Abstract
Regulatory CD4+CD25Hi T cells (Treg) and programmed death-1 (PD-1) molecule have emerged as pivotal players in immune regulation. However, the underlying mechanisms by which they impact antigen-specific CD8+ immune responses in cancer patients and how they interact with each other under physiologic conditions remain unclear. Herein, we examined the relationship of PD-1 and its abrogation to the function of Treg in patients with melanoma using short-term in vitro assays to generate melanoma-specific T cells. We identified Treg in the circulation of vaccinated melanoma patients and detected PD-1 expression on vaccine-induced melanoma antigen-specific CTLs, as well as on and within Treg from patients’ peripheral blood. Programmed death ligand (PD-L) 1 expression was also detected on patients’ Treg. PD-1 blockade promoted the generation of melanoma antigen-specific CTLs and masked their inhibition by Treg. The mechanisms by which PD-1 blockade mediated immune enhancement included direct augmentation of melanoma antigen-specific CTL proliferation, heightening their resistance to inhibition by Treg and direct limitation of the inhibitory ability of Treg. PD-1 blockade reversed the increased expression of PD-1 and PD-L1 on melanoma antigen-specific CTL by Treg, rescued INF-γ and IL-2 or INF-γ and tumor necrosis factor-α co-expression and expression of IL-7 receptor by melanoma antigen-specific CTL which were diminished by Treg. PD-1 blockade also resulted in down-regulation of intracellular FoxP3 expression by Treg. These data suggest that PD-1 is importantly implicated in the regulation of Treg function in melanoma patients.
Keywords: anti-PD-1, CD4+CD25+ regulatory T cells (Treg), CTL, melanoma
Introduction
Anti-tumor immune responses mediated by T cells play an important role in the growth of primary tumors and controlling metastases in experimental animal systems. Patients may fail to reject established tumors successfully because of a decreased magnitude of anti-tumor immunity, as well as extensive immune evasion and immune inhibitory mechanisms concomitant with tumor growth. The description and regulation of the mechanisms involved in immune inhibition in cancer patients have been investigated extensively, and their abrogation has been explored in order to improve immunotherapeutic approaches to cancer.
Melanoma is among the few cancer types in which immunotherapeutic approaches with cytokines or adoptive cell therapy have been shown to confer clinical benefit (1–4). CD8+ CTLs are known to play an important role in anti-melanoma tumor immunity in animal systems and in patients. During chronic infections and in the setting of tumor growth, CD8+ T cells display poor proliferation and impaired effector function (5–9). The magnitude and longevity of melanoma antigen-specific CTL responses is compromised by multiple immunosuppressive pathways during progression of melanoma, including transforming growth factor-β, IL-10, regulatory T cells and myeloid-derived suppressor cells. This loss of CTL function directly facilitates tumor growth. As one of the vital components of immune inhibition, regulatory CD4+CD25Hi T cells (Treg) play a crucial role in controlling tumor immunity and are likely to adversely affect immune therapeutic efficacy (10, 11). The number and frequency of Treg were shown to be elevated in the peripheral blood of cancer patients and especially within the tumor microenvironment (12–14). Treg hinder the induction of anti-melanoma effector CD8+ T cells, prevent tumor-specific CTL from exerting effector function (15, 16) and severely impair CTL memory responses (17). Intra-tumoral Treg completely inhibit proliferation of and cytolytic granule production by infiltrating CD8+ cells (18). Hence, Treg likely play a dominant role in decreasing immunity during tumor development and progression and may contribute to a poor outcome in cancer patients (19–21).
Programmed death-1 (PD-1) (CD279) (22) is a negative co-stimulatory molecule that plays an important role in the balance and regulation of adaptive immune responses. PD-1 engagement can down-regulate T cell activation. Programmed death ligand (PD-L) 1 is broadly and constitutively expressed by B cells, dendritic cells (DCs), macrophages (Mφ) and T cells. PD-L1 is further up-regulated upon activation (23), which appears to depend on toll like receptor 4 and signal transducers and activators of transcription protein 1 signaling (24). PD-1 is highly expressed on ‘exhausted’ T cells from virus-infected patients (23,25–27) and contributes to their poor proliferative capacity and incompetent effector function (28). Up-regulation of PD-1 and its ligand not only facilitates persistence of pathogens during chronic infection but also might be associated with immune evasion and inhibition in tumor-bearing hosts. Levels of T cells expressing PD-1 were increased in patients with high-risk renal cell carcinoma. Patients with PD-1-positive T cells were at significant risk of cancer-specific death compared with patients whose T cells were low expressors of PD-1 (29). Blockade of the PD-1/B7-H1 interaction restored effector CD8+ T cell responses in chronic infection, such as HIV, hepatitis C and lymphocytic choriomeningitis virus (27, 30, 31). The blockade of the PD-1/PD-L1 pathway using anti-PD-L1 mAbs abrogated Treg-mediated immune regulation in vitro and tolerance induction in vivo in mice (32). PD-1-mediated inhibition may also naturally prevent immunopathology by turning off CD8+ T cells in conditions of chronic stimulations with either foreign or self-antigens (33). Treg and PD-1 pathway signals have been studied in tumor-bearing patients; however, the relationship and function of Treg and PD-1 in cancer are not known.
In this study, we detected and characterized the function and phenotype of Treg and measured the expression of PD-1 and PD-L1 molecules on different cell populations from the peripheral blood of high-risk-resected stage III and IV melanoma patients. We demonstrated that PD-1 blockade augmented the generation of melanoma antigen-specific CTL directly by stimulating their proliferation and indirectly by masking their suppression by Treg. PD-1 blockade of Treg also diminished their inhibitory function. Therefore, the function of PD-1 blockade, by alleviating suppression by Treg, as well as promoting the proliferation and function of CD8+ responder T cells in melanoma patients, supports the role of PD-1 blockade as a promising component of immunotherapy against cancers and chronic infections.
Methods
PBMC, CD8+ and Treg purification
Patients with resected stage III/IV melanoma with no evidence of disease who were HLA-A *0201 were vaccinated with a cocktail of peptides including the glycoprotein 100 (gp100) 209-217 (210M) (gp100-2M) and MART-1 26-35 (27L) (MART 27L) heteroclitic peptide analogs emulsified in Montanide ISA 51 (Seppic Inc., Rutherford, NJ, USA). All clinical protocols were approved by the Cancer Therapy Evaluation Program of the National Cancer Institute, as well as the local Institutional Review Boards, and were conducted under an investigational new drug application from the US Food and Drug Administration. Written informed consents were obtained from all patients. PBMC from melanoma patients were isolated from pre- and post-vaccination aphaeresis specimens by purification using Lymphoprep (Greiner Bio-One, Longwood, FL, USA) density gradient centrifugation. PBMC were counted, then frozen in 40% human AB serum (HS), 50% AIM-V and 10% dimethyl sulfoxide (DMSO) (Sigma, St Louis, MO, USA) and stored in secured liquid nitrogen freezers at −168°C until used. CD8+, CD4+CD25+ and CD4+CD25− populations were purified from patients’ PBMC using magnetic CD8 isolation beads (Miltenyi Biotec, Auburn, CA, USA). The CD8− population was further purified to yield CD4+ cells by negative selection. The pre-enriched CD4+ population was either further separated using CD25 magnetic beads into CD4+CD25+ and CD4+CD25− population (human T regulatory CD4+CD25+ isolation kit, Miltenyi Biotec) or further fractionated by sorting using an FAC Aria cell sorter (BD Bioscience, San Jose, CA, USA) into CD4+CD25High, CD4+CD25Low and CD4+CD25Neg populations as described by Baecher-Allan et al. (34). Briefly, CD4+CD25Hi regulatory cells were gated on the tail which was to the right from the major population containing CD4+CD25Low and CD4+CD25− cells, as shown in Fig. 1(A), left panel. The final purity of each portion was typically ≥90% by flow cytometry.
Fig. 1.
Detection and characterization of Treg. (A) The phenotype of Treg from melanoma patients (A, left), Memory/naïve phenotype of the Treg (A, middle and right). Dot plots showed one representative profile from 14 patients. (B) CD27-, CTLA-4-, GITR-, PD-1- and PD-L1-specific staining on gated CD4+CD25Hi Treg (solid black lines), CD4+CD25Low T cells (gray dashed lines) and CD4+CD25Negative T cells (gray dotted lines) overlaid with isotype-matched control antibodies (shaded gray histograms). (C) Intracellular FoxP3, PD-1 and CTLA-4 staining on gated CD4+CD25Hi Treg (solid black lines), CD4+CD25Low T cells (gray dashed line) and CD4+CD25Negative T cells (gray dotted line) overlaid with isotype-matched control antibodies (shaded gray histograms). (D) PD-1 and PD-L1 expression on melanoma antigen-specific CD8+ cells, shown in the upper right quadrant was the percentage of PD-1/PD-L1-positive tetramer + CD8+ cells.
Tetramers, mAbs and flow cytometry immunofluorescence analyses
HLA-A *0201/PE–melanoma antigen recognized by T cell (MART)-1/gp100-2M tetramers were purchased from Beckman Coulter (Fullerton, CA, USA). All fluorochrome-conjugated antibodies and various isotype controls used for flow cytometry experiments were purchased from BD Bioscience PharMingen (San Diego, CA, USA) unless otherwise specified. The expression of cell surface and intracellular molecules was determined using an FC500 Flow Cytometer (Beckman Coulter) and FAS Calibur (BD Bioscience) and analyzed using CXP software (Beckman Coulter) and Cell Quest (BD Bioscience). PECy5-CD45RA, PECy7-CD45RO and FITC-CCR7 were purchased from R&D Systems (Minneapolis, MN, USA). Allophycocyanin (APC)-labeled antibodies against glucocorticoid induced tumor necrosis factor receptor (GITR) (R&D Systems), cytotoxic T lymphocyte antigen-4 (CTLA-4) and CD27 were used for detection of surface molecules on Treg. PD-1, PD-L1 and PD-L2 surface staining was performed on sorted CD4+CD25Hi, CD4+CD25Low and CD4+CD25Neg populations and melanoma antigen-specific CTL recognized by specific tetramer staining. Dead cells were excluded from all flow analyses by using a viability dye (Invitrogen Corporation, Carlsbad, CA, USA). mAb to human PD-1 (clone 5C4) and a matching IgG4 isotype control antibody were kindly provided by Allen Korman (Medarex Incorporated, Milpitas, CA, USA) and were used at a concentration of 10 μg ml−1. PD-1-blocking antibody and isotype IgG4 control antibody were added either to in vitro T cell cultures at day 0 or used for pre-incubation with Teff or Treg cell populations at 37°C, with 5% CO2 for 4 h. Pre-incubated cells were extensively washed with PBS before further experiments were conducted.
Generation of DCs
PBMC were cultured in X-VIVO 15 (Cambrex, East Rutherford, NJ, USA) at 37°C, 5% CO2 for 1–3 h. Non-adherent cells were removed and the adherent cells were cultured in X-VIVO 15 supplemented with 1000 U ml−1 rh granulocyte macrophage colony stimulating factor (GM-CSF) (R&D System) and 1000 U ml−1 rh IL-4 (R&D System). On day 6 of culture, rh GM-CSF and rh IL-4 were replenished. On day 7 of culture, DCs were pulsed with gp100-2M and/or MART 27L peptide at 10 μg ml−1. Four hours later, a cytokine cocktail was added to the culture at the final concentrations of 10 ng ml−1 of tumor necrosis factor (TNF) (R&D System), 10 ng ml−1 of IL-1β (R&D System), 100 IU ml−1 of IL-6 (R&D System) and 1 μg ml−1 of prostaglandin E2 (R&D System). After overnight maturation, DCs were harvested by growing in warm PBS for 30 min, washed and re-suspended in AIM V medium supplemented with 5% HS for further use.
Melanoma antigen-specific CTL generation and inhibition assay
Magnetically enriched CD8+ cells used as effector cells (Teff) were stimulated with mature DC loaded with melanoma peptides (Mart 27L and/or gp100-2M) at a 100:1 ratio in AIM-V media (Invitrogen Corporation, Grand Island, NY, USA) supplemented with 5% HS (Omega Scientific, Tarzana, CA, USA) in 24-well plates (Greiner Bio-One, Monroe, NC, USA) and incubated at 37°C, 5% CO2 for 10 days. A total of 100 U ml−1 IL-2 (R&D Systems) and 100 U ml−1 IL-15 (R&D Systems) were pulsed into the culture on day 4 and added every 3 days thereafter.
HLA-A *0201 restricted melanoma epitope peptides were synthesized by the USC-Norris Core lab (Norris Cancer Center, University of Southern California, Los Angeles, CA, USA).
Melanoma antigen-specific CTL generation was measured by tetramer staining and absolute cell count. For in vitro inhibitory assays, bead-isolated CD4+CD25+/CD4+CD25− populations or flow-sorted CD4+CD25High, CD4+CD25Low and CD4+CD25Neg cells were added to CD8+ T cells in the following ratios (CD4+/CD8+ = 1/2 for beads isolation; CD4+/CD8+ = 1/4 for flow cytometry sorting).
5,6-Carboxy fluorescein diacetate succinimidyl ester labeling of cells
The purified Teff were adjusted to 2 × 107 cells ml−1 in PBS. The stock solution of 5,6-carboxy fluorescein diacetate succinimidyl ester (CFSE) (Vynramt CFDA SE cell tracer kit, Invitrogen Corporation) was diluted in DMSO and PBS following the manufacturer's protocol to a concentration of 0.5 μM and then mixed 1:1 with the cells suspended and incubated at room temperature for 10 min and extensively washed with PBS before use.
Intracellular protein and cytokine staining
For intracellular cytokine staining, targeted cell populations were stimulated with phorbol myristate acetate (PMA) (Sigma, 5 ng ml−1) and ionomycin (250 ng ml−1) for 6 h in the presence of brefeldin A (10 μg ml−1) for the last 4 h. The stimulated cells were fixed, permeabilized and stained with the interesting cytokines antibodies and granzyme B-FITC. Intracellular FoxP3-APC (eBioscience, San Diego, CA, USA), CTLA-4-PECy5, PD-1-FITC and Ki67-FITC (Abcam) staining were performed using human FoxP3 staining kit (eBioscience) according to the manufacturer's direction.
IFN-γ enzyme-linked immunosorbent spot assay
Immunospot plates (Millipore) were coated with 10 μg ml−1 mouse anti-human IFN-γ mAb (Clone 1-D1K, Mabtech, Cincinnati, OH, USA) in sterile PBS at 4°C overnight. The plates were then blocked at 37°C for 2 h with AIM-V medium containing 5% human serum AB. Antigens-loaded K562-A2 cells and CTLs were then placed in each well and cultured for 18 h at 37°C in 5% CO2. After six washings with PBS + 0.05% Tween 20, 1 μg ml−1 of biotinylated anti-human IFN-γ detection mAb (7-B6-1-1 biotin, Mabtech) was added and incubated for 2 h at 37°C. The plates then were washed another four times in phosphate buffered saline + Tween 20 and incubated with streptavidin–HRP solution at room temperature for 1 h. Then after 2× plate wash, add AEC solution (BD Bioscience) to detect wells for 4 min. After rinsing with water, the resulting spots were counted on an ELISPOT Reader (Zeiss Inc., Munich, Germany). The result was expressed as the number of cytokine-producing spots per 10 000 or 1000 cells.
Statistical analysis
The statistical analysis of the data was done by RATIO paired t test and the Wilcoxon sign rank test and presented as box plots which indicated the minimum, maximum, 95% confidence intervals, mean and median; scattered dot plots which presented the min, max, mean and median and bar graphs with mean ± SD. A P value <0.05 was considered statistically significant. All in vitro data shown were representative of at least three independent experiments.
Results
Detection and characterization of Treg from stage III/IV melanoma patients
The phenotype of the Treg population from PBMC of normal and melanoma patients as described in Methods was characterized by flow cytometry. In our study comprising 10 normals (Supplementary Figure 1 is available at International Immunology Online) and 14 metastatic melanoma patients (Fig. 1), Treg were predominantly effector–memory and central memory-type cells (Fig. 1A middle panel, Supplementary Figure 1 middle panel, available at International Immunology Online). In order to confirm specific staining of CD45RA, CD45RO staining was also performed on the same samples, shown as the mirror staining pattern of CD45RA (Fig. 1A right panel, Supplementary Figure 1 right panel, available at International Immunology Online). Normals and melanoma patients have a similar Treg phenotype pattern (Supplementary Table 1 is available at International Immunology Online). Further characterization of surface markers on Treg (Fig. 1B) showing all CD4+ T cells expressed high levels of CD27; compared with the CD4+CD25Neg and CD4+CD25Low population, Treg expressed 3- to 4-fold higher GITR and CTLA-4, ∼2-fold higher PD-1 and 2- to 9-fold higher PD-L1 on their surface. Further characterization was done using intercellular markers (Fig. 1C): More than 90% of Treg expressed intracellular FoxP3, but the CD4+CD25Neg and CD4+CD25Low populations expressed very little FoxP3 intracellularly; Treg expressed almost 2-fold higher intracellular CTLA-4 than the CD4+CD25Neg and CD4+CD25Low populations; these three populations all had high levels of intracellular PD-1. Significant PD-1 expression was also detected on melanoma antigen-specific CD8+ T cells, as has been shown previously (35) (Fig. 1D, left panel). PD-L1 and PD-L2 expression was not detected on melanoma antigen-specific CD8+ cells from >20 patients tested (Fig. 1D, right panel and data not shown).
PD-1 blockade augmented melanoma antigen-specific CTL generation and overcame Treg suppression
To determine whether PD-1 blockade altered the inhibition of melanoma antigen-specific CTL generation by Treg, we added PD-1-blocking antibody or isotype control IgG4 antibody to an in vitro Treg inhibitory assay. Briefly, magnetic bead-isolated CD8+ T cells from patient PBMC were cultured with mature autologous DC loaded with melanoma-specific antigenic peptides. Magnetic bead/flow cytometry-purified Treg and CD4+CD25− T cells (which served as a cell control) were added to CD8+ cultures in order to measure the impact of Treg on melanoma antigen-specific CTL generation. The frequency and absolute cell count of melanoma antigen-specific CTL were monitored by performing MHC-class I tetramer and CD8 staining. Suppression of the generation of melanoma antigen-specific CTL by Treg was shown by the decreased frequency and overall cell count of melanoma antigen-specific CTL from CD8+ cells cultured with Treg, as compared with CD8+ T cells cultured alone or with CD4+CD25− cells. When Treg were separated from CD8+ T cells in transwell assays in the same experimental setting, the inhibitory effect of Treg was completely abolished (data not shown). One representative experiment of 14 is shown in Fig. 2(A): upper row in which the frequency of gp100-2M-specific tetramer-positive CD8+ T cells was reduced significantly from 5.6 to 0.6% when CD8+ T cells were co-cultured with Treg cells with isotype control IgG4 antibody compared with CD8+ T cells alone with isotype control. The addition of CD4+CD25− cells increased the frequency of gp100-2M-specific tetramer-positive CD8 T cells. Strikingly, when anti-PD-1 antibody was added to the same cultures at 10 μg ml−1, the frequency of tetramer-positive gp100-2M cells increased to a greater degree in each culture condition. It is notable that effectors co-cultured with Treg treated with anti-PD-1 antibody generated a higher level (6.8%) of melanoma antigen-specific CTL than the CD8+ alone group treated with control antibody (5.0%). These data suggest that PD-1 blockade augmented melanoma antigen-specific CTL generation and overcame the inhibition of CTL generation by Treg. The impact of PD-1 blockade on the frequency of melanoma antigen-specific CTL generation and the inhibition of Treg were summarized from 14 patients shown as the box plot in Fig. 2(B). The mean frequency of melanoma antigen-specific CTLs from CD8+ co-cultured with Treg is statistically significantly lower than the mean frequency of melanoma antigen-specific CTLs from CD8+ alone and CD8+ cultured together with CD4+CD25− cells. The augmentation of melanoma antigen-specific CTL in each culture condition by PD-1 blockade was demonstrated by comparing the means in every pair of boxes which represented isotype IgG4 (white box) or anti-PD-1 antibody (gray box) treatment. The rescue of the inhibitory effect of Treg by PD-1 blockade was shown when the mean from CD8+ co-cultured with Treg with anti-PD-1 antibody treatment was compared with the mean from CD8+ cultured by itself with control antibody treatment (P < 0.05 by Wilcoxon test).
Fig. 2.
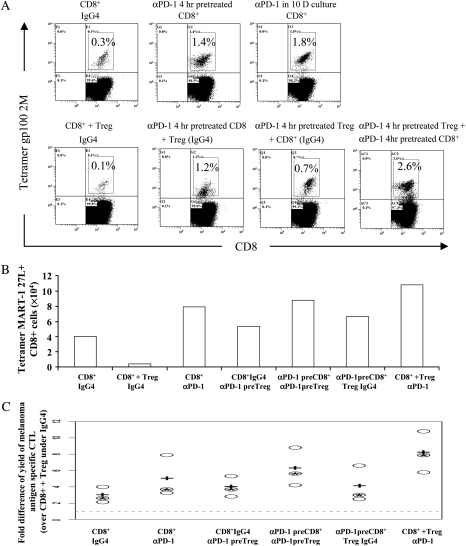
PD-1 blockade augmented generation of melanoma antigen-specific CTL and masked inhibition by Treg. (A) IgG4 isotype control antibody was added to all culture conditions in upper row, and anti-PD-1 antibody was added at all conditions continuously in lower row. Fig. 2A was a representative experiment of 14. (B) Summary of the frequency of melanoma antigen-specific CTL from 14 individual experiments; + represented the median and − represented the mean frequency of melanoma antigen-specific CTL generated from each culture condition. (C) The fold difference of the yield of tetramer gp 1002M+CD8+ cells from each culture was shown. Fig. 2C was a representative experiment from 11. (D) The summary of the fold difference of the yield of melanoma antigen-specific CTL from 11 melanoma patients. The dotted line was one; + represented the median and − represented the mean of each ratio. (E) Impact of anti PD-1 on the frequency of melanoma antigen-specific CTL generated from each culture condition from 14 melanoma patients. The doted line was one; + represented the median and − represented the mean of each ratio. (F) Summary of the impact of PD-1 blockade on the absolute yield of melanoma antigen-specific CTL generated from each culture condition from 11 individual experiments. The dotted line was one; + represented the median and − indicated the mean of each ratio. (G) CSFE-labeled CD8+ cells were cultured with autologous mature gp 100-2M-loaded DCs. Human IgG4 isotype control antibodies (upper rows) or anti PD-1 antibody (lower rows) were added into the culture. The numbers represented the percentage of dividing CFSE-labeled CD8+ T cells relative to total CFSE-labeled CD8+ T cells.
To examine the effect of PD-1 abrogation on the yield of melanoma antigen-specific CTL, we calculated the change in the yield of tetramer-positive cells by setting the total number of melanoma antigen-specific CTL generated from CD8+ T cells co-cultured with Treg with isotype control antibody to be one (Fig. 2C the second white dotted bar and Fig. 2D the dotted line). The fold difference was the yield of melanoma antigen-specific CTL from different culture conditions divided by the yield from CD8+ cells co-cultured with Treg with control antibody. We observed a discernible increase in the total yield of gp100-2M CD8+ cells when cultures were treated with anti-PD-1 antibody (Fig. 2C black dotted bars) compared with the yield from cultures treated with control antibody (Fig. 2C white dotted bars). The yield from CD8+ cells co-cultured with Treg treated with anti-PD-1 antibody (Fig. 2C middle black dotted bar) was equal to or greater than the yield from CD8+ cells cultured with control antibody (Fig. 2C left white dotted bar) or even CD8+ cells cultured with anti-PD-1 antibody (Fig. 2C left black dotted bar). Figure 2(C) showed one representative experiment from 11. Figure 2(D) showed the summary of the fold difference of the yield of melanoma antigen-specific CTL from 11 experiments. The yield of melanoma antigen-specific CTL from CD8+ co-cultured with Treg with Isotype antibody was set as one. The boxes represent the 95% confidence intervals of the ratio (the yield of melanoma antigen-specific CTL from all the other culture conditions divided by the yield of melanoma antigen-specific CTL from CD8+ co-cultured with Treg with control antibody). Comparing the means from the paired culture conditions (IgG4 and anti-PD-1) and the mean from CD8+ co-cultured with Treg with anti-PD-1 antibody with the baseline and the mean from CD8+ cultured alone with control antibody, we concluded that PD-1 blockade augmented the yield of melanoma antigen-specific CTL and masked its inhibition by Treg.
The frequency/absolute cell count of melanoma antigen-specific CTL from the anti-PD-1 antibody-treated culture groups divided by the frequency/absolute cell count of melanoma antigen-specific CTL from the same culture condition with control antibody was calculated. As illustrated in Fig. 2(E and F), the most potent effect of anti-PD-1 antibody treatment occurred when CD8+ T cells were co-cultured with Treg compared with CD8+ T cells cultured alone or CD8+ cells co-cultured with CD4+CD25− T cells. The box plots summarized from 14 individual experiments are shown in Fig. 2(E and F), in which the dotted line was set to 1, and the boxes represented the calculated ratios. All the means of the ratios were statistically different from baseline, and the second mean value was significant higher than all the other means, indicating that PD-1 blockade augmented the generation of melanoma antigen-specific CTL under each culture condition and the most significant influence occurred with CD8+ and Treg co-culture condition.
The masking of the inhibition by Treg of antigen-specific CTL with anti-PD-1 antibody was further confirmed by measuring the proliferation of CFDA SE cell tracer-labeled antigen-specific CD8+ T cells from melanoma patients. The enhanced rate of proliferation by CD8+ T cells after anti-PD-1 treatment was shown by comparing CFSE staining in the cultures exposed to anti-PD-1 antibody continuously with that of cultures exposed to control antibody. The inhibition by Treg of the Teff proliferation was clearly illustrated in Fig. 2(G). The proportion of proliferating Teff was reduced from 63.4% (Fig. 2G upper row, left panel) when CD8+ were cultured with control antibody to 49% (Fig. 2G upper row, middle panel) when Treg added to CD8+ culture and exposed to control antibody. Notably, the proportion of proliferating cells from the middle panel in the lower row (76.9%) in which CD8+ cells were co-cultured with Treg with anti-PD-1 treatment was much higher than the middle panel in the upper row representing the same culture condition with control antibody (49%) and even greater than CD8+ cultured alone or CD8+ effectors cultured with CD4+CD25− cells (75.6%, Fig. 2G upper row, right panel). Taken together, these results indicated that PD-1 blockade masked the Treg-induced suppression of proliferation and growth of antigen-specific CTL from melanoma patients.
PD-1 blockade directly targeted effector CD8+ T cells to increase their resistance to inhibition by Treg and directly targets Treg to limit their suppressive ability
To understand how PD-1 blockade reversed the suppression of antigen-specific CTL by Treg, CD8+ T cells and Treg were isolated and then separately pre-treated with anti-PD-1 antibody or isotype control IgG4 antibody at 37°C, 5% CO2 for 4 h. After extensive washing to remove non-specifically binding antibody, pre-treated cells were incubated in vitro in a melanoma antigen-specific CTL assay separately or combined together. We determined whether the fraction of melanoma antigen-specific CD8+ T cells was significantly increased in the presence of anti-PD-1 antibody pre-treatment compared with the control antibody-treated groups. The data shown in Fig. 3(A) indicated that anti-PD-1 pre-treatment of Teff for 4 h augmented the proportion of melanoma antigen gp100 209-217 (210M)-specific CD8+ T cells 4.7-fold (Fig. 3A upper middle panel) compared with Teff cultured alone and treated with control antibody (Fig. 3A upper left panel). When Teff were treated with anti-PD-1 antibody continuously in a 10-day culture, the increase was 6-fold (Fig. 3A upper right panel). The fraction of gp100-2M CD8+ cells generated from Teff co-cultured with Treg with control antibody treatment (Fig. 3A lower left panel) was reduced 3-fold compared with Teff cultured alone with control antibody_treatment. Teff pre-treated with anti-PD-1 antibody were resistant to inhibition by Treg. When Teff were pre-treated with anti-PD-1 antibody for 4 h and washed and then co-cultured with control antibody_pre-treated Treg, the fraction of tetramer gp100-2M+CD8+ cells (Fig. 3A lower second panel) was 12-fold higher than CD8+ T cells treated with control antibody and co-cultured with Treg. Pre-treatment of Treg with anti-PD-1 antibody also limited their inhibitory effects on CTL generation. This was calculated by comparing the proportion of gp100-2M tetramer-positive cells generated from Teff co-cultured with Treg pre-treated with PD-1-blocking antibody for 4 h then washed extensively (Fig. 3A lower third panel) to Teff co-cultured with Treg pre-treated with control antibody (Fig. 3A lower first panel). When PD-1-blocking antibody pre-treated Teff and Treg were co-cultured together in vitro, the fraction of gp100-2M-specific CD8+ T cells was greatly increased compared with Teff cultured with control antibody (8.7-fold higher) or Teff co-cultured with Treg treated with control antibody (26-fold higher). Moreover, the absolute number of melanoma antigen-specific CTL from different treatments (Fig. 3B) in one representative experiment of four indicated that PD-1 blockade of Teff alone augmented melanoma antigen-specific CTL generation and heightened their resistance to inhibition by Treg. PD-1 blockade of Treg limited their suppression of melanoma antigen-specific CTL. This observation was further clarified by summarizing three individual experiments in Fig. 3(C). When we set the yield of melanoma antigen-specific CTL harvested from Teff co-cultured with Treg as one, the index number was calculated by dividing the number of melanoma antigen-specific CTL from all other treatments by the number of antigen-specific CTL harvested from Teff co-cultured with Treg in which both were pre-treated with control antibody. The data were represented as the scattered dot plot of three individual experiments (Fig. 3C). The mean of all the cultures was clearly >1. Collectively, these results suggested that anti-PD-1 antibody could directly target Teff to enhance their proliferation and enable Teff to overcome suppression by Treg. Moreover, PD-1-blocking antibody also directly limited the suppressive effect of Treg.
Fig. 3.
PD-1 blockade increased the resistance of CD8 T cells to inhibition by Treg and directly limited the suppressive ability of Treg. (A) The impact of 4-h pretreatment of PD-1 blockade on the frequency of gp100-2M-specific CD8+ T cells. (B) Total yield of MART 27L tetramer + CTLs generated from different culture conditions; (B) Showed one representative experiment of four. (C) Dot plot summarized three experiments. The yield of melanoma antigen-specific CTL generated from Teff co-cultured with Treg and control antibody was arbitrarily set as 1 (the dotted line). Each circle indicates the index number; + was the median and * was the mean of the index number from each culture condition.
PD-1 blockade augmented functional melanoma antigen-specific CTL and rescued the impairment of effector function by Treg
To address the impact of PD-1 blockade on the function of melanoma antigen-specific CTL and to elucidate possible mechanisms by which PD-1 blockade altered T cell growth and reversed Treg suppression, we analyzed the expression of a set of surface and intracellular markers on/in melanoma antigen-specific CTL generated from co-culture of Teff and Treg treated with anti-PD-1 antibody or control antibody. To determine whether expression of PD-1/PD-L1 would be influenced by PD-1-blocking antibody, we measured PD-1 and PD-L1 expression on melanoma antigen-specific CTL (Fig 4A and B). When control antibody was added to the 10-day Teff and Treg co-culture, melanoma antigen-specific CTL expressed significantly higher level of PD-1 and PD-L1 on their surface than Teff were grown without Treg or co-cultured with CD4+CD25− cells. Anti-PD-1-blocking mAb treatment reversed the up-regulation of PD-1 and PD-L1 on melanoma antigen-specific CTL induced by Treg. The mean value of PD-1/PD-L1 expression on melanoma antigen-specific CTL generated from Teff and Treg co-culture with anti-PD-1 antibody treatment decreased significantly (P < 0.05) comparing with that on melanoma antigen-specific CTL generated from CD8+ and Treg co-culture with control antibody treatment. These results suggest that alteration of PD-1 and PD-L1 expression on CTL might be in part responsible for their suppression by Treg, and its reversal might account for the ability of PD-1 blockade to overcome suppression of CTL by Treg.
Fig. 4.
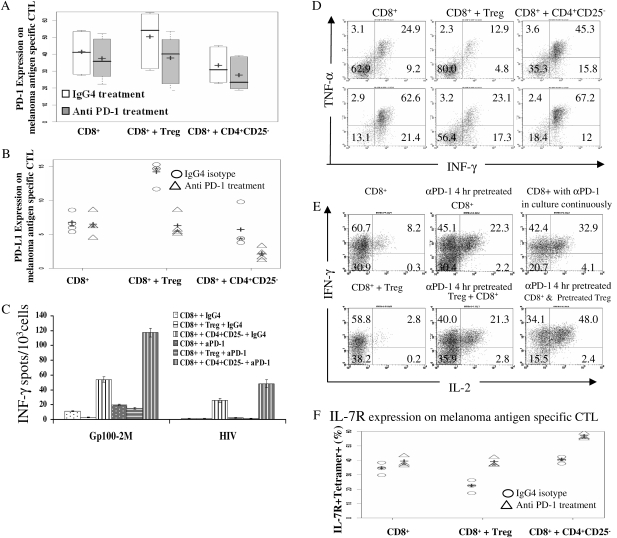
PD-1 blockade augmented the function of melanoma antigen-specific CTLs and reversed the impairment of effector function by Treg. (A) PD-1 blockade reduced PD-1 expression on melanoma antigen-specific CTL induced by Treg; + represented the median and − represented the mean PD-1 expression on melanoma antigen-specific CTL. Data were summarized from five separate experiments. (B) PD-1 blockade decreased PD-L1 expression on melanoma antigen-specific CTL up-regulated by Treg; + was the median and * was the mean of PD-L1 expression on melanoma antigen-specific CTL. The data shown as scatter dot plots from four individual experiments. (C) Anti PD-1 antibody reversed the impairment of INF-γ secretion by CTL induced by Treg. INF-γ secretion was presented as mean ± SD. This figure showed one representative experiment from six. (D) The impact of PD-1 blocking antibody on intracellular INF-γ and TNF-α secretion by melanoma antigen-specific CTL. The upper row represents cultures treated with control antibody and the lower row was the cultures treated with PD-1-blocking antibody for 10 days. (E) PD-1 blockade increased intracellular INF-γ and IL-2 co-expression in melanoma antigen-specific CTLs under different culture conditions. Dot plot is gated on tetramer gp100-2M+CD8+ T cells. (F) PD-1-blocking antibody treatment up-regulated the IL-7R expression on melanoma antigen-specific CTL; + was the median and * was the mean of the IL-7R expression on melanoma antigen-specific CTL. Data were shown as scatter plots from three individuals experiments.
To examine whether PD-1 blockade had any effect on the function and cytokine secretion of melanoma antigen-specific CTL, we assessed melanoma antigen-specific CTL harvested from different culture conditions by enzyme-linked immunosorbent spot (ELISPOT) assay and by intracellular cytokine staining. PD-1-blocking antibody treatment of CTL increased the number of INF-γ-secreting cells. The addition of Treg decreased the number of INF-γ-secreting cells detected by ELISPOT assay (Fig. 4C), compared with Teff cultured alone, which was significantly less than Teff co-cultured with CD4+CD25− T cells. PD-1-blocking antibody treatment (Fig. 4C gray bars) increased the number of INF-γ-secreting cells, compared with the cultures treated with control antibody (Fig. 4C the white bars). INF-γ-secreting cells increased 5-fold when Teff co-cultured with Treg and treated with anti-PD-1 antibody compared with Teff co-cultured with Treg with control antibody. When Teff were cultured alone or Teff were co-cultured with CD4+CD25− cells, PD-1-blocking antibody treatment resulted in an increase of INF-γ-secreting cells by 2-fold. The data show one representative experiment from six and was presented as mean ± SD. The suppression of INF-γ by Treg, enhancement of its secretion and the masking of its inhibition by Treg with anti-PD-1 antibody were all statistically significant (P < 0.05).
The effector cytokines generated by melanoma antigen-specific CTL included not only INF-γ but also TNF-α. In Fig. 4(D), the impact of PD-1-blocking antibody on the secretion of TNF-α and INF-γ by antigen-specific CTL was studied by intracellular staining and flow cytometry. Cells harvested after anti-PD-1 antibody and isotype control antibody treatment were further stimulated with PMA and ionomycin. Melanoma antigen-specific tetramer-positive CD8+ T cells were gated for TNF-α and INF-γ expression analysis. PD-1-blocking antibody treatment increased the co-expression of TNF-α and INF-γ by melanoma antigen-specific CTL compared with control antibody treatment. Adding Treg to Teff culture reduced the TNF-α and INF-γ co-expressing melanoma antigen-specific CTL 2-fold. Anti-PD-1 antibody treatment rescued TNF-α and INF-γ co-expression by melanoma antigen-specific CTL generated from Teff co-cultured with Treg, which was 2-fold higher than that from Teff co-cultured with Treg with control antibody and matched the TNF-α and INF-γ co-expression by melanoma antigen-specific CTL generated from Teff cultured alone with control antibody.
To test the idea that PD-1-blocking antibody might be able to influence longevity signals on/in melanoma antigen-specific CTL, we tested IL-2 and INF-γ co-expression by and the expression of IL-7 receptor on melanoma antigen-specific CTL harvested from different culture conditions. As displayed in Fig. 4(E), PD-1-blocking antibody treatment augmented a population of INF-γ and IL-2 co-expressing melanoma antigen-specific CTL. Treg reduced the INF-γ and IL-2 co-expressing melanoma antigen-specific CTL 2.9-fold, as compared with Teff cultured alone with control antibody or Teff co-cultured with Treg exposed to control antibody. Anti-PD-1 antibody treatment of Teff enhanced INF-γ and IL-2 co-expressing melanoma antigen-specific CTL by 2.7-fold if Teff were pre-treated with anti-PD-1 antibody for 4 h or by 4-fold if anti-PD-1 antibody treatment was continuously present in the Teff culture. When Treg were pre-treated with anti-PD-1 antibody for 4 h, extensively washed and added into Teff culture, the INF-γ and IL-2 co-expressing melanoma antigen-specific CTL were increased by 7.6-fold compared with Teff co-cultured with Treg with control antibody directly and even further increased by 2.6-fold compared with Teff cultured alone with control antibody treatment. When both Treg and Teff were pre-treated with anti-PD-1 antibody for 4 h, extensively washed and co-cultured, the INF-γ and IL-2 co-expressing melanoma antigen-specific CTL harvested from the culture were 17-fold higher than Teff co-cultured with Treg with control antibody and 6-fold higher than Teff cultured alone with control antibody. Taken together, anti-PD-1 antibody treatment either of Treg or Teff promoted the generation of an IL-2/INF-γ co-expressing melanoma antigen-specific T-cell population. CD127 (IL-7 receptor α) is felt to be critical for the maintenance of memory T cells. PD-1-blocking antibody-treated cell cultures had more IL-7 receptor-positive melanoma antigen-specific CTL (Fig. 4F triangles) compared with control antibody-treated cultures (Fig. 4F circles). Adding Treg to Teff cultured with control antibody reduced the frequency of CD127+ melanoma antigen specific CTL. Mean IL-7R expression was also reduced in Teff co-cultured with Treg with control antibody compared with Teff cultured with CD4+CD25Neg T cells with control antibody. When anti-PD-1 antibody was used to treat all the cultures, the frequency of CD127+ melanoma antigen-specific CTL was heightened by 10–40%, and the mean fluorescence intensity of the CD127 on melanoma antigen-specific CTL was also higher by 2–17% (data not shown), compared with the relevant cultures treated with control antibody. The frequency of CD127+ melanoma antigen-specific CTL was higher when anti-PD-1 antibody was used to treat Teff co-cultured with Treg than Teff co-cultured with Treg with control antibody treatment and even higher than Teff cultured alone with control antibody. Taken together, PD-1 blockade resulted in the generation of increased cytokine-secreting, antigen-specific functional T cells with higher IL-7R expression. These effectors might be less receptive to inhibitory signals from Treg. Anti-PD-1 antibody treatment rescued the impaired effector function and survival signals of CD8+ T cells reduced by Treg. The increased generation of melanoma antigen-specific CTL by PD-1 antibody did not appear to result from improvement of early activation events since no discernible alteration of the reduced early activation markers CD69 and CD25 on CD8+ T cells by Treg was observed (data not shown).
The impact of PD-1 blockade on Treg phenotype
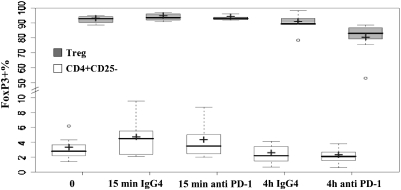
To explore the mechanisms by which PD-1 blockade might impact on the function of Treg, we treated Treg with PD-1-blocking antibody and isotype control antibody for 15 min and also for 4 h and then examined the changes in FoxP3 in Treg. CD4+CD25− T cells were used as a cell control in each experiment. A statistically significant down-regulation of FoxP3 in Treg was observed after 4 h of PD-1 blockade by comparing the mean value of FoxP3 expression in Treg with 4 h anti-PD-1 treatment with all the other mean values of FoxP3 expression in freshly isolated Treg or all control antibody-treated conditions (15 min and 4 h) or anti-PD-1_antibody treatment for 15 min (Fig. 5 gray boxes were summarized from six independent experiments). FoxP3 expression was barely detected in the CD4+CD25− population, which was not impacted by PD-1-blocking antibody significantly (Fig. 5 white boxes). The expression of Ki67 and extensive intracellular expression of PD-1 and CTLA-4 by Treg was not impacted by 4 h of anti-PD-1 antibody treatment dramatically (data not shown). Virtually, no granzyme B was detected in the Treg isolated from melanoma patients, and this was not changed by 4 h exposure to either control or anti-PD-1 antibody (data not shown). We did not observe a clear impact of PD-1-blocking antibody on PD-1, PD-L1, CTLA-4, GITR, OX40 and 4-1BB expression on Treg cells (data not shown). Furthermore, PD-1-blocking antibody treatment did not induce or reduce the apoptosis rate by Treg because we did not neither observe changes in Annexin-V and 7-AAD staining nor was there an impact on intracellular-cleaved caspase-3 expression in Treg treated with anti-PD-1 antibody. Taken together, attenuated expression of FoxP3 by Treg after PD-1-blocking antibody treatment suggested a potential mechanism by which PD-1 abrogation might regulate the inhibitory function of Treg.
Fig. 5.
The impact of anti PD-1 antibody on FoxP3 expression by Treg; + represented the median of FoxP3 expression and − indicated the mean of FoxP3 expression in Treg.
Discussion
In our study, we identified and characterized Treg in peptide vaccine-treated stage III- and IV-resected melanoma patients by flow cytometry analysis and established an in vitro assay showing that Tregs can suppress the generation of CD8+ T cells recognizing melanoma-specific antigens. We demonstrated that CD4+CD25+FoxP3+ Treg derived from the peripheral blood could suppress the generation of melanoma-specific CTL without in vitro pre-activation. PD-1 blockade promoted melanoma antigen-specific CTL generation, enhanced the resistance of CD8+ cells to Treg suppression and masked the inhibitory effect of Treg cells. PD-1 blockade directly targeted both Teff and Treg cells. These data demonstrated that blockade of PD-1 not only might directly increase the frequency of functional tumor-specific T cells but also may inactivate or overcome the inhibitory effects of regulatory T-cell populations.
The Treg in our study were either magnetic bead-isolated or flow cytometry-purified CD4+CD25High cells from the PBMC of melanoma patients. The characterization of the Treg in the current study is consistent with the results of other Treg studies in cancer patients (36–38). The Treg in our study exhibited inhibitory function in a standard Treg inhibitory assay (Supplementary Figure 2 is available at International Immunology Online). But the Treg in current study was quite unlike the Treg from a recent study (14) in metastatic melanoma and kidney cancer. In that study, the Treg also constitutively expressed intracellular FoxP3 and CTLA-4 and produced high amount of IL-10 but were generally CCR7+, with 50% naive and 50% central memory T cells. (In healthy donors, Treg were defined as naive in 45% and central memory in 53.9% of the samples. Similarly, Treg were characterized as naive in 51.4% and central memory in 46.3% of our melanoma patients.) In the current study, a majority of Treg expressed a memory phenotype (both central and effector), but a much smaller proportion had a naive phenotype. Because of the difference, we carefully characterized 14 melanoma patients, including metastatic patients and 10 healthy individuals and found consistent results in all of our analyses (Fig. 1A, Supplementary Figure 1 and Table 1 are available at International Immunology Online).
Treg require activation via their TCR to manifest suppressor function. Following activation, suppressor function is antigen-non-specific and does not require re-stimulation via the TCR. In previous studies, Treg were activated in a non-antigen-specific manner, such as exposure to CD3 and CD28 antibodies. In our study, Treg were not previously activated in vitro but were still inhibitory. One reason may be that in tumor-bearing patients, Treg are constitutively activated. In cancer patients, tumors promote the activation of Treg through several mechanisms involving activation of naturally occurring Treg cells as well as conversion of CD4+CD25− cells into Treg (39). The increase in PD-1 expression on Treg is also consistent with their activation. Normally, PD-1 is located intracellularly and is up-regulated only on the surface of activated T cells (38). We did detect extensive (>90%) intracellular PD-1 expression in and 10–30% of surface PD-1 expression on isolated Treg (Fig. 1B and C). In a murine model, Zhou et al. (39) demonstrated the tumor-induced regulatory T cells (TMTregs) arose both from pre-committed ‘natural’ regulatory T cells and CD4+CD25− GITR low precursors. Once induced, TMTregs were capable of maintaining suppressor activity long after transfer into antigen-free recipients. Vaccination of tumor-bearing hosts concomitantly expanded and activated TMTregs and effector cells. In our experiments, after 10 days of culture, not only were CD8+ T cells activated but also CD4+ T cells because Treg and CD4+CD25− cells expressed high CD25 and intracellular FoxP3 (data not shown). Induction of CD25, the high affinity IL-2 receptor, confirms the activation of CD4+ T cells in the current in vitro study. Activation may have occurred non-specifically from mature DCs loaded with melanoma peptides, proteins from culture media or from human serum. FoxP3 has also been shown to be expressed by CD4+CD25− T cells, at a low level, and is not associated with any suppressive function (40).
PD-1 surface expression was significantly higher on Treg than either on CD4+CD25Low or on CD4+CD25negative populations as has been recently shown (32). The PD-1/PD-L1 pathway interactions might be responsible at least in part for the suppressive function of Treg. PD-L1 on Treg could bind directly to melanoma antigen-specific CD8+ T cells which expressed high levels of PD-1 on their surface (35). There is a direct interaction between Treg and Teff as claimed by Dieckmann et al. (41). In our study, 10-day cultured melanoma antigen-specific CTL showed higher PD-1, PD-L1 (Fig. 4A and B) and CTLA-4 expression (data not shown) on their surface if Teff were co-incubated with Treg than if Teff were cultured alone, with CD4+CD25− T cells or with CD4+CD25Low cells. PD-1-blocking antibody might prevent PD-L1 on Treg/DC from binding PD-1 expressed on melanoma antigen-specific CTL.
Anti-PD-1 antibody treatment and anti-PD-1 antibody pre-treatment of Treg and of CD8+ T cells may activate nearby DCs, which might have been suppressed by PD-1 on Treg and melanoma antigen-specific CTL binding to PD-L1 on the DCs, allowing them to induce a stronger adaptive immune response. In our culture system, mature DC expressed very high levels of PD-L1 (data not shown); Treg expressed low to moderate amount of PD-L1; Teff expressed no PD-L1 on their surface. It is notable that anti PD-L1 antibody can directly block the PD-1/PD-L1 pathway via DC to enhance melanoma antigen-specific CTL generation (Supplementary Figure 3A and B is available at International Immunology Online) and via Treg too (Supplementary Figure 3C is available at International Immunology Online). Treg-directed inhibition of DC function has been demonstrated in vitro (42–44). Tumor-associated Treg may contribute to the suppressive milieu in which intra-tumoral DC remain immature and do not activate effectors (45, 46). Treg may exert suppressive activity on APCs through B7-H4 induction as shown in human ovarian cancer (21). Suppressive effects of Tregs on DC have been observed including down-regulation of MHC-II and co-stimulatory molecules or inhibition of cytokine production by DC (43, 47). Treg could also inhibit the contact between Ag-specific Teff and peptide-pulsed DC (48), and thereby convert them into sub-optimal or even tolerogenic APC. Treg suppression of Teff proliferation in vivo is to a lesser extent mediated through DC and only a partial suppressive effect results from the direct influence of Treg on Teff (49).
In this study, we demonstrated that PD-1-blocking antibody not only augmented melanoma antigen-specific CTL responses by promoting their proliferation, strengthening their cytolytic function and heightening their resistance to inhibition by Treg but also it directly limited the suppressive ability of Treg. Abrogation of the PD-1-PD-L1 pathway enhanced CD8+ T cell responses, showing that not only did the number of antigen-specific CD8+ T cells increase dramatically (Figs 2 and 3) but also their function was improved (Fig. 4). Specific CD8+ T cells treated with PD-1 abrogation produced more INF-γ and TNF-α on a per cell basis (23). There are some published reports suggesting that blockade of the PD-1/PD-L1 pathway impacts Treg cells. Anti-PD-L1 mAbs were found to inhibit Treg suppression and restore CD4+CD25− T cell proliferation in vitro. Blockade of the PD-1/PD-L1 pathway abrogated Treg-mediated immunoregulation, suggesting that the PD-1/PD-L1 pathway is required for Treg suppression of alloreactive responses by CD4+CD25− T cells (32). PD-1 is not the only co-stimulatory molecule which can function on both Teff and Treg. An agonist anti OX40 mAb (OX86) has also been shown to be capable of inhibition of Treg cell function and boosting effector T cell activity (50).
A pre-requisite for the utility of anti-PD-1 mAb is the expression of PD-1 on the surface of target cells. By utilizing flow cytometry, we showed that there were high levels of expression of PD-1 on both CTL and Treg in peripheral blood from melanoma patients. Tumor-infiltrating effector cells may be in a tolerant state that can be actively reversed by PD-1 abrogation so that PD-1 antibody might boost effector cells and limit Treg cell inhibitory function in the tumor microenvironment. Memory CD8+ T cells were capable of making IL-2 (51) and had down-regulated PD-1 expression (52). We observed reduced PD-1 expression on melanoma antigen-specific CTL harvested from anti-PD-1 antibody-treated cultures. PD-1 blockade induced a higher fraction of IL-2-secreting melanoma antigen-specific CTL (Fig. 4E). Memory cells were characterized by IL-2 production and by down-regulation of activation markers such as Ki67, CD38, HLA-DR and PD-1 and bimodal expression of CCR7, CCR5, CD62L, CD28 and granzyme B (52). We did observe down-regulation of granzyme B in melanoma antigen-specific CTL treated with anti-PD-1 antibody (data not shown). Classically, memory T cells express receptors for the homeostatic cytokines IL-7 and IL-15 (53, 54). Accordingly, we did observe a bigger fraction of IL-7 receptor expressing melanoma antigen-specific CTL after anti-PD-1 antibody treatment. It is very possible that PD-1 blockade in the culture resulted in more memory precursor cells. Anti-PD-L1 antibody treatment did not augment the proportion of memory T cells when used in a murine model of lymphocytic choriomeningitis virus (LCMV) infection. Although a single injection of PD-L1-specific antibody boosted CD8+ T cell numbers and function for at least 100 days, none of the LCMV-specific CD8+ T cells down-regulated the inhibitory receptor PD-1 (27), and effectors did not acquire the phenotype of memory T cells that is typically observed after successful clearance of LCMV and contraction of the immune response. It is tempting to speculate that anti-PD-1 antibody treatment might be broadly applicable to infectious diseases and tumor immunotherapy both by directly augmenting CTL numbers and function and inhibiting the function of Treg, supporting a potential therapeutic strategy with anti-PD-1-blocking antibody to abrogate PD-1/PD-L1 interactions.
Supplementary data
Supplementary Figures 1–3 and Table 1 are available at International Immunology Online.
Disclosures
Alan Korman is an employee of Medarex Incorporated. Dr. Jeffrey Weber has accepted honoraria of less than 10,000 dollars in two years from Medarex Incorporated.
Funding
National Institute of Health (6 P30 CA14089) to USC/Norris Cancer Center and (2 P30 CA076292 and RO1 CA 109307) to Moffitt Cancer Center.
Acknowledgments
We are grateful to James J. Mulé, Martin Kast and Dixon J. Gray for helpful discussions and suggestions to Lisa M. Martin and Shari A. Pilon-Thomas for technical support to Jodie Kroger for technical assistance and to the Moffitt Flow Cytometry Core Facility.
Glossary
Abbreviations
- APC
allophycocyanin
- CFSE
carboxy fluorescein diacetate succinimidyl ester
- CTLA-4
cytotoxic T lymphocyte antigen-4
- DC
dendritic cell
- DMSO
dimethyl sulfoxide
- ELISPOT
enzyme-linked immunosorbent spot assay
- gp100
glycoprotein 100
- HS
human AB serum
- LCMV
lymphocytic choriomeningitis virus
- MART
melanoma antigen recognized by T cell
- PD-1
programmed death-1
- PD-L
programmed death ligand
- PMA
phorbol myristate acetate
- TMTreg
tumor-induced regulatory T cell
- TNF
tumor necrosis factor
- Treg
regulatory CD4+CD25Hi T cells
References
- 1.Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J. Clin. Oncol. 2008;26:3445. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science (New York) 2006;314:126. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM. Cancer immunotherapy—the endgame begins. N. Engl. J. Med. 2008;358:2664. doi: 10.1056/NEJMp0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008;358:2698. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 2005;6:873. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 8.Zippelius A, Pittet MJ, Batard P, et al. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J. Exp. Med. 2002;195:485. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittet MJ, Zippelius A, Speiser DE, et al. Ex vivo IFN-gamma secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectious and malignant diseases. J. Immunol. 2001;166:7634. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- 10.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. 2006;6:715. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 11.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 2007;178:6730. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 12.Miller AM, Lundberg K, Ozenci V, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 2006;177:7398. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J. Clin. Oncol. 2006;24:1169. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 15.Zippelius A, Batard P, Rubio-Godoy V, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 16.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 17.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 18.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66:10145. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 20.Kordasti SY, Ingram W, Hayden J, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 21.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 22.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. 2004;4:336. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 23.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 2006;203:2223. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl Acad. Sci. USA. 2003;100:5336. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radziewicz H, Ibegbu CC, Fernandez ML, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 2007;81:2545. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 2007;81:9249. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 28.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J. Immunol. 2007;178:2714. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 29.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin. Cancer Res. 2007;13:1757. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 30.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J. Immunol. 2008;180:4875. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 32.Kitazawa Y, Fujino M, Wang Q, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83:774. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 33.Shin EC, Rehermann B. Taking the brake off T cells in chronic viral infection. Nat. Med. 2006;12:276. doi: 10.1038/nm0306-276. [DOI] [PubMed] [Google Scholar]
- 34.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 35.Wong RM, Scotland RR, Lau RL, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int. Immunol. 2007;19:1223. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 36.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 37.Ruprecht CR, Gattorno M, Ferlito F, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 2005;201:1793. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J. Immunol. 2006;176:2808. doi: 10.4049/jimmunol.176.5.2808. [DOI] [PubMed] [Google Scholar]
- 39.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longhi MS, Hussain MJ, Mitry RR, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J. Immunol. 2006;176:4484. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 41.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 2001;193:1303. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J. Immunol. 2006;176:6202. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 44.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr. Opin. Immunol. 2006;18:496. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Vicari AP, Chiodoni C, Vaure C, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 2002;196:541. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 2005;65:8479. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- 47.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J. Immunol. 2004;172:4676. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 48.Tadokoro CE, Shakhar G, Shen S, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 2006;203:505. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanig J, Lutz MB. Suppression of mature dendritic cell function by regulatory T cells in vivo is abrogated by CD40 licensing. J. Immunol. 2008;180:1405. doi: 10.4049/jimmunol.180.3.1405. [DOI] [PubMed] [Google Scholar]
- 50.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 2008;205:825. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 2008;205:625. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp. Med. 2002;195:1523. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.