bovine) was assessed by rabbit anti-endopin 2 detected with anti-rabbit IgG Alexa Fluor 488 (green fluorescence), and detection of NPY with sheep anti-NPY detected with anti-sheep IgG Alexa 594 (red fluorescence). The colocalization of endopin 2 and NPY is illustrated by overlay of the images, illustrated by yellow fluorescence.
c. Endopin 2 and chromogranin A. Immunofluorescence colocalization of endopin 2 (E2) with the secretory vesicle marker chromogranin A (CgA) in chromaffin cells was assessed by rabbit anti-endopin 2 detected with anti-rabbit IgG Alexa Fluor 488 (green fluorescence), and detection of CgA with mouse anti-CgA detected with anti-mouse IgG Alexa 594 (red fluorescence). The colocalization of endopin 2 and CgA is illustrated by the overlay of the images, illustrated by yellow fluorescence.
Distribution of endopins 1 and 2 in neuroendocrine tissues
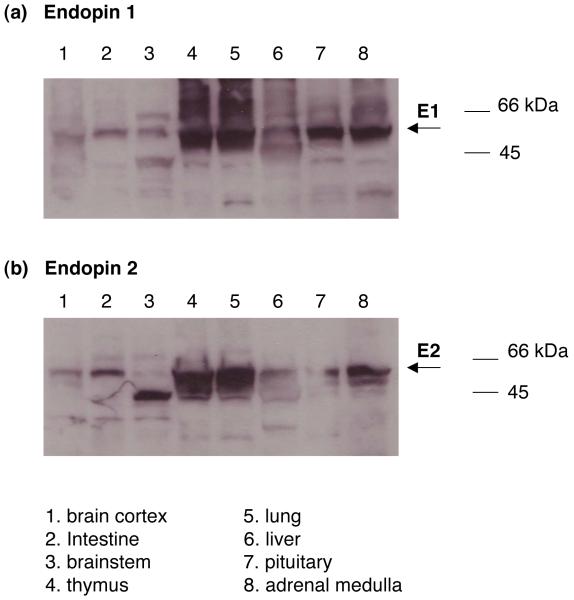
To gain knowledge of the potential roles of endopins 1 and 2 for protease regulation, it is necessary to examine their tissue distribution among neurodocrine cell types. For this reason, tissue distribution of endopin 1 and 2 was conducted. Endopin 1 and 2 are present in adrenal medulla and pituitary at comparable levels (fig. 5). Both endopins are expressed in cortical and brainstem brain regions. Furthermore, expression of endopin 1 and 2 occurs in lung where neuropeptides are present [13-15].
Figure 5. Endopin 1 and endopin 2 in neuroendocrine tissues.
a. Endopin 1. Anti-endopin 1 westerns blots were utilized to assess the distribution of endopin 1 in bovine neuroendocrine tissue regions. Tissue homogenates were prepared as described in the methods, and subjected to SDS-PAGE (50 μg protein per lane) for anti-endopin 1 (1:500 dilution) western blots. Lanes 1-8 represent tissue homogenates from (1) brain cortex, (2) intestine, (3) brainstem, (4) thymus, (5) lung, (6) liver, (7) pituitary, and (8) adrenal medulla, respectively. Results demonstrate the neuroendocrine tissue distribution of endopin 1.
b. Endopin 2. Anti-endopin 2 western blots assessed the bovine tissue distribution of endopin 2. Tissue homogenates were prepared as described in the methods, and samples were subjected to SDS-PAGE (50 μg protein per lane) for anti-endopin 2 western blots. Lanes 1-8 represent tissue homogenates from (1) brain cortex, (2) intestine, (3) brainstem, (4) thymus, (5) lung, (6) liver, (7) pituitary, and (8) adrenal medulla, respectively. Results demonstrate the neuroendocrine tissue distribution of endopin 2.
Western blots of endopin 1 and endopin 2 showed apparent molecular weights (MW) of ∼55-60 kDa. With the knowledge that the primary sequences of endopins 1 and 2 possess predicted glycosylation sitse, the larger in vivo MWs of the endopins compared to recombinant endopins (fig. 1) suggest possible glycosylation in vivo (fig. 5). Interestingly, western blots of brainstem and thymus show endopin 1 as bands of ∼66 kDa, as well as ∼55-60 kDa, suggesting possible differential glycosylation. Overall, western blot results demonstrated the presence of endopin 1 and endopin 2 serpins in neuroendocrine tissues.
Discussion
Implementation of selective antisera to the serpins endopin 1 and endopin 2 demonstrated their localization in secretory vesicles of bovine adrenal medulla (also known as chromaffin granules), the primary subcellular site for production of neuropeptides including (Met)enkephalin and NPY. High resolution immunoelectron microscopy demontrated the presence of endopins 1 and 2 within chromaffin granules that contain the neuropeptide (Met)enkephalin. Cellular immunofluorescence studies illustrated the colocalization of endopins 1 and 2 with (Met)enkephalin and NPY neuropeptides that are produced and stored in secretory vesicles. The secretory vesicle localization of endopin 1 and endopin 2 was further illustrated by colocalization with chromogranin A, a marker for secretory vesicles. Tissue distribution studies indicated the expression of endopin 1 and endopin 2 in multiple neuroendocrine regions including adrenal medulla, pituitary, brain, lung, thymus, and intestine tissues (bovine). These findings demonstrate the unique functional role of endopin serpin protease inhibitors in secretory vesicles of neuroendocrine tissues.
Previous molecular and biochemical studies of bovine endopin 1 and endopin 2 serpins have illustrated similarities in their primary sequences, but they each possess unique reactive site loop (RSL) domains that participate in defining target proteases. Their novel RSL domains predicted their distinct target protease specificities. Recombinant endopin 1 inhibits the serine protease trypsin that cleaves at basic residues, but endopin 1 does not inhibit PC1/3 or PC2 (PC, prohormone convertases) that also cleave at basic residues. Recombinant endopin 2 displays the serpin property of cross-class inhibition of papain-like cysteine proteases and the serine protease elastase. Notably, a recently identified isoform of endopin 2, known as endopin 2C, shows efficient inhibition of the cysteine protease cathepsin L [3]. Secretory vesicle cathepsin L has been demonstrated to participate in the proteolytic processing of proprotein precursors for the production of enkephalin and other neuropeptides [9,16,17]. Demonstration of the in vivo localization of endopin 2 within neuropeptide-containing secretory vesicles in endocrine and neuronal tissues suggests a role for endopin 2 in regulating secretory vesicle cathepsin L for neuropeptide production.
Based on the localization of endopin 2 in neuropeptide-containing secretory vesicles, endopin 2 inhibition of cathepsin L, and participation of cathepsin L in secretory vesicles for neuropeptide production [9,18], it may be proposed that endopin 2 represents an endogenous protease inhibitor of cathepsin L for production of neuropeptides in secretory vesicles. Thus, the cathepsin L with endopin 2 inhibitor may be proposed as a cysteine protease pathway for prohormone processing. This cysteine protease pathway is distinct from the subtilisin-like prohormone convertases PC1/3 and PC2 [18-21].
In summary, endopin 1 and endopin 2 have been demonstrated to reside within secretory vesicles that produce and secrete neuropeptides as peptide neurotransmitters and hormones. Endopin 1 and 2 are expressed among numerous endocrine and nervous system regions in bovine. These findings support the proposed roles of endopin serpins for regulating proteases in secretory vesicles of neuroendocrine tissues.
Acknowledgments
This research was supported by grants from the National Institutes of Health. Technical assistance by Joe Thoits and Katie Troutner is appreciated.
References
- [1].Hwang SR, Steineckert B, Yasothornsrikul S, Sei CA, Toneff T, Rattan J, Hook VY. Molecular cloning of endopin 1, a novel serpin localized to neurosecretory vesicles of chromaffin cells. J Biol Chem. 1999;274:34164–34173. doi: 10.1074/jbc.274.48.34164. [DOI] [PubMed] [Google Scholar]
- [2].Hwang SR, Steineckert B, Toneff T, Bundey R, Logvinova A, Goldsmith P, Hook VY. The novel serpin endopin 2 demonstrates cross-class inhibition of papain and elastase: localization of endopin 2 to regulated secretory vesicles of neuroendocrine chromaffin cells. Biochemistry. 2002;41:10397–10405. doi: 10.1021/bi020088o. [DOI] [PubMed] [Google Scholar]
- [3].Hwang SR, Stoka V, Turk V, Hook VY. The novel bovine serpine endopin 2C demonstrates selective inhibition of the cysteine protease cathepsin L compared to the serine protease elastase, in cross-class inhibition. Biochemistry. 2005;44:7757–7767. doi: 10.1021/bi050053z. [DOI] [PubMed] [Google Scholar]
- [4].Hwang SR, Hook VY. Multiple domains of endopin 2A for serpin cross-class inhibition of papin. Arch Biochem Biophys. 2007;461:219–224. doi: 10.1016/j.abb.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chandra T, Stackhouse R, Kidd VJ, Robson KJH, Woo SLC. Sequence homology between human α1-antichymotrypsin, α1-antitrypsin, and antithrombin III. Biochemistry. 1983;22:5055–5061. doi: 10.1021/bi00291a001. [DOI] [PubMed] [Google Scholar]
- [6].Wei A, Rubin H, Cooperman BS, Christianson DW. Crystal structure of uncleaved serpin reveals the conformation of an inhibitory reactive loop. Nat Struct Biol. 1994;1:251–258. doi: 10.1038/nsb0494-251. [DOI] [PubMed] [Google Scholar]
- [7].Cooperman BS, Stavridi E, Nickbarg E, Rescorla E, Schechter NM, Rubin H. Antichymotrypsin interaction with chymotrypsin. J Biol Chem. 1993;268:23616–23625. [PubMed] [Google Scholar]
- [8].Hook VY, Noctor S, Sei CA, Toneff T, Yasothornsrikul S, Kang YH. Evidence for functional localization of the proenkephalin-processing enzyme, prohormone thiol protease, to secretory vesicles of chromaffin cells. Endocrinology. 1999;140:3744–3754. doi: 10.1210/endo.140.8.6926. [DOI] [PubMed] [Google Scholar]
- [9].Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mende-Mueller LM, Toneff T, Hwang SR, Chesselet MF, Hook VY. Tissue-specific proteolysis of huntingtin (htt) in human brain: evidence of enhanced levels of N- and C-terminal htt fragments in Huntington’s Disease striatum. J Neurosci. 2001;21:1830–1837. doi: 10.1523/JNEUROSCI.21-06-01830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hwang SR, Steineckert B, Kohn A, Palkovits M, Hook VY. Molecular studies define the primary structure of alpha1-antichymotrypsin (ACT) protease inhibitor in Alzheimer’s disease brains: comparison of ACT in hippocampus and liver. J Biol Chem. 1999;274(3):1821–7. doi: 10.1074/jbc.274.3.1821. [DOI] [PubMed] [Google Scholar]
- [12].Rubin R, Wang Z, Nickbarg EB, McLarney S, Naidoo N, Schoenberger OL, Johnson JL, Cooperman BS. Cloning, expression, purification, and biological activity of recombinant native and variant human alpha1-antichymotrypsins. J. Biol. Chem. 1990;265(2):1199–1207. [PubMed] [Google Scholar]
- [13].Moody TW. Peptide hormones and lung cancer. Panminerva Med. 2006;48:19–26. [PubMed] [Google Scholar]
- [14].Hastings RH. Parathyroid hormone-related protein and lung biology. Respir. Physiol. Neurobiol. 2004;142:95–113. doi: 10.1016/j.resp.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [15].Barnes PJ. Neuropeptides in human airways: function and clinical implications. Am Rev Respir Dis. 1987;136:77–83. doi: 10.1164/ajrccm/136.6_Pt_2.S77. [DOI] [PubMed] [Google Scholar]
- [16].Hook VY. Unique neuronal functions of cathepsin L and cathepsin B in secretory vesicles: biosynthesis of peptides in neurotransmission and neurodegenerative disease. Biol Chem. 2006;387:1429–1439. doi: 10.1515/BC.2006.179. [DOI] [PubMed] [Google Scholar]
- [17].Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook V. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretions. J Biol Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- [18].Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Tox. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- [20].Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- [21].Scamuffa N, Calvo F, Chrétien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]