Abstract
Objective
Macrophage apoptosis plays important roles in atherosclerosis. Bcl-2 is a key cell survival molecule, but its role in macrophage apoptosis in atherosclerosis is not known. The goal herein was to determine the effect of macrophage-targeted deletion of Bcl-2 on macrophage apoptosis in atherosclerotic lesions of Apoe-/- mice.
Methods and Results
Bcl2flox-LysMCre mice were created as a model of macrophage Bcl-2 deficiency. Macrophages from these mice were more susceptible to apoptosis than those from control Bcl2WT-LysMCre mice. The mice were bred onto the Apoe-/-background and fed a Western-type diet for 4 or 10 weeks. Apoptotic cells were equally very rare in the lesions of both groups of the 4-wk-diet mice, and there was no difference in lesion area. However, Bcl2flox-LysMCre;Apoe-/- plaques from the 10-wk-diet protocol had a 40-45% increase in apoptotic cells and, in female mice, a ~25% increase in plaque necrosis (P<0.05) compared with Bcl2WT-LysMCre lesions.
Conclusions
Macrophage Bcl-2 plays a protective role against macrophage apoptosis specifically in advanced atherosclerotic lesions of Apoe-/- mice.
Keywords: Atherosclerosis-Pathophysiology, Apoptosis, Macrophage, Animal models of human disease
Macrophage apoptosis can be a critical event in atherosclerosis and occurs at all stages of disease development.1 In early lesions, macrophage apoptosis is associated with a decrease in lesion cellularity and plaque progression,2, 3 which could be due to rapid phagocytic clearance or egress of the apoptotic cells and/or decreased influx of macrophages. In advanced lesions, however, clearance of apoptotic cells is defective, which leads to secondary necrosis of apoptotic cells.4-6 Accordingly, we have proposed that macrophage apoptosis in advanced lesions leads to plaque necrosis, which is thought to promote plaque disruption and acute clinical events in humans.7 In support of this idea, vulnerable, necrotic human plaques have increased macrophage apoptosis.8 Moreover, genetic or pharmacological manipulations that decrease macrophage apoptosis in advanced murine lesions decrease plaque necrosis, and vice versa9.
Bcl-2 has been on the forefront of cell survival signaling since its discovery more than 20 years ago, yet there is an absence of in-vivo causation studies assessing the role of Bcl-2 in atherosclerosis using genetically altered mouse models. This point is critical, because there are a number of other cell survival molecules in lesional cells, including Bcl-xL, apoptosis inhibitor expressed by macrophages (AIM; SPα), Mcl-1, and members of the inhibitor-of-apoptosis (IAP) family, and so redundancy might negate the effect of deletion of any one cell survival molecule. Because Bcl-2 is expressed in other lesional cell types and holo-Bcl2-/- mice have multiple abnormalities at birth10, we created macrophage-targeted Bcl-2-deficient mice and studied the effect of this mutation on atherosclerosis in Western diet-fed Apoe-/- mice. Our studies indicate that macrophage Bcl-2 plays a protective role against macrophage apoptosis specifically in advanced atherosclerotic lesions. Moreover, in the more advanced lesions of female mice, macrophage Bcl-2 also has a modest protective effect against plaque necrosis.
Materials and Methods
Materials and methods related to cultured macrophages, genetically altered mice, plasma lipid analysis, laser capture microdissection, quantification of atherosclerotic lesions, and statistics appear in Supplementary Materials.
In-situ TUNEL Assays and Plaque Necrosis
Apoptotic cells in atherosclerotic lesions were detected by the TUNEL (TdT-mediated dUTP nick end labeling) technique using the TMR red in-situ cell death detection kit (Roche). Nuclei were stained with DAPI. TUNEL-positive nuclei were counted under an Olympus IX-70 inverted fluorescent microscope. For assessment of macrophage co-localization, macrophages were detected using a rabbit anti-macrophage antibody (AIA31240) from Accurate Chemical and Scientific Corporation, and nuclei were stained with Hoechst. Plaque necrosis was quantified by measuring the area of hematoxylin and eosin-negative acellular areas in the intima, as described previously.11
Results
Intimal Cell Apoptosis is Increased in Lesions of Bcl2flox-LysMCre;Apoe-/- Mice Fed A Western Diet for 10 Weeks But Not 4 Weeks
Mice with Bcl-2 deficiency in macrophages using the cre-lox strategy (Bcl2flox-LysMCre) were created as described in Supplementary Materials (Supplementary Results and Figure IA-B). Peritoneal macrophages from these mice were more susceptible than control Bcl2flox-LysMCre mice to a variety of apoptosis inducers (Supplementary Results and Figure IC-D). The two groups of mice were bred onto the Apoe-/- background and fed a Western-type diet for 4 or 10 weeks, and the aortic root was examined. The 4-wk lesions showed similar area between the two groups of mice, and only rare examples of lesional macrophage apoptosis (Supplementary Results).
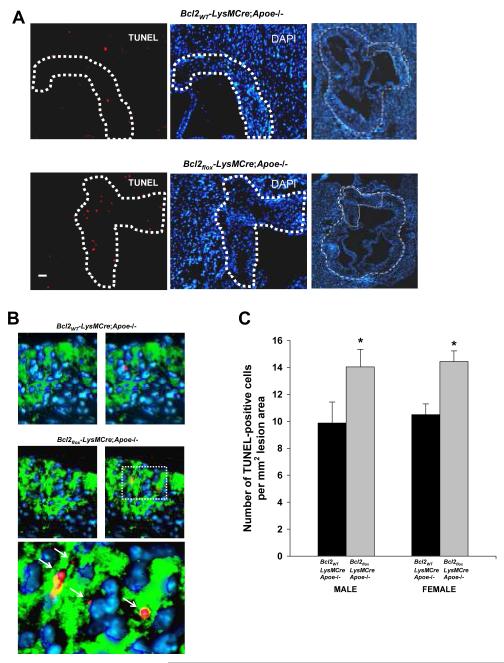
Background data for the 10-wk-diet study are described in Supplementary Results and Supplementary Figure II. Figure 1A demonstrates and increase in TUNEL-positive nuclei, a measure of apoptosis, in Bcl2flox-LysMCre;Apoe-/- vs. Bcl2WT-LysMCre;Apoe-/-lesions. As expected, the areas of TUNEL staining correlated with macrophage-rich areas in these lesions (Figure 1B). The quantified data for the full cohort of mice is shown in Figure 1C. Examples of hematoxylin and eosin-stained aortic root sections of from female Bcl2WT-LysMCre;Apoe-/- and Bcl2flox-LysMCre;Apoe-/- mice are shown in Figure 2A. Total lesion area per se was not affected by macrophage Bcl-2 depletion (Figure 2B). Also not affected by macrophage Bcl2 deficiency were plasma levels of TNFα and IL-6 (data not shown).
Figure 1. In-situ TUNEL staining of aortic root lesions of Bcl2WT-LysMCre;Apoe-/-and Bcl2flox-LysMCre;Apoe-/- mice fed a Western-type diet for 10 wks.
A, The left and middle panels show TUNEL- and DAPI-stained aortic root lesions from 10-wk-diet-fed female Bcl2WT-LysMCre;Apoe-/- and Bcl2flox-LysMCre;Apoe-/- mice. The full aortic root sections are shown in the right panel (outlined by the dashed lines). Bar, 20 μm. B, Macrophage (green) and TUNEL (red) staining of female Bcl2WT-LysMCre;Apoe-/- and Bcl2flox-LysMCre;Apoe-/- lesions. Hoechst-stained nuclei are blue. The two left images show only macrophage and nuclei. The bottom image is a high-magnification of the area designated by the box in the right image from the Bcl2flox-LysMCre;Apoe-/- lesion. The arrows in this image show apoptotic macrophages. C, Quantification of TUNEL-positive nuclei per mm2 lesion area. The differences between the two genotypes for both males and females were statistically significant (asterisks, P<0.05).
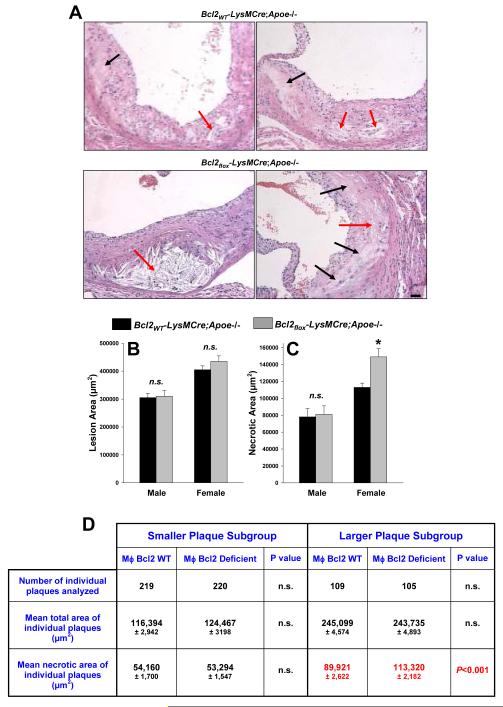
Figure 2. Images and quantifications of lesion and necrotic areas of aortic root lesions of Bcl2WT-LysMCre;Apoe-/- and Bcl2flox-LysMCre;Apoe-/- mice fed a Western-type diet for 10 wks.
A, Images of hematoxylin- and eosin-stained aortic root lesions from 10-wk-diet-fed female Bcl2WT-LysMCre;Apoe-/- and Bcl2flox-LysMCre;Apoe-/- mice. Black arrows, acellular necrotic areas; red arrows, necrotic areas with cholesteryl crystals. Bar, 20 μm. B, Quantification of cross-sectional lesion area. The differences between female vs. male lesions in both genotypes are statistically different by Mann-Whitney analysis (P<0.05). C, Quantification of cross-sectional necrotic area. Asterisk, P<0.05. D, Individual plaques in aortic root cross sections from female mice were separated into two groups based on plaque area, <200,000 and >200,000 μm2, and then analyzed for plaque necrosis For each mouse, six cross sections were analyzed, and each section had, on average, three individual plaques. The differences in necrotic area between larger and smaller plaques for each genotype were statistically significant (P<0.001 by Mann-Whitney).
Increases in macrophage apoptosis are translated into increases in plaque necrosis only after further lesion progression.12 In the current study, the female mice as a group had larger and more advanced lesions than male mice, and female Bcl2flox-LysMCre;Apoe-/- lesions appeared to have more acellular necrotic areas (black arrows in Fig. 2A) as well as necrotic areas with cholesterol crystals (red arrows in Fig. 2A). Quantification showed a ~25% increase in necrotic area in Bcl2flox-LysMCre;Apoe-/- vs. Bcl2WT-LysMCre;Apoe-/- female mice (p < 0.05) (Figure 2C). Neither fibrous cap thickness nor lesional collagen content were affected by macrophage Bcl-2 deficiency (data not shown).
The difference in the affect of macrophage Bcl-2 deficiency on plaque necrosis could be due to a direct effect of sex differences, e.g., sex steroids, and/or to the fact that female lesions were more advanced (see Fig. 2B).12 In attempt to sort out these possibilities, individual plaques from the female mice were divided into two sub-groups based on plaque area: <200,000 and >200,000 μm2. We found a statistically significant difference in necrotic area between the genotypes only in the larger plaque subgroup (Table in Figure 2D). This finding is consistent with the conclusion that macrophage Bcl-2 deficiency has a selective effect on more advanced lesions. There were not enough larger plaques among the male lesions for analysis, and so pending future studies with more advanced male lesions, there is also the possibility of a direct sex effect as well. In summary, macrophage Bcl-2 deficiency is associated with increased advanced lesional macrophage apoptosis and, in large lesions in female mice, an increase in plaque necrosis.
Discussion
See Supplementary Material for a discussion of the findings in this report in relationship to relevant articles in the literature.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by an American Heart Association Scientist Development Grant 0435364T (to Y.L.), an American Heart Association Postdoctoral Training Grant (to. E.T.), NIH grant HL084312 (to E.A.F.), and NIH grants HL54591 and HL75662 (to I.T.).
Footnotes
Disclosures None.
References
- 1.Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- 2.Arai S, Shelton JM, Chen M, et al. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metabolism. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 5.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 6.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 8.Tyurina YY, Serinkan FB, Tyurin VA, et al. Lipid antioxidant, etoposide, inhibits phosphatidylserine externalization and macrophage clearance of apoptotic cells by preventing phosphatidylserine oxidation. J Biol Chem. 2004;279:6056–6064. doi: 10.1074/jbc.M309929200. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I. Mouse models of apoptosis and efferocytosis. Curr Drug Targets. 2008 doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- 10.Kamada S, Shimono A, Shinto Y, et al. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 11.Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim WS, Timmins JM, Seimon TA, et al. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.