Introduction
Osteosarcoma
Osteosarcoma (OS) is one of the most common, non-hematologic primary bone tumors. OS can occur at any age but most patients are between 12 and 25 years of age. A second peak occurs above the age of 50 years (29, 63, 93, 145). The definition of OS is deceptively straightforward. It is a malignant tumor of connective tissue (mesodermal) origin within which the tumor cells produce bone or osteoid. The tumor arises typically in the metaphyseal region of long bones and most frequently metastasizes to the lungs. OS may affect any bone and often produces various kinds of extracellular matrices while displaying different degrees of differentiation. This has led to the practice of sub-classifying OS. A detailed review on its anatomic and histological variations has recently been published (70).
Most patients with OS have no specific clinical symptoms. However, when present the most frequent symptom is pain, usually present several weeks or months prior to diagnosis. The most frequent presenting clinical sign is a firm and tender mass, often brought to attention to by a minor trauma. Next to the devastating effect of the primary tumor on the musculoskeletal system, lung metastasis eventually causes the patient’s death in the vast majority of cases which develop metastatic disease. The diagnosis can be strongly suggested by conventional radiography in about half of the patients while more detailed imaging techniques may be required.
The exact cause of OS remains unknown. OS are tumors with complex unbalanced karotypes and other chromosomal aberrations. During the past decades many genetic and epigenetic factors have been speculated to be linked to the development of OS. What is clear is that numerous inherited syndromes place individuals at heighten risk for OS. Among these are, the Rothmund Thomson syndrome (128, 140), Bloom syndrome (11) and Li-Fraumeni syndrome (83). Other abnormalities linked to a predilection for the development of OS include Paget disease and fibrous dysplasia. Furthermore, OS frequently occurs in patients with an inherited mutation in the retinoblastoma gene (Rb), since patients affected with hereditary Rb mutations have a 1000-fold higher change of developing OS (51).
The current treatment of OS involves an integrated strategy incorporating chemotherapy and surgery. Localized tumors limited to the bone of origin have the best prognosis; 5-year survival varies between 60–75% (4, 89, 90, 98, 124, 134). Patients with metastatic disease (20% at presentation) are typically treated by surgical resection of the primary tumor. As noted above, bone and lung are the two most common sites of metastases. In the latter case, where possible, treatment consists of thoracotomy for removal of pulmonary metastases, followed by postoperative combination chemotherapy. These patients still have a poor overall two-year survival of 55% (4). In patients with local recurrences (30% of the patients with OS) survival has been reported as being below 30% (4, 19, 32, 97, 138).
Despite the fact that introduction of chemotherapy has improved the survival of patients with OS dramatically, there are still patients who cannot benefit from these improvements. Regardless of all the efforts in the field of chemotherapy and aggressive surgery in the past decade, no major changes in treatment and outcome have been achieved. The emerging knowledge of the pathogenesis and genetic abnormities associated with OS has exposed the field to new potential molecular targets. The next challenge is to translate this knowledge into anticancer tools to benefit patients.
Gene therapy using viral vectors can be such a tool. Numerous cancer gene therapy strategies using both viral and non-viral vectors to deliver genetic material have been developed during the past two decades. Furthermore, the increasing knowledge of the viral genome and its function as well as the mechanisms of tumor cell infection and viral replication has led to the development of a novel approach in cancer therapy. Known as virotherapy, which employs the inherent cytopathic activity of candidate viruses to kill tumor cells (Table I). The current review focuses on the latest developments, efforts and future directions in gene therapy research for OS, with special emphasis on adenovirus targeting strategies and oncolytic viral therapy approaches.
Gene Therapy
Gene therapy relies on the delivery of foreign DNA into human cells. This has led to the engineering of effective and efficient vehicles for transferring genes into cells. The transfer of DNA can be accomplished by non-viral vectors utilizing liposome delivery or DNA protein complexes and by viral vectors. Viral vectors are genetically modified viruses, which are still able to transfer their genetic material to a host cell. The viral vectors used in experimental OS studies include integrating vectors based on retroviruses (Rv) and adeno-associated viruses (AAV), as well as non-integrating vectors based on adenoviruses (Ad), lentivirus and herpes viruses. Following is a brief description of the commonly used vectors for gene transfer described in this review.
Vectors
Non-viral vectors
The simplest method to deliver DNA is by plasmid DNA expression vectors driven by eukaryotic promoters. Nonviral vectors for delivery of DNA can be classified into 2 major types: polymeric delivery systems (DNA polymer complexes) and liposomal delivery systems (DNA entrapped in and/or complexed to liposomes) (114). Polymeric delivery systems deliver DNA based on the general mechanism of cellular uptake of a positively charged complex owing to electrostatic interaction with anionic DNA. This polyplex can be manipulated to interact with a negatively charged cell surface to maximize DNA uptake. Polymeric matrices with varying properties can be designed by choosing molecules with an appropriate distribution of different molecular weights and degrees of cross- linking of the polymer and by the incorporation of targeting ligands (114). Alternatively, liposomes are vesicles that consist of an aqueous compartment enclosed in a phospholipid bilayer. DNA can be entrapped inside the aqueous core or complexed to the phospholipid lamellae. Liposome delivery systems can be easily engineered to yield a desired size, surface charge, composition and morphology (114).
In comparison to viral vectors the transfection efficiency of non-viral vectors is significantly lower. Another drawback is their rapid inactivation in the presence of serum. The major advantage of non-viral vectors lies in the fact that they are generally safer to administer as they will not elicit major immune responses or integrates into the host genome potentially resulting in oncogenic molecular events.
Viral vectors
Retroviral vectors
Initial gene therapy trials utilized retroviruses as the preferred vectors as their molecular sequencing has previously been well worked out and because retroviruses can integrate into the host genome. The retrovirus is a double stranded RNA virus (12). Typically the murine leukemia virus (MLV) was used. However the inability of retrovirus vectors to infect non-dividing cells has restricted their potential application (74). Since retroviruses are capable of integrating into the host genome, they are suitable vectors when the long-term expression of a foreign gene is needed (92). The main concerns regarding retroviruses remain the accidentally random integration of them into the host chromosome resulting in deleterious and potentially life-threatening effects (131).
Adeno-associated viral vectors
Adeno-associated viral vector (AAV) is one of the most commonly used gene delivery systems. It is a small DNA virus belonging to the parvoviridae family. AAV efficiently expresses transgenic DNA in various cell types in many tissues (131). The disadvantages of AAV are two fold; one is its restricted packaging capacity and the second is its inefficient large-scale production capability. In addition, AAV needs a helper virus for productive viral replication. Furthermore, the pre-existing immunity to human AAV vectors is comparable to adenovirus (131).
Adenovirus
The ability of adenoviruses to be grown to a high-titer and to engage in high-level heterologous gene expression has made human adenoviruses popular gene therapy vectors. Adenoviral vectors used in clinical trials have historically been largely derived from adenovirus serotype 5 that causes mild upper respiratory infections in infants (131). The wild type adenovirus is a non-enveloped virus containing a single linear fragment of double stranded DNA. Adenoviruses can infect a variety of cell types (127). However, the use of adenoviruses has been limited as they do not integrate in the host chromosome and endogenously home to their liver. This results in short-term expression of the transduced gene. In addition, adenoviruses may elect a strong immunological response, which can potentially cause harmful effects in patients (20).
Herpes simplex virus
The Herpes simplex viruses belong to the family of herpesviridae. They have a relatively large genome size, demonstrate neurotropism and exhibit a latent phase in their life cycle, which allows their genome to remain episomally in the cell nucleus for the lifetime of the host. Features of Herpes simplex viruses include their ability to grow to high titers, their ability to infect non-dividing cells and their ability to successfully package large exogenous inserts (61). However, when using herpes simplex viral vectors it is difficult to completely eliminate lytic viral gene expression, vector induced cytotoxicity and transient gene expression limiting their usefulness for use in gene therapy (92).
Lentiviruses
Lentiviruses are a subset of the retrovirus group. Many of the lentivirus vectors used in gene therapy are based on human immunodeficiency virus (HIV) (75). HIV vectors can accommodate fairly large gene inserts and can provide long-term expression through chromosomal integration. However, in contrast to conventional retrovirus particles, lentivirus vectors can also deliver foreign genes to non- dividing cells. In addition, they can be targeted to hematopoietic cells expanding their value in clinical human therapy trial design (131). Table 1 summarizes the different viral vectors used for gene delivery.
Table 1.
Properties of viruses used for gene delivery
| Vector | Packaging capacity | Genetic material | Genome size (kb) | Diameter(nm) | Host range | Features |
|---|---|---|---|---|---|---|
| Retrovirus | 8 kb | Linear single stranded RNA | 7–11 | 100–145 | Constricted, dividing cells only | Long term expression
Genome integration |
| Adeno-associated virus | 4.7 kb | Linear single stranded DNA | 4.7 | 20–22 | Extensive, both non-dividing and dividing cells | Genome integration
Long term expression Inefficient large scale production |
| Adenovirus | 8–9 kb | Linear double stranded DNA | 36 | 80–100 | Extensive, both non-dividing and dividing cells | Transient expression
Strong immunogenicity |
| Herpes virus | 30 kb | Linear double stranded DNA | 152 | 200 | Extensive | Long term expression
Low toxicity Latent infection |
| Lenitvirus | 8 kb | Linear double stranded DNA | 7–11 | 80–100 | Extensive | Genome integration
Long term expression Inefficient production Safety concerns |
Rather than focus on different viral vector usage in gene therapy one can also classify approaches based on the transferred genes utilized. Three main strategies for human gene therapy in the OS field can be segregated into mutation compensation of oncogenes or tumor suppressor genes, suicide gene therapy, and immuno-potentiation (see figure 1).
FIGURE 1.
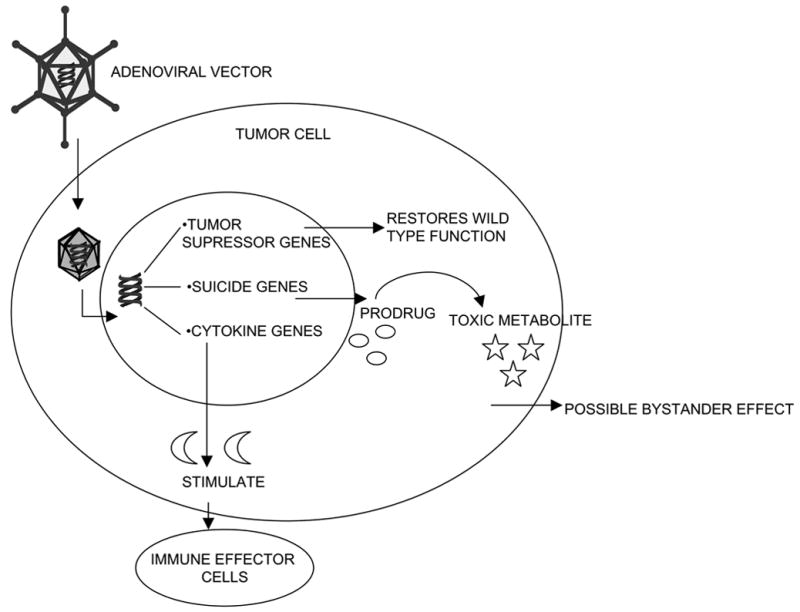
GENE THERAPY STRATEGIES; MUTATION COMPENSATION, SUICIDE GENE THERAPY AND IMMUNO-POTENTIATION
Mutation Compensation Gene Therapy
Mutation compensation is based on the idea that a disease results from single genetic alteration. Since this is not the case in cancer, where the disease is the result of a cascade of genetic abnormalities (rearrangements, deletions, frame shifts mutations etc), gene replacement will be theoretically effective when it replaces a gene pivotal in the cancer development cascade, such as p53 or pRb. An exogenously delivered wild type gene thereby restoring its original function to the cell/tissue replacing the mutated or deleted gene. Osteosarcomas are known to have complex unbalanced karyotypes, and the p53 and retinoblastoma (Rb) gene pathways are altered in most cases (33, 48, 91, 94). These tumor suppressor pathway anomalies provide significant opportunities for compensation of disrupted cell functions in OS tumor cells.
Mutations associated with pRb pathway are the most frequently observed changes in OS (6, 37, 51). Several groups have tried to restore the pRb pathway by the use of a replication-deficient recombinant adenovirus vector encoding the full-length pRb (158) or p16INK4A (22). These strategies were able to suppress tumorgenicity in nude mice. However this treatment was less effective in well-established xenografts. It became rapidly clear that the effect was strongly associated with the presence of functional pRb in the cancer cells, and that the most pronounced effect could be observed in pRb null tumor cells. Moreover, to increase pRb mutation compensation efficacy, improved transfection efficiency and expression of the pRb gene was warranted.
Since p53 alterations are common in OS (17, 35, 99, 100), restoring p53 function was another attractive target in cancer gene therapy. In several cancer models, p53 gene transfer reduced DNA damage, enhanced induction of apoptosis, inhibited tumor growth and increased survival of tumor-bearing mice (39, 52, 107, 108). In OS, the group of Phelan et al. (115) delivered functional p53 by the use of herpes protein VP22 in OS cell lines. They showed that this protein efficiently induced apoptosis in p53 negative human OS cells. Moreover in an established OS lung tumor model, nude mice were treated with aerosol delivery of plasmid DNA containing the p53 gene. This resulted in a significant reduction of tumor nodules and in the average size of the tumors (25). Since SAOS-LM6 was used to develop this tumor model, these results were not entirely unexpected, as SAOS-LM6 cells are derived from SAOS-2, a p53 null line.
Another interesting feature of restoring wild type p53 was an increasing sensitivity of OS cells to chemotherapeutic agents. This improvement in sensitivity was observed in combination with cisplatin (141) and adriamycin (135). In addition Ganjavi et al. (40) showed that cell viability decreased after treatment with an adenoviral vector encoding p53 and that the sensitivity of the OS cell lines to cisplatin and doxorubicin increased.
Despite the fact that these strategies showed efficacy in vitro and in vivo, the concept of mutation compensation mandates transduction of every tumor cell, in the absence of lateralization or a bystander effect (see below), in order to eradicate a complete tumor population. Furthermore, the application of wild type tumor-suppressor gene therapy on genetically variable OS may only be effective in genetic subgroups of this tumor, reducing but not eliminating the entire tumor population (58). Alternative gene therapy approaches that are less dependent on the genetic background of the target cell population and with more widespread anti-tumor effects have, therefore, been developed.
Suicide gene therapy
Suicide gene therapy (also called; gene-directed enzyme prodrug therapy, molecular chemotherapy) relies on the capacity of the gene product of a transduced cell to convert a non-toxic prodrug into a toxic compound. This approach has the advantage that not every single tumor cell needs to express this product to eradicate a tumor population, due to the so-called bystander effect. This phenomenon can be explained by diffusion (extracellularly or via gap junctions) of toxic compounds, formed in the transduced cell, to neighboring, non-transduced or transfected cells. Several prodrug-converting enzymes have been extensively studied, including Herpes Simplex Thymidine Kinase (HSV-TK), bacterial cytosine deaminase (CD), carbocylesterase-2 (CE-2), bacterial nitroreductase/CB1954 and cytochrome P450/cyclophosphamide. The latter two have not been applied to the field of OS and will not be discussed further.
Herpes Simplex thymidine Kinase
The HSV-TK gene is the most widely studied suicide gene therapy system. Expression of the HSV-TK gene confers enhanced tumor sensitivity to nucleoside analogues such as ganciclovir (GCV) and acyclovir (ACV). HSV-TK converts GCV or ACV to their non-phosphorylated form, which is trapped in the cytoplasm and subsequently, becomes further phosphorylated to the triphosphorylated (GCV-3P/AVC-3P) form by cellular kinases of the host cells. Upon division of the tumor cell, GCV-3P or ACV-3P is incorporated into the cellular DNA, thereby inhibiting DNA replication and RNA polymerase activity, eventually leading to cell death. The HSV-TK/GCV strategy has demonstrated strong anti-tumor activity in numerous tumor models including ovarian carcinoma, malignant glioma, hepatocellular carcinoma and prostate cancer (17, 30, 118, 121, 126, 136). This system was first tested in OS by Grossin et al. (15). Using a retroviral vector, they were able to sensitize OS cell lines and generate a strong bystander effect, which for this class of compounds was shown to be due to gap junction-mediated diffusion of the toxic drug to neighboring tumor cells. With the use of ex vivo transduced tumor cells inoculated in xenografts on a rat hind limb, they showed that this system reduced the tumor mass and prevented the appearance of lung metastases (15). In addition, Ketola et al. studied the efficacy of adenoviral/lentiviral delivery for HSV-TK/GCV gene therapy in OS cell lines and reported sufficient HSV-TK expression yielding both cytotoxicity and a bystander effect (67). They reported that the cell lines tested were transduced at high efficiency and that HSV-TK expression was comparable to that with adenovirus vectors. However these results were not confirmed with in vivo studies. Although these studies showed anti-tumor efficacy in vitro and in vivo, a major drawback of this approach, from a clinical point of view, was that this anti-tumor strategy was based on a two-step system approach which was highly limiting in the clinical setting.
Bacterial cytosine deaminase
A second enzyme prodrug therapy is based on the conversion of the prodrug 5-FC to the cytotoxic drug 5-fluorouracil (5-FU) by the enzyme cytosine deaminase. 5-FC is deaminated by CD into the cytotoxic drug 5-FU. Administration of 5-FU causes toxic side effects and high doses are required for tumor response. Designing therapies where CD is expressed in localized areas of cancer is attractive as such treatments could deliver very high local concentrations of 5-FU to tumor sites while avoiding toxic systemic levels. CD genes from Escherichia coli and Saccharomyces cerevisiae have been evaluated and found effective in various experimental animal models for enzyme/prodrug therapy of tumors (62, 66, 102, 109). The main advantage of CD/5-FC suicide gene therapy is that no cell-to-cell contact is required for the bystander effect, since 5-FU can diffuse in and out of cells by non-facilitated diffusion. A study of Ramnaraine et al. (120) showed that using this suicide gene therapy approach, bone sarcomas in 9 of 10 treated mice could be eliminated. In addition they studied the efficacy of osteoclast precursor cells transduced with the CD gene as a gene delivery system. Their results indicate that transduced osteoclasts could promote killing of cancer cells and that the bystander effect remained present (119). Despite these encouraging early results it, should be emphasized that in the clinical context of OS this was not seen to be an active agent and other enzyme prodrug therapies were seen to be needed and thus of greater interest.
Carboxylesterase
The third prodrug-converting enzyme that has been incorporated into gene therapeutic strategies is carboxylesterase (CE), which is involved in the conversion of the prodrug CPT-11 to its toxic derivative SN-38. CPT-11 is a semi-synthetic, water-soluble derivative of camptothecin that belongs to the class of topoisomerase I inhibitors. Topoisomerase I acts through stabilization of a drug-induced cleavable complex and formation of irreversible double-stranded DNA breaks which are toxic for the cell. CPT-11 is a prodrug that undergoes de-etherification into the much more potent topoisomerase I inhibitor SN-38. Although SN-38 can be detected in the plasma of cancer patients only minutes after the administration of CPT-11, 90% of the administered CPT-11 is not converted. Conversion of CPT-11 is achieved by carboxylesterase (CE), which has been found in liver and lung (130). Koijma et al. and Wierdl et al. have shown that certain solid tumors could be sensitized to CPT-11 after adenoviral vector mediated CE gene transfer (72, 152). Since conversion of CPT-11 to SN-38 in the extracellular space between cells would allow a larger bystander effect than would be possible when relying only on intracellular conversion alone, Oosterhoff et al. constructed an adenoviral vector encoding a secreted from of CE (sCE). This construct was successfully used on cells originating from various tumor types (111). In the context of OS, adenoviral gene transfer of sCE in combination with CTP-11 treatment delayed OS xenograft growth significantly (113). Since there are no reported clinical data of SN38 as an active agent for OS, the studies reviewed above have shown this approach to be effective only in the preclinical setting. Thus, the efficacy of SN38 has not yet been shown to be beneficial for patients with OS.
Immunopotentiation gene therapy
The third main gene therapeutic approach to osteosarcoma involved strategies to achieve anti-tumor immunity. Although tumors display a variety of tumor-associated antigens that can be recognized by T cells, most of them escape host cellular immune surveillance. Two basic approaches have traditionally been utilized: (i) enhancement of tumor cell recognition; and (ii) augmentation of the innate efficacy of the immune system. In the specific context of OS, recently a comprehensive review has been written on this topic by Mori et al. (101).
Tumor cell recognition can be enhanced by improving tumor antigen presentation by transduction with MHC-class I molecules, or by transferring tumor antigens to antigen presenting (dendritic) cells (36, 105). In the context of OS, research has also focused on enhancing the efficacy of the immune system. Examples of this approach include; genetically modifying T-lymphocytes to enable their receptors to recognize tumor cells more efficiently, or a general boosting of the immune system by introducing genes coding for cytokines and other co-stimulatory molecules (76, 143, 156). The cytokine IL-12 is a key intermediary in numerous immune functions including stimulation of T cells and NK cells and the regulation of several key cell adhesion molecules. Specifically, it promotes the production of IFN-gamma by T and NK cells. The mechanisms underlying this anti-tumor activity are incompletely understood but may be related to the ability of IL-12 to both inhibit angiogenesis as well as stimulate T cells and NK cells. The transduction of OS cells with the IL-12 gene reduced the ability of these cells to form lung metastases in nude mice (76, 156). Aerosol gene therapy with PEI (a liposomal DNA carrier) showed effective gene transfer of IL-12. Mice bearing OS lung metastasis treated with PEI:IL-12 had significant tumor regression (38, 64).
Liebau et al. extensively studied immunotherapy strategies using plasmid-mediated gene transfer. The effect of NK-mediated tumor cell lysis in vitro was studied. The results demonstrated that IL-12 can enhance the expression of ICAM-1 in the presence of IFN-gamma and, with IL-18, enhances NK anti-tumor activity (87). Of the cytokines IL-12, IL-18 and IL-23, the last appeared to be a less effective immuno-therapeutic agent for adjuvant treatment of OS than IL-12 and IL-18 (88).
Co-stimulatory molecules promote T-cell proliferation and cytokine production and thereby induce a more potent anti-tumor immune response. One of the co-stimulatory molecules that are well characterized is B7-1 (CD80). Successful induction of an anti-tumor immune response has been reported in a number of tumor models. Tsuji et al. found that adenovirus-mediated rat B7-1 gene transfer induced expression of B7-1, curative immunity against pre-established primary OS, systemic immunity against pre-established pulmonary metastasis and activation of regional lymph nodes CD4+ T cells (143). In order to increase the efficacy of this approach, a B7-1/Fas chimeric gene was transferred via an adenoviral vector to OS cells. This resulted in expression, activation of T-cells, apoptosis of OS cells and a stronger inhibitory effect on the development of pulmonary metastasis compared to B7-1 alone (142).
Together, the results of above-described gene therapeutic strategies for treatment of OS have yielded promising results. However, efficient gene transfer to tumor cells has proven to be more difficult than originally foreseen. Moreover, gene delivery to non-target tissue with possible resultant side effects remains an issue requiring attention. In addition, in the adenoviral context, it has become apparent that a major determinant of gene transfer efficacy is the expression of the Coxsackie Adenovirus Receptor (CAR) (8), on target cells’ surfaces. It has become evident that CAR expression is limited on tumor cells. This has lead to the practical and biological need to develop new vectors. Strategies to improve these Ad vectors are to target gene delivery to tumor cells thereby also inducing a more tumor specific gene transfer. This field has particular exploded in the context of adenovirus-mediated gene transfer.
Targeting to tumor cells
The broad tropism of adenoviral vectors can be a potential disadvantage, since in cancer gene therapy; more selective gene transfer in specific cell types is warranted. In the context of osteogenesis for example, others have shown the utility of adenovirus-mediated gene therapy in osteoblasts (96, 159, 163). These studies have shown effective adenovirus transfection ex vivo as well as in vivo in normal bone cells, with minimal toxicity. Therefore in order to establish an effective and safe gene therapy approach, tumor selectivity of the gene transfer or expression is a prerequisite. In addition, retargeting allows for the administration of lower vector dose to achieve a similar therapeutic effect while minimizing the direct cytotoxic effects of adenoviral vectors. Further more retargeting might prevent inadvertent adenoviral transduction into antigen-presenting cells and consequently diminish inflammatory responses to the vector (20). This retargeting can be achieved by limiting the infection to the tumor cell (transductional targeting) or by limiting the gene expression to the tumor cell (transcriptional targeting).
Transductional targeting
Transductional targeting involves the modification of viral tropism in order to limit infection to certain cell types, and to improve infection efficiency of cancer cells. This can be achieved by redirecting binding of the vector to molecules highly expressed on tumor cell surfaces. This approach has been most widely applied in the context of Ad, as reviewed by Everts et al. (31). The adenovirus capsid is comprised of three major protein components- the hexon, penton base and fiber/knob. The knob is responsible for binding to the host primary cellular receptor (60). To enter the cell the adenovirus binds via its binding domain (fiber/knob) to the Coxsackie Adenovirus Receptor (CAR) (8). Following viral attachment, internalization is accomplished through cellular αv integrins (cell adhesion molecules) (151). This schematically is depicted in figure 2.
FIGURE 2.
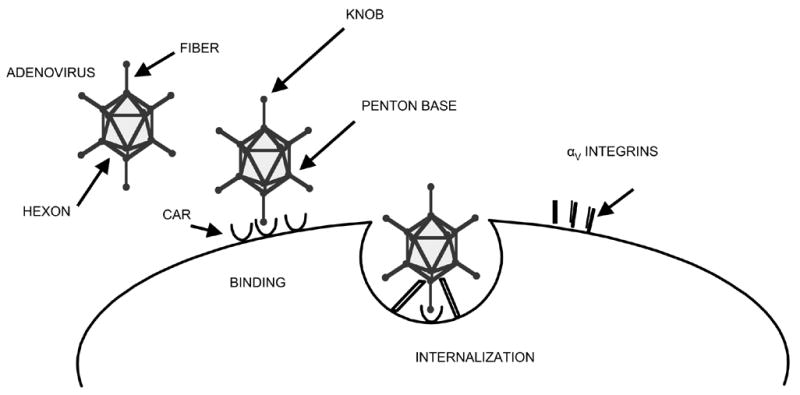
SCHEMATIC ADENOVIRAL BINDING AND INTERNALIZATION VIA CAR AND INTEGRINS
As noted above, studies on various types of tumors have shown that application of adenoviral vectors for cancer gene therapy is hampered by the lack of CAR expression on primary tumor cells (30, 46, 85, 150). The recognition that CAR deficiency is a nearly universal feature of primary tumors has predicated the need for adenoviral vectors capable of CAR independent gene delivery. Furthermore, to enhance the selectivity of transduction of tumor cells, vectors should be targeted towards specific tumor cell receptors. Selectivity can be achieved by using ligands for cellular receptors or by using antibodies against cellular antigens linked to an antibody against the binding domain of the viral vector. Such complexes; known as bispecific antibodies, were first described by Douglas et al. (30). An alternative approach was presented by Krasnyck et al. (73), who incorporated the targeting peptide Arg-Gly-Asp (RGD-4C) into the HI loop of the fiber knob of the adenoviral vector. This peptide sequence is known to interact strongly with αv integrins and RGD-4C modified adenoviral vectors demonstrated highly efficient infection efficiencies on various tumors (23, 78). These strategies are depicted in figure 3.
FIGURE 3.
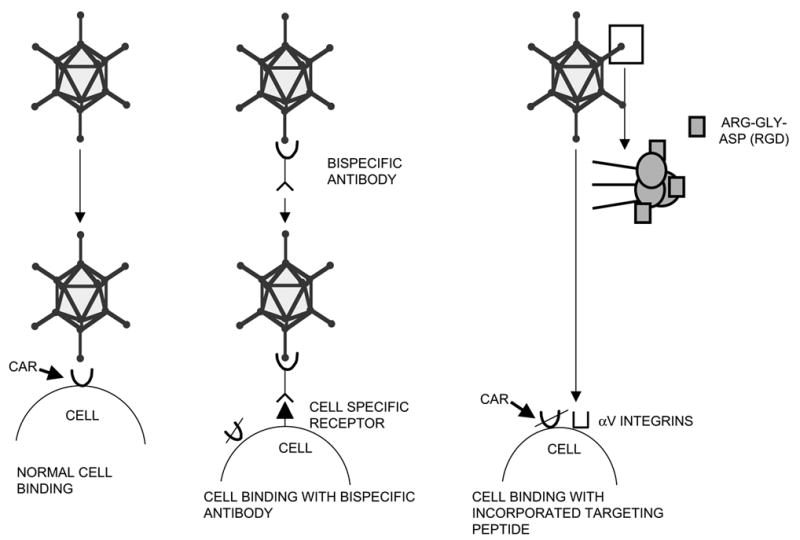
SCHEMATIC ADENOVIRAL CELL BINDING
Of the tumor cell receptors, the epidermal growth factor receptor (EGFR) has been studied extensively in the context of redirecting Ad. EGFR is over expressed in a large number of tumors, including head and neck, breast, colorectal, lung, prostate, kidney, ovary, brain, pancreas and bladder carcinomas (69, 99, 100). Targeting adenoviral vectors towards EGFR was achieved by using a bispecific single-chain antibody (scFv), which targets adenoviral vectors towards tumor cells that express the EGFR (425-s11). The molecular adapter 425-s11 binds to the knob of the adenovirus and to the EGFR on the (tumor) cell membrane (50) thereby increasing transduction efficiency. A comparable approach used a recombinant soluble form of truncated CAR (sCAR) and a ligand targeting EGFR (28). This approach was found to be effective in meningioma, bladder cancer, pancreatic cancer, squamous cell carcinoma and glioma (10, 27, 46, 147, 150).
Primary OS are also known to have low or absent expression levels of CAR whereas EGFR is widely expressed on OS. (44, 155). Ad targeting to EGFR using bispecific 425-s11 improved gene transfer to a panel of primary patient-derived OS cells 10 fold. Moreover, we have shown that enhanced infectability translates to improved cell killing. Using EGFR-targeted suicide gene therapy with an adenoviral vector containing the HSV-TK gene under control of the cytomegalovirus (CMV) promoter (AdCMVHSV-TK) in combination with ganciclovir enhanced the anti-tumor effect on OS cell lines and primary short-term cultures compared to the non targeted AdCMVHSV-TK (155).
In addition, we showed that the ανβ3 and ανβ5 integrins are expressed abundantly on OS cells and that insertion of an RGD integrin-targeting motif into the adenovirus fiber knob augmented adenovirus infection of primary OS cells by two orders of magnitude. (154). Alternative molecules that have been proven as useful for redirecting adenoviral attachment and entry and which may be of potential interest to targeting OS, include fibroblast growth factor receptor (47), folate receptor (30), transferrin receptor (157) and vascular endothelial growth factor receptor (34). Targeting towards the transferrin receptor was shown to be effective in OS: Nakase et al. studied liposome-p53 gene delivery targeted towards transferrin in vitro and in vivo and demonstrated reduced tumor growth of established xenografts (103).
Transcriptional targeting
Modulating gene expression may also be achieved by delivering genes under the control of an appropriate tissue- or tumor cell type-specific promoter. Several tissue and tumor specific promoters have been reported (reviewed by Haviv et al. (54) such as Survivin, COX-2 and PSA. In OS, the osteocalcin (OC) promoter is of particular interest. OC is a major noncollagenous bone protein whose expression is limited almost exclusively to osteogenic tumors and mature calcified tissue. Osteocalcin is expressed in several solid tumors, including OS (71). The efficacy and specificity of OC promoter-driven therapeutic gene constructs for treatment of OS were shown in vitro (18) as well as in vivo (18, 132). In the latter case, 80% of animals bearing subcutaneous OS xenografts that were treated with an adenovirus encoding HSV-TK under the OC promoter (Ad-OC-Tk) survived, and survival rates were 100% when this therapeutic approach was combined with metrotrexate (18). Furthermore, Ad-OC-TK in combination with ACV was also effective in treating OS lung metastatic disease in mice, as treated animals showed a significant decreased number of tumor nodules, decreased net lung weight, and significantly increased survival rates (132). Of note is the finding of Pollmann et al. (116) that OS-specific promoters should not only be evaluated for the target cells, but also for neighboring normal tissues. Barnet et al (5) combined transductional and transcriptional targeting by using a bispecific antibody conjugate redirecting towards EGFR and placing the reporter gene under the control of osteocalcin. They demonstrated a higher level of specificity for cancer cells (including OS cell lines) than either approach alone. Despite promising results as described above, OS being a highly mixed morphologic tumor with an unstable and variably mineralized matrix, it is not surprising that OC gene transcription has been shown to be extremely variable. These data should further be interpreted with caution since OC in the cell lines studied was highly expressed whereas, in general, in primary OS expression of OC is generally low. Therefore, the cell lines used in the studies discussed above are not truly representative of the tumor cells in the in vivo situation. Therefore studies using alternative and more tumor specific promoters of the in situ condition are warranted.
Virotherapy
The main difficulties encountered in solid tumor therapy with replication-incompetent viruses, has been the inability to efficiently disperse and infect the majority of tumor cells within the tumor mass. Replication and spread of a virus within a solid tumor could overcome the problem of inefficient cancer cell infection seen with non-replicating viruses. Since viral replication leads to amplification of the input dose of the virus, after tumor cell lysis, these progeny viruses will spread and infect surrounding cells. Several rounds of replication will thus eventually destroy the tumor. This is schematically depicted in figure 4.
Figure 4.
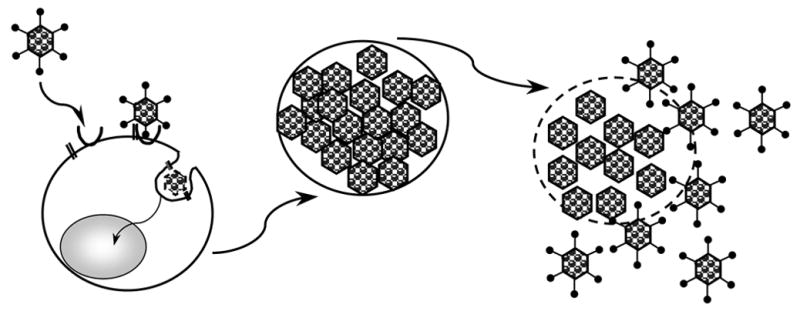
schematic presentation of CRAd infection, replication tumor cell lysis and lateral spread
This idea of using replication competent viruses for treatment of cancer, originates from the 1950’s. Oncolytic viral vectors based on adenovirus, herpes virus, poliovirus, measles and reovirus have all been described as therapeutic agents for various malignancies. In particular, oncolytic adenoviruses have received widespread attention and were rapidly translated from pre-clinical to clinical testing. (69). Oncolytic adenoviruses, or conditionally replicating adenoviruses (CRAds) with tumor specificity, have been obtained in two ways; 1) by deletions in viral genes encoding proteins that interact with cellular proteins necessary to complete the viral lytic cycle in normal cells, but not in tumor cells, and 2) by placing the control of early viral genes under a tumor specific promoter.
Strategies to Increase the oncolytic potency of CRAds has led to development of a new generation of oncolytic viruses. These generations of oncolytic viruses are combining the cytotoxic effects of the lytic cycle with the delivery of transgenes with established anti-tumor efficacy and are known as armed therapeutic viruses.
Deletion of essential early genes
In the first approach, specific mutations or deletions are made in the E1 region of the adenoviral genome. This region contains two genes, E1A and E1B, which encode proteins required for a productive adenovirus lytic cycle. Bischoff et al. were the first to describe the construction of a CRAd based on a deletion of the E1B-55kDa gene (9). This gene product was known to bind p53 (in complex with E4orf6), leading to p53 inhibition and/or degradation. In theory, E1B-55 kDa-deleted adenoviruses such as dl1520 (Onyx-015) would not be able to inactivate p53 in normal cells and, as a result, the viral replication cycle would not be completed (56). In cancer cells lacking normal p53 function, however, replication was predicted to proceed. In this way, replication would be restricted to tumor cells. It now appears that this is not the mechanism by which ONYX-015 achieves selective replication capacity in malignant cells [101–102]. Nevertheless, this oncolytic adenovirus proved to be very effective in a wide range of preclinical models for various tumor types, including cervical carcinoma, laryngeal carcinoma, colorectal carcinoma, head and neck cancer, and glioma, with reports on complete regression of xenografts tumors and long-term survival (9, 42, 56, 57).
Employing a similar approach, adenoviruses were engineered to replicate selectively in tumor cells with lesions in the retinoblastoma tumor repressor (pRb) pathway. This was accomplished by deleting 24 bp from the E1A gene, which abolishes the pRb binding capacity of the E1A protein (38, 55). This adenovirus mutant, known as AdΔ24 or Addl922-947, demonstrated greatly reduced replication in non-proliferating normal cells relative to other gene-deleted and wild type adenoviruses. The anti-cancer efficacy of these agents was confirmed in vitro and in heterotopic xenograft animal models of uman glioma and breast cancer (38, 55). Of these first generation CRAds only AdΔ24 was studied in the context of OS. Infection of human OS cell lines, primary cell cultures as well as subcutaneous OS xenografts with AdΔ24 demonstrated limited anti-tumor activity at low-doses of the virus. However, when tropism of AdΔ24 was expanded to αv integrins by capsid insertion of the Arg-Gly-Asp motif (Ad5-Δ24RGD), OS cells were killed highly efficiently. Furthermore, intratumoral injections with Ad5-Δ24RGD into established human OS xenografts derived directly from a patient with OS, caused a significant tumor growth delay [88].
Promoter-driven viral replication
Additional means to achieve tumor selectivity is by transcriptionally regulating the expression of essential early viral genes by using tumor specific promoters. Conditional replication may be enforced on the virus by placing the transcription of replication-essential genes under the control of tumor-specific promoters (133). The first CRAd developed via this approach was CN706 (CV706) that specifically replicates in prostate-specific antigen (PSA) expressing cells (125). Based on the same principle, CRAds with the viral essential genes under the COX-2 promoter, the human tyrosine promoter, the midkine promoter and the hTERT promoter demonstrated efficacy in respectively pancreas cancer and gastric cancer (110, 160), metastatic melanoma (106) pediatric solid tumors (2) and small cell lung cancer (144). The hTERT promoter-driven CRAd is a possible candidate for OS as telomerase expression is frequently observed in OS and correlates with a poor prognosis (129).
Another CRAd, which embodies this principle, and of special interest for OS, uses the bone matrix osteocalcin (OC) promoter to drive expression of E1A (AdOC E1a) (95). Another example of one such replicative agent is the OCaP1; this is a CRAd with the essential genes under the control of OC (7). In 2001, a phase I/II dose escalation and activity study of intravenous injections of OCaP1 for subjects with refractory OS metastases to the lung was proposed. Yamamura et al. exploited this concept to develop a conditionally replicating herpes vector. The calponin (expressed in OS) promoter drives expression of ICP4, a major trans-activating factor for viral genes. In vivo data demonstrated that this vector promotes oncolysis in tumor cells and spares normal vascular smooth muscle cells, Furthermore, mice treated with this vector showed replication in the distant non-treated tumor (161). In order to study oncolytic virotherapy in a large animal model, we (59) developed a nonhuman canine CRAd. Since OS in dogs is virtually identical to human OS this is a valuable model to increase our understanding of sarcomas translate CRAd effectivety to the human situation and provides a unique immunocompetent context. We have advanced our work in this area by demonstrating that chimeric fiber derived from both Canine Adenovirus Types 1 and 2-display novel tropism (43, 137). Furthermore, we have shown that the canine adenovirus can be fluorescently tagged for use in imaging studies (81) and that novel constructs result in infectivity enhancement of canine OS cells (82). Lastly, we have discovered that human type 5 Ad can engage in productive replication in canine OS cells, suggesting cross-species CRAd therapy may be possible for OS (139).
Armed Therapeutic viruses
To enhance therapeutic efficacy, “armed” replicative viruses (ATV), incorporating therapeutic transgenes, have been introduced for cancer gene therapy. These ATVs embody two potential advantages. First, the gene product can enhance the oncolytic capacity; second the CRAd has a very potent level of transgene expression relative to the replication-defective vector counterparts. However, premature cell death during viral DNA replication compromises virus production, therefore timing of apoptosis induction is essential. Increased oncolytic capacity has been shown by van Beusechem et al. The expression of p53 in the E3 region (AdΔ24-p53) exhibited enhanced oncolytic potency compared to the control vector on a majority of tested tumor cell lines and xenografts models (41, 146). Sauthoff et al. constructed a CRAd that expressed p53 during the late replication phase of an Ad without E3 and showed that the CRAd expressing p53 compared to a wild type CRAd was more effective in tumor cell killing. The approach, which combined oncolytic properties of a CRAd with an anticancer therapeutic gene, was first exploited in the context of HSV-TK in combination with ganciclovir. This approach was shown to be effective and increased oncolytic potency (153). In addition Conrad et al. showed that the expression of CD (Δ24-hyCD) in combination with 5-FC resulted in improved oncolytic tumor activity compared to the CRAd therapy alone (21). Furthermore, Oosterhoff et al. designed a CRAd Ad5-Δ24.E3-sCE2, which in combination with CPT-11 showed augmented cytotoxicity against colon cancer cell lines. However, low concentrations of CPT-11 inhibited propagation (112). Based on these observations we showed with CE/CPT-11 in a non-replicative viral vector against OS, that this might be a promising agent against these tumor cells. The fact that increased oncolytic capacity should not interfere with viral propagation has led to the development of ATVs expressing transgenes with an indirect anti-tumor activity. An example of those ATVs are Ad5-Δ24TIMP-3 which showed enhanced oncolytic activity on a panel of primary cell cultures and two glioma cell lines compared with the control oncolytic virus AdΔ24Luc (77).
Toxicity and delivery
Gene therapy is regarded by many as a potential revolution in medicine. This is because gene therapy initially aimed at treating or eliminating the cause of the disease whereas other strategies often treat only the symptoms, signs or sequelae. Thus, gene therapy was received with enormous optimism, suggesting it was a curative strategy for all kinds of diseases including cancer. However, since its introduction several draw backs have hampered progress and acceptance into main-stream therapy. Chief among these is vector related toxicity. A prerequisite for clinical trials is the assurance that appropriate safety standards are met. In close relation to safety issues is the activation of innate immunity to some of the vectors described above (20). Apart from inducing an acute inflammatory response immunologic stimulation may drastically limit vector transduction efficiency and the duration of transgene expression. Furthermore, availability problems of vectors can be due to lack of no specificity of targeting to tissues, insolubility in water, inability to cross transport barriers in target tissue and rapid clearance from the systemic circulation
Gene delivery
The efficacy of gene therapy is not only determined by the efficiency of the vector itself but also by the efficiency of vector delivery to tumor cells. To date several routes of delivery have been evaluated, including intratumorally, intraperitoneally, intra-arterially and intravenously. Initially, clinical trials were limited to cancers for which intra tumoral injection was feasible in order to maximize safety. In this regard, head a neck cancers and prostate cancer were studied extensively (26, 68) (104). Next, intraperitoneal injections were performed in patients with ovarian carcinoma (148). Since this appeared safe and feasible the next step of intravascular administration was contemplated. The feasibility of vascular delivery to tumors was “unknown territory” due to the innate immunity and neutralizing antibodies in the context of adenovirus and adeno-associated virus. Due to ubiquitous nature of adenovirus, a majority of the human population is exposed to adenoviruses leading to the development of an adenoviral specific immune response (53). Pre-existing immunity against adenovirus would theoretically significantly reduce the uptake of the vector. In addition, the cellular immune response, mediated through adenoviral specific CD8 positive T cells, eliminate the cells’ expressing viral and transgene products (24). Overall the undesirable acute immune response to viral proteins was considered the main drawback of virus-based gene therapy (13, 14, 16).
In the context of OS, delivery of gene therapy was seen in the perspective of two problems; destruction of the primary tumor and the need to successfully block or destroy lung metastasis. Since pulmonary metastasis is the key determinant of mortality from OS, delivery, it was rationalized, should be adapted to target these lesions. Further, the nature of these warranted intra-vascular administration. Alternatively, in the context of pulmonary disease aerosolized gene therapy was seen as an attractive administration route (80). The advantages of this form of administration are the non-invasive means for targeted delivery to the majority of the lung parenchyma; moreover this route of administration was shown to be capable of delivering a high dose to the target site. A final potential advantage of this administration route is the belief that this would result in fewer adverse effectors compared to intra-venous delivery (80).
Since delivery strategies warrant the availability of representative animal models, two of these should be mentioned. The group of Kleinerman et al. has developed an OS lung metastasis model in which delivery methods and efficacy can be studied extensively (65). Recently in this model the CRAd Ad-OC-E1a construct was evaluated and showed a significant decrease in tumor nodules. Moreover, normal lung tissue was not affected when using this intravenous administrated vector (84). In addition, the canine model developed by us (59) should be a valuable model to study delivery in an immune-competent context as canine OS bears a striking resemblance to human OS and thus will be a useful model for testing gene therapy approaches. Wold and his colleagues have also present evidence for yet another (immunologically intact) animal model to examine adenoviral vectors. In conclusion, delivery methods for OS gene therapy are being developed and pre- clinical evaluation in representative animal models is ongoing and should lead to significant advances in the not to distant future.
Safety issues
The tragic death of an 18 year old patient on an early adenoviral gene therapy trial for transcabamylase deficiency raised serious concerns about the safety of that vector in the context of human disease. From that period Reid et al. summarized the clinical utility of intravascular delivery in cancer patients (123). Based on those studies, it became clear that adenoviruses could be administrated into the bloodstream at doses that result in gene transduction/replication. In the case of the young adult patient it became clear that he experienced an acute immune response to the Ad because of a previous infection. More recently other deaths have been reported including an unexplained gene-therapy trial death which raised new questions as one of three individuals enrolled in a trial to treat the rare immunodeficiency disease, chronic granlomatous disease died due to septic shock leading to organ failure (1, 45).
Next to these tragic events, one of the first success of gene therapy also brought a major tragedy as was reported in 2000 when a group in Paris succeeded in totally correcting Severe Combined Immune Deficiency using a retroviral vector in children, but 2 of the treated children later developed leukemia (49). SCID is characterized by a total lack of T lymphocytes and natural killer cells, which normally defends the body against infections. The disease was corrected by using a retrovirus containing the normal gene transferred to the patient blood cells which were re-infused. In short, the mechanism of the leukemiogenesis was due to the fact that the integrating normal gene was inserted near an oncogene. In fact, this normal gene could effectively act as an oncogene under the control of the retroviral promoter, further suggesting that the transduced cells were only one mutation away from tumor development. It has thus become clear that gene therapy based therapies have unlocked the potential of treating fatal hereditary disorders heretofore considered untreatable and offers enhanced hope for successful treatment of more complex diseases including OS. However, this carries with it grave ethical questions and obligations concerning the well being of the patient, the impacted family members, as well as the clinical investigators. A comprehensive review on this topic has been written by Arkin et al. (3). Furthermore, in order to improve the clinical application and safety of gene therapy additional research has been focused on reducing or evading the immune response and enhancing target cell transduction. These strategies have been recently reviewed by Wang et al.(149).
Conclusions and perspectives
The main challenge to developing novel gene therapies including oncolytic virology therapies for OS will be to combine the expanding knowledge and techniques of molecular biology with modern evidence-based medical anti-tumor treatments. The understanding of OS biology by array data offers the hope of customized information for each tumor, thereby increasing the potential of tumor-specific treatment strategies. The development of valid animal tumor models (most especially the dog) should provide the much-needed means to study new treatment options in a pre-clinical setting. However, it should be recognized there are important limitations regarding the in vitro and in vivo models discussed here. The chromosomal and molecular genetic aberrations in OS are underrepresented in cell culture and xenograft animal models. Therefore, translation of these results to the clinical situation should be performed with reticence. In addition, the natural course of metastatic OS (primarily but exclusively to the lung) represent the clinical challenge. Despite the advent of lung metastatic models (65) the problem of micrometastasis in the clinical situation cannot yet be sufficiently mirrored. Given these concerns, data presented in the in vitro and in vivo studies described in this article may only be partial translatable to the human condition.
To assess the value of a new treatment modality, it will remain useful and adventagous in the foreseeable future to investigate its efficacy in combination with conventional anticancer agents that constitute standard therapy. This is particularly relevant for clinical development. Conventional chemotherapy for OS often includes cisplatin and doxorubicin. A synergistic anti-tumor effect of CRAds with doxorubicin or cisplatin has been reported previously for other types of cancer (56, 86, 117, 162) and CRAd therapy combined with chemotherapy has already shown promising results in solid tumors in phase I and II clinical trials (68, 79, 122). Furthermore, the recognition that tumor cells develop genetic mechanisms that override apoptosis caused by chemotherapy damage to their DNA dictates the finding of alternate pathways that would complement therapeutic induction of apoptosis. Therefore, the development of near future clinical trials will be that of gene therapy in combination with chemotherapy, radiation therapy and conventional surgery. The ultimate goal will be to prolong and improve the survival of patients with OS. Despite the fact that the pre-clinical studies discussed above have not yet led to development of advanced human clinical trials, the efforts in this field of cancer research will eventually result in progression towards clinical application for OS. Especially in the context of a rare tumor type such as OS this will surely be in the setting of intense collaboration and continuous joint efforts between scientists and clinicians to complement current treatment strategies.
Acknowledgments
M.A. Witlox is supported by the Netherlands Organization of Scientific Research (NOW-AGIKO 920-03-167). D.T. Curiel is supported by grant NIH-R01CA093796 from the National Cancer Institute, USA. G.P. Siegal is supported by NIH CA93796, CA098543 CA098543 and Haley’s Hope Memorial Support Fund for Osteosarcoma Research at the University of Alabama at Birmingham, USA.
Footnotes
Conflict of Interest
All authors have declared that they have no real or potential conflict of interest associated with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott A. Questions linger about unexplained gene-therapy trial death. Nat Med. 2006;12:597. doi: 10.1038/nm0606-597a. [DOI] [PubMed] [Google Scholar]
- 2.Adachi Y, Reynolds PN, Yamamoto M, Wang M, Takayama K, Matsubara S, Muramatsu T, Curiel DT. A midkine promoter-based conditionally replicative adenovirus for treatment of pediatric solid tumors and bone marrow tumor purging. Cancer Res. 2001;61:7882–8. [PubMed] [Google Scholar]
- 3.Arkin LM, Sondhi D, Worgall S, Suh LH, Hackett NR, Kaminsky SM, Hosain SA, Souweidane MM, Kaplitt MG, Dyke JP, Heier LA, Ballon DJ, Shungu DC, Wisniewski KE, Greenwald BM, Hollmann C, Crystal RG. Confronting the issues of therapeutic misconception, enrollment decisions, and personal motives in genetic medicine-based clinical research studies for fatal disorders. Hum Gene Ther. 2005;16:1028–36. doi: 10.1089/hum.2005.16.1028. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, Manfrini M, Galletti S. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14:1126–34. doi: 10.1093/annonc/mdg286. [DOI] [PubMed] [Google Scholar]
- 5.Barnett BG, Tillman BW, Curiel DT, Douglas JT. Dual targeting of adenoviral vectors at the levels of transduction and transcription enhances the specificity of gene expression in cancer cells. Mol Ther. 2002;6:377–85. doi: 10.1006/mthe.2002.0670. [DOI] [PubMed] [Google Scholar]
- 6.Benassi MS, Molendini L, Gamberi G, Sollazzo MR, Ragazzini P, Merli M, Magagnoli G, Sangiorgi L, Bacchini P, Bertoni F, Picci P. Altered G1 phase regulation in osteosarcoma. Int J Cancer. 1997;74:518–22. doi: 10.1002/(sici)1097-0215(19971021)74:5<518::aid-ijc7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin R, Helman L, Meyers P, Reaman G. A phase I/II dose escalation and activity study of intravenous injections of OCaP1 for subjects with refractory osteosarcoma metastatic to lung. Hum Gene Ther. 2001;12:1591–3. [PubMed] [Google Scholar]
- 8.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell JL, Miller CR, Douglas JT, Li H, Reynolds PN, Carroll WR, Peters GE, Strong TV, Curiel DT. Retargeting to EGFR enhances adenovirus infection efficiency of squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1999;125:856–63. doi: 10.1001/archotol.125.8.856. [DOI] [PubMed] [Google Scholar]
- 11.Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am J Dis Child. 1954;88:754–8. [PubMed] [Google Scholar]
- 12.Bonnet MC, Tartaglia J, Verdier F, Kourilsky P, Lindberg A, Klein M, Moingeon P. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol Lett. 2000;74:11–25. doi: 10.1016/s0165-2478(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 13.Bowers WJ, Olschowka JA, Federoff HJ. Immune responses to replication-defective HSV-1 type vectors within the CNS: implications for gene therapy. Gene Ther. 2003;10:941–5. doi: 10.1038/sj.gt.3302047. [DOI] [PubMed] [Google Scholar]
- 14.Brown BD, Lillicrap D. Dangerous liaisons: the role of “danger” signals in the immune response to gene therapy. Blood. 2002;100:1133–40. doi: 10.1182/blood-2001-11-0067. [DOI] [PubMed] [Google Scholar]
- 15.Charissoux JL, Grossin L, Leboutet MJ, Rigaud M. Treatment of experimental osteosarcoma tumors in rat by herpes simplex thymidine kinase gene transfer and ganciclovir. Anticancer Res. 1999;19:77–80. [PubMed] [Google Scholar]
- 16.Chen D, Murphy B, Sung R, Bromberg JS. Adaptive and innate immune responses to gene transfer vectors: role of cytokines and chemokines in vector function. Gene Ther. 2003;10:991–8. doi: 10.1038/sj.gt.3302031. [DOI] [PubMed] [Google Scholar]
- 17.Cheon J, Kim HK, Moon DG, Yoon DK, Cho JH, Koh SK. Adenovirus-mediated suicide-gene therapy using the herpes simplex virus thymidine kinase gene in cell and animal models of human prostate cancer: changes in tumour cell proliferative activity. BJU Int. 2000;85:759–66. doi: 10.1046/j.1464-410x.2000.00516.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheon J, Ko SC, Gardner TA, Shirakawa T, Gotoh A, Kao C, Chung LW. Chemogene therapy: osteocalcin promoter-based suicide gene therapy in combination with methotrexate in a murine osteosarcoma model. Cancer Gene Ther. 1997;4:359–65. [PubMed] [Google Scholar]
- 19.Chi SN, Conklin LS, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R. The patterns of relapse in osteosarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2004;42:46–51. doi: 10.1002/pbc.10420. [DOI] [PubMed] [Google Scholar]
- 20.Chuah MK, Collen D, VandenDriessche T. Biosafety of adenoviral vectors. Curr Gene Ther. 2003;3:527–43. doi: 10.2174/1566523034578140. [DOI] [PubMed] [Google Scholar]
- 21.Conrad C, Miller CR, Ji Y, Gomez-Manzano C, Bharara S, McMurray JS, Lang FF, Wong F, Sawaya R, Yung WK, Fueyo J. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12:284–94. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 22.Craig C, Kim M, Ohri E, Wersto R, Katayose D, Li Z, Choi YH, Mudahar B, Srivastava S, Seth P, Cowan K. Effects of adenovirus-mediated p16INK4A expression on cell cycle arrest are determined by endogenous p16 and Rb status in human cancer cells. Oncogene. 1998;16:265–72. doi: 10.1038/sj.onc.1201493. [DOI] [PubMed] [Google Scholar]
- 23.Cripe TP, Dunphy EJ, Holub AD, Saini A, Vasi NH, Mahller YY, Collins MH, Snyder JD, Krasnykh V, Curiel DT, Wickham TJ, DeGregori J, Bergelson JM, Currier MA. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61:2953–60. [PubMed] [Google Scholar]
- 24.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–10. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 25.Densmore CL, Kleinerman ES, Gautam A, Jia SF, Xu B, Worth LL, Waldrep JC, Fung YK, T’Ang A, Knight V. Growth suppression of established human osteosarcoma lung metastases in mice by aerosol gene therapy with PEI-p53 complexes. Cancer Gene Ther. 2001;8:619–27. doi: 10.1038/sj.cgt.7700343. [DOI] [PubMed] [Google Scholar]
- 26.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, Hamper U, DeJong R, Detorie N, Rodriguez R, Haulk T, DeMarzo AM, Piantadosi S, Yu DC, Chen Y, Henderson DR, Carducci MA, Nelson WG, Simons JW. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–72. [PubMed] [Google Scholar]
- 27.Dirven CM, Grill J, Lamfers ML, Van der Valk P, Leonhart AM, Van Beusechem VW, Haisma HJ, Pinedo HM, Curiel DT, Vandertop WP, Gerritsen WR. Gene therapy for meningioma: improved gene delivery with targeted adenoviruses. J Neurosurg. 2002;97:441–9. doi: 10.3171/jns.2002.97.2.0441. [DOI] [PubMed] [Google Scholar]
- 28.Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–84. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorfman Bone tumors. St. louis: Mosby; 1997. [Google Scholar]
- 30.Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–8. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 31.Everts M, Curiel DT. Transductional targeting of adenoviral cancer gene therapy. Curr Gene Ther. 2004;4:337–46. doi: 10.2174/1566523043346372. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari S, Bacci G, Picci P, Mercuri M, Briccoli A, Pinto D, Gasbarrini A, Tienghi A, Brach del Prever A. Long-term follow-up and post-relapse survival in patients with non-metastatic osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Ann Oncol. 1997;8:765–71. doi: 10.1023/a:1008221713505. [DOI] [PubMed] [Google Scholar]
- 33.Feugeas O, Guriec N, Babin-Boilletot A, Marcellin L, Simon P, Babin S, Thyss A, Hofman P, Terrier P, Kalifa C, Brunat-Mentigny M, Patricot LM, Oberling F. Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. J Clin Oncol. 1996;14:467–72. doi: 10.1200/JCO.1996.14.2.467. [DOI] [PubMed] [Google Scholar]
- 34.Fischer A. Gene therapy: some results, many problems to solve. Cell Mol Biol (Noisy-le-grand) 2001;47:1269–75. [PubMed] [Google Scholar]
- 35.Florenes VA, Maelandsmo GM, Forus A, Andreassen A, Myklebost O, Fodstad O. MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst. 1994;86:1297–302. doi: 10.1093/jnci/86.17.1297. [DOI] [PubMed] [Google Scholar]
- 36.Frolkis M, Fischer MB, Wang Z, Lebkowski JS, Chiu CP, Majumdar AS. Dendritic cells reconstituted with human telomerase gene induce potent cytotoxic T-cell response against different types of tumors. Cancer Gene Ther. 2003;10:239–49. doi: 10.1038/sj.cgt.7700563. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs N, Winkler K. Osteosarcoma. Curr Opin Oncol. 1993;5:667–71. doi: 10.1097/00001622-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti- glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 39.Gallardo D, Drazan KE, McBride WH. Adenovirus-based transfer of wild-type p53 gene increases ovarian tumor radiosensitivity. Cancer Res. 1996;56:4891–3. [PubMed] [Google Scholar]
- 40.Ganjavi H, Gee M, Narendran A, Parkinson N, Krishnamoorthy M, Freedman MH, Malkin D. Adenovirus-mediated p53 gene therapy in osteosarcoma cell lines: sensitization to cisplatin and doxorubicin. Cancer Gene Ther. 2005 doi: 10.1038/sj.cgt.7700909. [DOI] [PubMed] [Google Scholar]
- 41.Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Terrier-Lacombe MJ, Bressac De-Paillerets B, Barrois M, Feunteun J, Kirn DH, Vassal G. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–72. [PubMed] [Google Scholar]
- 42.Geoerger B, Vassal G, Opolon P, Dirven CM, Morizet J, Laudani L, Grill J, Giaccone G, Vandertop WP, Gerritsen WR, van Beusechem VW. Oncolytic activity of p53-expressing conditionally replicative adenovirus AdDelta24-p53 against human malignant glioma. Cancer Res. 2004;64:5753–9. doi: 10.1158/0008-5472.CAN-04-0499. [DOI] [PubMed] [Google Scholar]
- 43.Glasgow JN, Kremer EJ, Hemminki A, Siegal GP, Douglas JT, Curiel DT. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology. 2004;324:103–16. doi: 10.1016/j.virol.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Graat HC, Wuisman PI, van Beusechem VW, Carette JE, Gerritsen WR, Bras J, Schaap GR, Kaspers GJ, Ogose A, Gu W, Kawashima H, Hotta T. Coxsackievirus and adenovirus receptor expression on primary osteosarcoma specimens and implications for gene therapy with recombinant adenoviruses. Clin Cancer Res. 2005;11:2445–7; author reply 2447–8. doi: 10.1158/1078-0432.CCR-04-2375. [DOI] [PubMed] [Google Scholar]
- 45.Grez M. Gene therapy was declared a success. However, one patient was already dead. MMW Fortschr Med. 2006;148:16. [PubMed] [Google Scholar]
- 46.Grill J, Van Beusechem VW, Van Der Valk P, Dirven CM, Leonhart A, Pherai DS, Haisma HJ, Pinedo HM, Curiel DT, Gerritsen WR. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin Cancer Res. 2001;7:641–50. [PubMed] [Google Scholar]
- 47.Gu Z, Kuntz-Simon G, Rommelaere J, Cornelis J. Oncogenic transformation-dependent expression of a transcription factor NF-Y subunit. Mol Carcinog. 1999;24:294–9. doi: 10.1002/(sici)1098-2744(199904)24:4<294::aid-mc7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 48.Guo W, Wang X, Feng C. P53 gene abnormalities in osteosarcoma. Chin Med J (Engl) 1996;109:752–5. [PubMed] [Google Scholar]
- 49.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval JL, Fraser CC, Cavazzana-Calvo M, Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 50.Haisma HJ, Grill J, Curiel DT, Hoogeland S, van Beusechem VW, Pinedo HM, Gerritsen WR. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 2000;7:901–4. doi: 10.1038/sj.cgt.7700198. [DOI] [PubMed] [Google Scholar]
- 51.Hansen MF, Koufos A, Gallie BL, Phillips RA, Fodstad O, Brogger A, Gedde-Dahl T, Cavenee WK. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985;82:6216–20. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris MP, Sutjipto S, Wills KN, Hancock W, Cornell D, Johnson DE, Gregory RJ, Shepard HM, Maneval DC. Adenovirus-mediated p53 gene transfer inhibits growth of human tumor cells expressing mutant p53 protein. Cancer Gene Ther. 1996;3:121–30. [PubMed] [Google Scholar]
- 53.Harvey BG, Worgall S, Ely S, Leopold PL, Crystal RG. Cellular immune responses of healthy individuals to intradermal administration of an E1-E3- adenovirus gene transfer vector. Hum Gene Ther. 1999;10:2823–37. doi: 10.1089/10430349950016555. [DOI] [PubMed] [Google Scholar]
- 54.Haviv YS, Curiel DT. Conditional gene targeting for cancer gene therapy. Adv Drug Deliv Rev. 2001;53:135–54. doi: 10.1016/s0169-409x(01)00225-3. [DOI] [PubMed] [Google Scholar]
- 55.Heise C, Ganly I, Kim YT, Sampson-Johannes A, Brown R, Kirn D. Efficacy of a replication-selective adenovirus against ovarian carcinomatosis is dependent on tumor burden, viral replication and p53 status. Gene Ther. 2000;7:1925–9. doi: 10.1038/sj.gt.3301319. [DOI] [PubMed] [Google Scholar]
- 56.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–45. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 57.Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999;59:2623–8. [PubMed] [Google Scholar]
- 58.Hellwinkel OJ, Muller J, Pollmann A, Kabisch H. Osteosarcoma cell lines display variable individual reactions on wildtype p53 and Rb tumour-suppressor transgenes. J Gene Med. 2005;7:407–19. doi: 10.1002/jgm.684. [DOI] [PubMed] [Google Scholar]
- 59.Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, Wang M, Desmond RA, Keriel A, Barnett B, Baker HJ, Siegal GP, Curiel DT. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–73. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 60.Henry LJ, Xia D, Wilke ME, Deisenhofer J, Gerard RD. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–46. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Prog Neurobiol. 1998;55:399–432. doi: 10.1016/s0301-0082(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 62.Huber BE, Austin EA, Good SS, Knick VC, Tibbels S, Richards CA. In vivo antitumor activity of 5-fluorocytosine on human colorectal carcinoma cells genetically modified to express cytosine deaminase. Cancer Res. 1993;53:4619–26. [PubMed] [Google Scholar]
- 63.Huvos AG. Diagnosis, treatment and prognosis. Philedelphia: 1991. Bone tumors. [Google Scholar]
- 64.Jia SF, Worth LL, Densmore CL, Xu B, Zhou Z, Kleinerman ES. Eradication of osteosarcoma lung metastases following intranasal interleukin-12 gene therapy using a nonviral polyethylenimine vector. Cancer Gene Ther. 2002;9:260–6. doi: 10.1038/sj.cgt.7700432. [DOI] [PubMed] [Google Scholar]
- 65.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–6. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 66.Kanai F, Lan KH, Shiratori Y, Tanaka T, Ohashi M, Okudaira T, Yoshida Y, Wakimoto H, Hamada H, Nakabayashi H, Tamaoki T, Omata M. In vivo gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by adenovirus-mediated transfer of cytosine deaminase gene. Cancer Res. 1997;57:461–5. [PubMed] [Google Scholar]
- 67.Ketola A, Maatta AM, Pasanen T, Tulimaki K, Wahlfors J. Osteosarcoma and chondrosarcoma as targets for virus vectors and herpes simplex virus thymidine kinase/ganciclovir gene therapy. Int J Mol Med. 2004;13:705–10. [PubMed] [Google Scholar]
- 68.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 69.Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225) Curr Opin Oncol. 2001;13:506–13. doi: 10.1097/00001622-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Klein MJaSGP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125(4):555–81. doi: 10.1309/UC6K-QHLD-9LV2-KENN. [DOI] [PubMed] [Google Scholar]
- 71.Koeneman KS, Kao C, Ko SC, Yang L, Wada Y, Kallmes DF, Gillenwater JY, Zhau HE, Chung LW, Gardner TA. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J Urol. 2000;18:102–10. doi: 10.1007/s003450050181. [DOI] [PubMed] [Google Scholar]
- 72.Kojima A, Hackett NR, Crystal RG. Reversal of CPT-11 resistance of lung cancer cells by adenovirus- mediated gene transfer of the human carboxylesterase cDNA. Cancer Res. 1998;58:4368–74. [PubMed] [Google Scholar]
- 73.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–52. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuznetsov YG, Victoria JG, Low A, Robinson WE, Jr, Fan H, McPherson A. Atomic force microscopy imaging of retroviruses: human immunodeficiency virus and murine leukemia virus. Scanning. 2004;26:209–16. doi: 10.1002/sca.4950260409. [DOI] [PubMed] [Google Scholar]
- 75.Kuznetsov YG, Victoria JG, Robinson WE, Jr, McPherson A. Atomic force microscopy investigation of human immunodeficiency virus (HIV) and HIV-infected lymphocytes. J Virol. 2003;77:11896–909. doi: 10.1128/JVI.77.22.11896-11909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lafleur EA, Jia SF, Worth LL, Zhou Z, Owen-Schaub LB, Kleinerman ES. Interleukin (IL)-12 and IL-12 gene transfer up-regulate Fas expression in human osteosarcoma and breast cancer cells. Cancer Res. 2001;61:4066–71. [PubMed] [Google Scholar]
- 77.Lamfers ML, Gianni D, Tung CH, Idema S, Schagen FH, Carette JE, Quax PH, Van Beusechem VW, Vandertop WP, Dirven CM, Chiocca EA, Gerritsen WR. Tissue inhibitor of metalloproteinase-3 expression from an oncolytic adenovirus inhibits matrix metalloproteinase activity in vivo without affecting antitumor efficacy in malignant glioma. Cancer Res. 2005;65:9398–405. doi: 10.1158/0008-5472.CAN-04-4264. [DOI] [PubMed] [Google Scholar]
- 78.Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, Alemany R, Fueyo J, Curiel DT, Vassal G, Pinedo HM, Vandertop WP, Gerritsen WR. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–42. [PubMed] [Google Scholar]
- 79.Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7:588–92. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- 80.Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy, and vaccination. Respir Care. 2005;50:1161–76. [PubMed] [Google Scholar]
- 81.Le LP, Li J, Ternovoi VV, Siegal GP, Curiel DT. Fluorescently tagged canine adenovirus via modification with protein IX-enhanced green fluorescent protein. J Gen Virol. 2005;86:3201–8. doi: 10.1099/vir.0.80968-0. [DOI] [PubMed] [Google Scholar]
- 82.Le LP, Rivera AA, Glasgow JN, Ternovoi VV, Wu H, Wang M, Smith BF, Siegal GP, Curiel DT. Infectivity enhancement for adenoviral transduction of canine osteosarcoma cells. Gene Ther. 2005 doi: 10.1038/sj.gt.3302674. [DOI] [PubMed] [Google Scholar]
- 83.Li FP, Fraumeni JF, Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, Miller RW. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48:5358–62. [PubMed] [Google Scholar]
- 84.Li X, Jung C, Liu YH, Bae KH, Zhang YP, Zhang HJ, Vanderputten D, Jeng MH, Gardner TA, Kao C. Anti-tumor efficacy of a transcriptional replication-competent adenovirus, Ad-OC-E1a, for osteosarcoma pulmonary metastasis. J Gene Med. 2006;8:679–89. doi: 10.1002/jgm.904. [DOI] [PubMed] [Google Scholar]
- 85.Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP, Wang Z, Hsieh JT. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–30. [PubMed] [Google Scholar]
- 86.Li Y, Yu DC, Chen Y, Amin P, Zhang H, Nguyen N, Henderson DR. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res. 2001;61:6428–36. [PubMed] [Google Scholar]
- 87.Liebau C, Merk H, Schmidt S, Roesel C, Karreman C, Prisack JB, Bojar H, Baltzer AW. Interleukin-12 and interleukin-18 change ICAM-I expression, and enhance natural killer cell mediated cytolysis of human osteosarcoma cells. Cytokines Cell Mol Ther. 2002;7:135–42. doi: 10.1080/13684730310001977. [DOI] [PubMed] [Google Scholar]
- 88.Liebau C, Roesel C, Schmidt S, Karreman C, Prisack JB, Bojar H, Merk H, Wolfram N, Baltzer AW. Immunotherapy by gene transfer with plasmids encoding IL-12/IL-18 is superior to IL-23/IL-18 gene transfer in a rat osteosarcoma model. Anticancer Res. 2004;24:2861–7. [PubMed] [Google Scholar]
- 89.Lindner NJ, Ramm O, Hillmann A, Roedl R, Gosheger G, Brinkschmidt C, Juergens H, Winkelmann W. Limb salvage and outcome of osteosarcoma. The University of Muenster experience. Clin Orthop. 1999:83–9. doi: 10.1097/00003086-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, Ayala A, Shuster J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop. 1991:8–14. [PubMed] [Google Scholar]
- 91.Lonardo F, Ueda T, Huvos AG, Healey J, Ladanyi M. p53 and MDM2 alterations in osteosarcomas: correlation with clinicopathologic features and proliferative rate. Cancer. 1997;79:1541–7. [PubMed] [Google Scholar]
- 92.Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–22. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 93.Marcove RC, Heelan RT, Huvos AG, Healey J, Lindeque BG. Osteoid osteoma. Diagnosis, localization, and treatment. Clin Orthop. 1991:197–201. [PubMed] [Google Scholar]
- 94.Masuda H, Miller C, Koeffler HP, Battifora H, Cline MJ. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987;84:7716–9. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsubara S, Wada Y, Gardner TA, Egawa M, Park MS, Hsieh CL, Zhau HE, Kao C, Kamidono S, Gillenwater JY, Chung LW. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–9. [PubMed] [Google Scholar]
- 96.Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, Gittes GK, Longaker MT. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999;14:1290–301. doi: 10.1359/jbmr.1999.14.8.1290. [DOI] [PubMed] [Google Scholar]
- 97.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–8. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 98.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–8. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 99.Miller CW, Aslo A, Tsay C, Slamon D, Ishizaki K, Toguchida J, Yamamuro T, Lampkin B, Koeffler HP. Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer Res. 1990;50:7950–4. [PubMed] [Google Scholar]
- 100.Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koeffler HP. Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol. 1996;122:559–65. doi: 10.1007/BF01213553. [DOI] [PubMed] [Google Scholar]
- 101.Mori K, Redini F, Gouin F, Cherrier B, Heymann D. Osteosarcoma: current status of immunotherapy and future trends (Review) Oncol Rep. 2006;15:693–700. [PubMed] [Google Scholar]
- 102.Mullen CA, Coale MM, Lowe R, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994;54:1503–6. [PubMed] [Google Scholar]
- 103.Nakase M, Inui M, Okumura K, Kamei T, Nakamura S, Tagawa T. p53 gene therapy of human osteosarcoma using a transferrin-modified cationic liposome. Mol Cancer Ther. 2005;4:625–31. doi: 10.1158/1535-7163.MCT-04-0196. [DOI] [PubMed] [Google Scholar]
- 104.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A, Romel L, Randlev B, Kaye S, Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 105.Nencioni A, Muller MR, Grunebach F, Garuti A, Mingari MC, Patrone F, Ballestrero A, Brossart P. Dendritic cells transfected with tumor RNA for the induction of antitumor CTL in colorectal cancer. Cancer Gene Ther. 2003;10:209–14. doi: 10.1038/sj.cgt.7700557. [DOI] [PubMed] [Google Scholar]
- 106.Nettelbeck DM, Rivera AA, Balague C, Alemany R, Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–70. [PubMed] [Google Scholar]
- 107.Nielsen LL, Dell J, Maxwell E, Armstrong L, Maneval D, Catino JJ. Efficacy of p53 adenovirus-mediated gene therapy against human breast cancer xenografts. Cancer Gene Ther. 1997;4:129–38. [PubMed] [Google Scholar]
- 108.Nielsen LL, Maneval DC. P53 tumor suppressor gene therapy for cancer. Cancer Gene Ther. 1998;5:52–63. [PubMed] [Google Scholar]
- 109.Ohwada A, Hirschowitz EA, Crystal RG. Regional delivery of an adenovirus vector containing the Escherichia coli cytosine deaminase gene to provide local activation of 5-fluorocytosine to suppress the growth of colon carcinoma metastatic to liver. Hum Gene Ther. 1996;7:1567–76. doi: 10.1089/hum.1996.7.13-1567. [DOI] [PubMed] [Google Scholar]
- 110.Ono HA, Davydova JG, Adachi Y, Takayama K, Barker SD, Reynolds PN, Krasnykh VN, Kunisaki C, Shimada H, Curiel DT, Yamamoto M. Promoter-controlled infectivity-enhanced conditionally replicative adenoviral vectors for the treatment of gastric cancer. J Gastroenterol. 2005;40:31–42. doi: 10.1007/s00535-004-1490-y. [DOI] [PubMed] [Google Scholar]
- 111.Oosterhoff D, Pinedo HM, van der Meulen IH, de Graaf M, Sone T, Kruyt FA, van Beusechem VW, Haisma HJ, Gerritsen WR. Secreted and tumour targeted human carboxylesterase for activation of irinotecan. Br J Cancer. 2002;87:659–64. doi: 10.1038/sj.bjc.6600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oosterhoff D, Pinedo HM, Witlox MA, Carette JE, Gerritsen WR, van Beusechem VW. Gene-directed enzyme prodrug therapy with carboxylesterase enhances the anticancer efficacy of the conditionally replicating adenovirus AdDelta24. Gene Ther. 2005;12:1011–8. doi: 10.1038/sj.gt.3302492. [DOI] [PubMed] [Google Scholar]
- 113.Oosterhoff D, Witlox MA, van Beusechem VW, Haisma HJ, Schaap GR, Bras J, Kruyt FA, Molenaar B, Boven E, Wuisman PI, Pinedo HM, Gerritsen WR. Gene-directed enzyme prodrug therapy for osteosarcoma: sensitization to CPT-11 in vitro and in vivo by adenoviral delivery of a gene encoding secreted carboxylesterase-2. Mol Cancer Ther. 2003;2:765–71. [PubMed] [Google Scholar]
- 114.Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. Aaps J. 2005;7:E61–77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Phelan A, Elliott G, O’Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–3. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 116.Pollmann A, Kabisch H, Block A, Muller J, Hellwinkel OJ. Limited specificity of promoter constructs for gene therapy in osteosarcoma. Int J Mol Med. 2004;14:737–42. [PubMed] [Google Scholar]
- 117.Portella G, Scala S, Vitagliano D, Vecchio G, Fusco A. ONYX-015, an E1B gene-defective adenovirus, induces cell death in human anaplastic thyroid carcinoma cell lines. J Clin Endocrinol Metab. 2002;87:2525–31. doi: 10.1210/jcem.87.6.8529. [DOI] [PubMed] [Google Scholar]
- 118.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 119.Ramnaraine M, Pan W, Clohisy DR. Osteoclasts direct bystander killing of cancer cells in vitro. Bone. 2005 doi: 10.1016/j.bone.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 120.Ramnaraine M, Pan W, Goblirsch M, Lynch C, Lewis V, Orchard P, Mantyh P, Clohisy DR. Direct and bystander killing of sarcomas by novel cytosine deaminase fusion gene. Cancer Res. 2003;63:6847–54. [PubMed] [Google Scholar]
- 121.Rancourt C, Rogers BE, Sosnowski BA, Wang M, Piche A, Pierce GF, Alvarez RD, Siegal GP, Douglas JT, Curiel DT. Basic fibroblast growth factor enhancement of adenovirus-mediated delivery of the herpes simplex virus thymidine kinase gene results in augmented therapeutic benefit in a murine model of ovarian cancer. Clin Cancer Res. 1998;4:2455–61. [PubMed] [Google Scholar]
- 122.Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L, Hatfield M, Rubin J, Kirn D. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8:1618–26. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002;9:979–86. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Renard AJ, Veth RP, Schreuder HW, Pruszczynski M, Bokkerink JP, van Hoesel QG, van Der Staak FJ. Osteosarcoma: oncologic and functional results. A single institutional report covering 22 years. J Surg Oncol. 1999;72:124–9. doi: 10.1002/(sici)1096-9098(199911)72:3<124::aid-jso3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–63. [PubMed] [Google Scholar]
- 126.Rosenfeld ME, Feng M, Michael SI, Siegal GP, Alvarez RD, Curiel DT. Adenoviral-mediated delivery of the herpes simplex virus thymidine kinase gene selectively sensitizes human ovarian carcinoma cells to ganciclovir. Clin Cancer Res. 1995;1:1571–80. [PubMed] [Google Scholar]
- 127.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 128.rothmund a. Ueber katarakt in verbindiung mit einer eigentuemlichen haut degeneration. J Arch Ophthalmol. 1887;4 [Google Scholar]
- 129.Sanders RP, Drissi R, Billups CA, Daw NC, Valentine MB, Dome JS. Telomerase expression predicts unfavorable outcome in osteosarcoma. J Clin Oncol. 2004;22:3790–7. doi: 10.1200/JCO.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 130.Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol. 1998;38:257–88. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- 131.Seth P. Vector-mediated cancer gene therapy: an overview. Cancer Biol Ther. 2005;4:512–7. doi: 10.4161/cbt.4.5.1705. [DOI] [PubMed] [Google Scholar]
- 132.Shirakawa T, Ko SC, Gardner TA, Cheon J, Miyamoto T, Gotoh A, Chung LW, Kao C. In vivo suppression of osteosarcoma pulmonary metastasis with intravenous osteocalcin promoter-based toxic gene therapy. Cancer Gene Ther. 1998;5:274–80. [PubMed] [Google Scholar]
- 133.Siders WM, Halloran PJ, Fenton RG. Transcriptional targeting of recombinant adenoviruses to human and murine melanoma cells. Cancer Res. 1996;56:5638–46. [PubMed] [Google Scholar]
- 134.Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R. Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop. 1999:120–7. [PubMed] [Google Scholar]
- 135.Song SU, Boyce FM. Combination treatment for osteosarcoma with baculoviral vector mediated gene therapy (p53) and chemotherapy (adriamycin) Exp Mol Med. 2001;33:46–53. doi: 10.1038/emm.2001.9. [DOI] [PubMed] [Google Scholar]
- 136.Stefani AL, Barzon L, Castagliuolo I, Guido M, Pacenti M, Parolin C, Farinati F, Palu G. Systemic efficacy of combined suicide/cytokine gene therapy in a murine model of hepatocellular carcinoma. J Hepatol. 2005;42:728–35. doi: 10.1016/j.jhep.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 137.Stoff-Khalili MA, Rivera AA, Glasgow JN, Le LP, Stoff A, Everts M, Tsuruta Y, Kawakami Y, Bauerschmitz GJ, Mathis JM, Pereboeva L, Seigal GP, Dall P, Curiel DT. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005;12:1696–706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- 138.Strauss SJ, McTiernan A, Whelan JS. Late relapse of osteosarcoma: implications for follow-up and screening. Pediatr Blood Cancer. 2004;43:692–7. doi: 10.1002/pbc.20154. [DOI] [PubMed] [Google Scholar]
- 139.Ternovoi VV, Le LP, Belousova N, Smith BF, Siegal GP, Curiel DT. Productive replication of human adenovirus type 5 in canine cells. J Virol. 2005;79:1308–11. doi: 10.1128/JVI.79.2.1308-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomson m. A hitherto undescribed familial disease. Br J Dermatol. 1923;135 [Google Scholar]
- 141.Tsuchiya H, Mori Y, Ueda Y, Okada G, Tomita K. Sensitization and caffeine potentiation of cisplatin cytotoxicity resulting from introduction of wild-type p53 gene in human osteosarcoma. Anticancer Res. 2000;20:235–42. [PubMed] [Google Scholar]
- 142.Tsuji H, Kawaguchi S, Wada T, Nagoya S, Inobe M, Yagita H, Okumura K, Yamashita T, Uede T. Concurrent induction of T-cell activation and apoptosis of osteosarcoma cells by adenovirus-mediated B7-1/Fas chimeric gene transfer. Cancer Gene Ther. 2003;10:717–25. doi: 10.1038/sj.cgt.7700624. [DOI] [PubMed] [Google Scholar]
- 143.Tsuji H, Kawaguchi S, Wada T, Nagoya S, Inobe M, Yamashita T, Ishii S, Uede T. Adenovirus-mediated in vivo B7-1 gene transfer induces anti-tumor immunity against pre-established primary tumor and pulmonary metastasis of rat osteosarcoma. Cancer Gene Ther. 2002;9:747–55. doi: 10.1038/sj.cgt.7700491. [DOI] [PubMed] [Google Scholar]
- 144.Uchino J, Takayama K, Harada A, Kawakami Y, Inoue H, Curiel DT, Nakanishi Y. Infectivity enhanced, hTERT promoter-based conditionally replicative adenoviruses are useful for SCLC treatment. Cancer Gene Ther. 2005;12:737–48. doi: 10.1038/sj.cgt.7700838. [DOI] [PubMed] [Google Scholar]
- 145.Dahlin’s Bone Tumors. philedelphia: Lippincott-raven; 1996. Unni General Aspects and data on 11,087 cases; pp. 143–183. [Google Scholar]
- 146.van Beusechem VW, van den Doel PB, Grill J, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165–71. [PubMed] [Google Scholar]
- 147.van der Poel HG, Molenaar B, van Beusechem VW, Haisma HJ, Rodriguez R, Curiel DT, Gerritsen WR. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J Urol. 2002;168:266–72. doi: 10.1097/00005392-200207000-00089. [DOI] [PubMed] [Google Scholar]
- 148.Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S, Kirn DH, O’Neill V, Siddiqui N, Seiden MV, Kaye SB. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–9. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y, Yuan F. Delivery of viral vectors to tumor cells: extracellular transport, systemic distribution, and strategies for improvement. Ann Biomed Eng. 2006;34:114–27. doi: 10.1007/s10439-005-9007-2. [DOI] [PubMed] [Google Scholar]
- 150.Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, Li H, Parameshwar M, Vickers SM, Jaffee EM, Huibregtse K, Curiel DT, Dmitriev I. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–76. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- 151.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–19. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 152.Wierdl M, Morton CL, Weeks JK, Danks MK, Harris LC, Potter PM. Sensitization of human tumor cells to CPT-11 via adenoviral-mediated delivery of a rabbit liver carboxylesterase. Cancer Res. 2001;61:5078–82. [PubMed] [Google Scholar]
- 153.Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–3. [PubMed] [Google Scholar]
- 154.Witlox AM, Van Beusechem VW, Molenaar B, Bras H, Schaap GR, Alemany R, Curiel DT, Pinedo HM, Wuisman PI, Gerritsen WR. Conditionally replicative adenovirus with tropism expanded towards integrins inhibits osteosarcoma tumor growth in vitro and in vivo. Clin Cancer Res. 2004;10:61–7. doi: 10.1158/1078-0432.ccr-0609-03. [DOI] [PubMed] [Google Scholar]
- 155.Witlox MA, Van Beusechem VW, Grill J, Haisma HJ, Schaap G, Bras J, Van Diest P, De Gast A, Curiel DT, Pinedo HM, Gerritsen WR, Wuisman PI. Epidermal growth factor receptor targeting enhances adenoviral vector based suicide gene therapy of osteosarcoma. J Gene Med. 2002;4:510–6. doi: 10.1002/jgm.308. [DOI] [PubMed] [Google Scholar]
- 156.Worth LL, Jia SF, Zhou Z, Chen L, Kleinerman ES. Intranasal therapy with an adenoviral vector containing the murine interleukin-12 gene eradicates osteosarcoma lung metastases. Clin Cancer Res. 2000;6:3713–8. [PubMed] [Google Scholar]
- 157.Xia CQ, Wang J, Shen WC. Hypoglycemic effect of insulin-transferrin conjugate in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2000;295:594–600. [PubMed] [Google Scholar]
- 158.Xu HJ, Zhou Y, Seigne J, Perng GS, Mixon M, Zhang C, Li J, Benedict WF, Hu SX. Enhanced tumor suppressor gene therapy via replication-deficient adenovirus vectors expressing an N-terminal truncated retinoblastoma protein. Cancer Res. 1996;56:2245–9. [PubMed] [Google Scholar]
- 159.Xu XL, Tang T, Dai K, Zhu Z, Guo XE, Yu C, Lou J. Immune response and effect of adenovirus-mediated human BMP-2 gene transfer on the repair of segmental tibial bone defects in goats. Acta Orthop. 2005;76:637–46. doi: 10.1080/17453670510041709. [DOI] [PubMed] [Google Scholar]
- 160.Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, Curiel DT. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–18. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 161.Yamamura H, Hashio M, Noguchi M, Sugenoya Y, Osakada M, Hirano N, Sasaki Y, Yoden T, Awata N, Araki N, Tatsuta M, Miyatake SI, Takahashi K. Identification of the transcriptional regulatory sequences of human calponin promoter and their use in targeting a conditionally replicating herpes vector to malignant human soft tissue and bone tumors. Cancer Res. 2001;61:3969–77. [PubMed] [Google Scholar]
- 162.You L, Yang CT, Jablons DM. ONYX-015 works synergistically with chemotherapy in lung cancer cell lines and primary cultures freshly made from lung cancer patients. Cancer Res. 2000;60:1009–13. [PubMed] [Google Scholar]
- 163.Zhu W, Rawlins BA, Boachie-Adjei O, Myers ER, Arimizu J, Choi E, Lieberman JR, Crystal RG, Hidaka C. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004;19:2021–32. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]


