Abstract
Objective
The International Association for the Study of Lung Cancer (IASLC) proposed a revision to the Union Internationale Contre le Cancer (UICC-6) staging system for non–small cell lung cancer. The goal of our study was to compare these systems in patients undergoing surgery for non–small cell lung cancer to determine whether one system is superior in staging operable disease.
Methods
Pathologic stages in 1154 patients undergoing complete resection over a 9-year period were analyzed. Patients were assigned a stage based on both IASLC and UICC-6 systems. We tested for statistically meaningful differences between the two staging systems using the Wilcoxon signed rank test and the permutation test.
Results
The IASLC system is more effective than the UICC-6 system at ordering and differentiating patients (P = .009). Application of the IASLC system resulted in 202 (17.5%) patients being reassigned to a different stage (P = .012), with the most common shifts occurring from IB to IIA and IIIB to IIIA. The 5-year and median survivals of the IASLC IIIA patients including those shifted from the UICC-6 IIIB were 37% and 35 months, respectively. Reclassifying UICC-6 IIIB to IASLC IIIA did not reduce survival for the newly characterized IIIA cohort.
Conclusion
Our data confirm that the proposed IASLC staging system is more effective at differentiating stage than the UICC-6 system. Reclassifying patients from UICC-6 IIIB to IASLC IIIA will shift some patients from a stage previously considered unresectable to a stage frequently offered surgical resection. Further study and validation of the IASLC system are warranted.
Despite the overall poor prognosis of patients with lung cancer, there are subsets of patients who benefit from treatment.1–4 Effective staging systems stratify patient survival and can be used to assess outcome of defined patient subgroups after treatment. The sixth edition of the Union Internationale Contre le Cancer (UICC-6) and the American Joint Committee on Cancer (AJCC) has served as the current tumor, node, metastases (TNM) staging system for non–small cell lung cancer (NSCLC) since 2002.5 The UICC-6 system is derived from the 1997 staging system proposed by Mountain.6 This staging system was based on 5319 patients treated for primary lung cancer at The University of Texas—M. D. Anderson Cancer Center (UTMDACC) (4351 patients) from 1975 to 1988 or by the National Cancer Institute Cooperative Lung Cancer Study Group (968 patients) from 1977 to 1982. This represents primarily a single-institution experience from a single country. The current staging system has considerable intrastage heterogeneity with groups within a stage varying widely in prognosis.
In 1998 the International Association for the Study of Lung Cancer (IASLC) staging project was initiated to develop the next revision of the current UICC-6 system.7–11 The proposed revision represents data collected from 100,869 patients from Europe, Australia, Asia, and North America. The data were analyzed by Cancer Research and Biostatistics and the IASLC International Staging Committee. The revised system proposes changes to the T and M classifications (Table 1) and overall stage groupings (Table 2). The revised TNM staging has been submitted for approval to the UICC. The IASLC system has yet to be independently evaluated.
TABLE 1.
Comparison of T and M stage of UICC-6 and IASLC staging systems
| A. UICC-6 staging system |
| Tx: Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0: No evidence of primary tumor |
| Tis: Carcinoma in situ |
| T1: Tumor 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, without evidence of invasion more proximal than the lobar bronchus |
T2: Tumor more than 3 cm in diameter; or tumor with any of the following features:
|
T3: Tumor more than 7 cm or
|
T4: Tumor of any size that invades any of the following:
|
| M1: Distant metastases |
| B. IASLC staging system |
| Tx: Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
| T0: No evidence of primary tumor |
| Tis: Carcinoma in situ |
| T1: Tumor 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, without evidence of invasion more proximal than the lobar bronchus |
| T1a: Tumor 2 cm or less in greatest dimension |
| T1b: Tumor more than 2 cm but not more than 3 cm in greatest dimension |
T2: Tumor more than 3 cm but not more than 7 cm or tumor with any of the following features:
|
| T2a: Tumor more than 3 cm but not more than 5 cm in greatest dimension |
| T2b: Tumor more than 5 cm but not more than 7 cm in greatest dimension |
T3: Tumor more than 7 cm or
|
| T4: Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body or carina Separate tumor nodule(s) in a different ipsilateral lobe |
| M1a: Tumor nodule in contralateral lung, tumor with pleural nodules, malignant effusion |
| M1b: Distant metastases |
UICC, Union Internationale Contre le Cancer; IASLC, International Association for the Study of Lung Cancer.
TABLE 2.
Comparison of TNM stage groupings of IASLC versus UICC-6 staging systems
| Stage | UICC-6 | IASLC |
|---|---|---|
| Stage IA | T1 N0 M0 | T1a N0 M0 |
| T1b N0 M0 | ||
| Stage IB | T2 N0 M0 | T2a N0 M0 |
| Stage IIA | T1 N1 M0 | T1a N1 M0 |
| T1b N1 M0 | ||
| T2a N1 M0 | ||
| T2b N0 M0 | ||
| Stage IIB | T2 N1 M0 | T2b N1 M0 |
| T3 N0 M0 | T3 N0 M0 | |
| Stage IIIA | T3 N1 M0 | T1a N2 M0 |
| T1–3 N2 M0 | T1b N2 M0 | |
| T2a N2 M0 | ||
| T2b N2 M0 | ||
| T3 N1 M0 | ||
| T3 N2 M0 | ||
| T4 N0 M0 | ||
| T4 N1 M0 | ||
| Stage IIIB | T4, Any N, M0 | T4 N2 M0 |
| Any T, N3 M0 | Any T, N3, M0 | |
| Stage IV | Any T, Any N, M1 | Any T, Any N, M1 a/b |
UICC, Union Internationale Contre le Cancer; IASLC, International Association for the Study of Lung Cancer.
The goal of our study was to apply the proposed changes to the current UICC-6 staging system to a cancer center population undergoing surgery for NSCLC and to directly compare the proposed IASLC and UICC-6 staging systems with respect to discrimination, monotonicity and intrastage heterogeneity.
PATIENTS AND METHODS
Population
This study analyzed data from a prospectively collected database of 1154 patients who underwent an R0 surgical resection for NSCLC at UTMDACC between 1998 and 2006. UTMDACC was a contributor of patient data for the IASLC study. Less than 5% of the patients in our study were the same patients as those in the IASLC study, and of those patients who were the same, the length of follow-up was different in the two studies. The two populations can be treated as two distinct data sets.
Institutional review board approval was obtained for this study, and informed consent was obtained from each participant.
Patients were excluded from analysis if histologic type was small cell carcinoma, neuroendocrine carcinoma, or predominantly bronchoalveolar carcinoma. Chemotherapy or radiation therapy administration did not exclude patients from analysis. A histologic classification of adenocarcinoma with bronchoalveolar features was included in the analysis. Pathologic staging of resected specimens was based on the application of the UICC-6 TNM staging system. Nodal (N) classification for each patient was determined either by systematic lymph node dissection or by lymph node sampling. Each specimen was then reassigned a surgical TNM classification and overall stage on the basis of the IASLC system.
Data on pathologic TNM classification, overall stage, and outcome for patients treated at UTMDACC were collected prospectively using Internet-based data collection entered by the health provider at the point of care. The data are subjected to periodic reviews for quality control.
Statistical Analyses
We tested for statistically meaningful differences between the UICC-6 and IASLC staging systems with respect to stage assignment using a Wilcoxon signed rank test. Statistical analysis was performed with SPSS software (SPSS, Inc, Chicago, Ill).
Survival probabilities were assessed by the Kaplan–Meier method calculated from the date of surgery until death or most recent follow-up. Operative mortality was excluded from survival analysis to allow for assessment of long-term rather than short-term mortality. Each patient was assigned a T classification and overall stage grouping on the basis of both UICC-6 and IASLC staging systems (Tables 1 and 2). The prognostic significance of overall stage using both the UICC-6 and IASLC systems was determined by univariate analysis. The effectiveness of each staging system was evaluated statistically by a log–rank trend test. In addition, we also assessed whether one staging system is more effective than the other via a permutation test in which we construct differences in log–rank trend test statistics under random rearrangements (ie, permutations) of the original labels assigned to the observed data. By repetition of this process many times (eg, 10,000 times), a null distribution is created and used to assess the difference between the log–rank trend statistic under the original labels and the randomly permuted labels.12 Please see the included appendix for complete description of the statistical analysis.
RESULTS
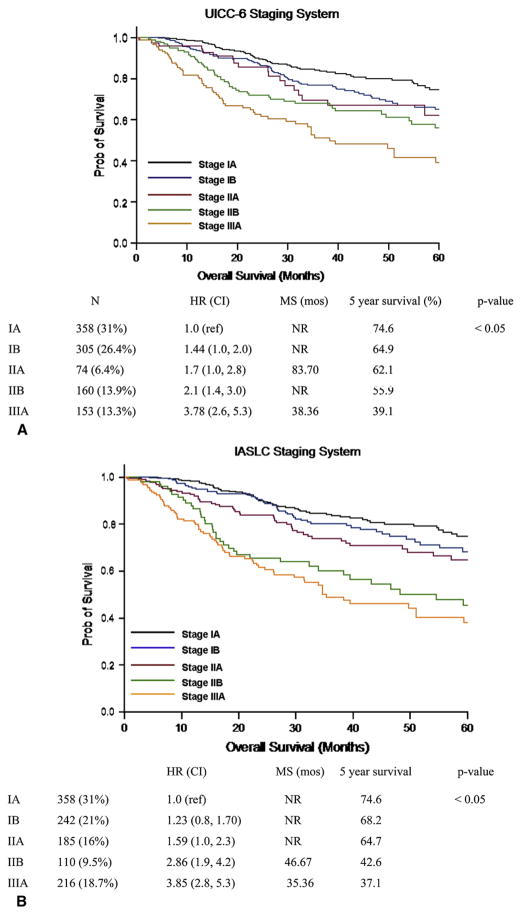
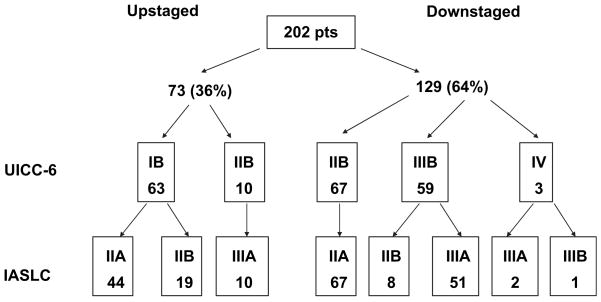
The population studied included all patients undergoing curative resection for NSCLC at UTMDACC between 1998 and 2006. The demographics of the study group are shown in Table 3. Each patient was assigned a pathologic T classification and overall stage on the basis of both the UICC-6 and IASLC staging systems. In 952 patients, application of the IASLC staging system resulted in no change from the UICC-6 assigned stage grouping. However, 202 (17.5%) patients were assigned a different stage grouping when the IASLC staging system was applied to their pathologic stage (Wilcoxon signed rank test; P = .012). Of these 202 patients with a change in stage grouping, 73 (36.2%) patients were upstaged and 129 (63.8%) patients were down-staged by application of the IASLC staging system (Figure 1). Patients assigned a higher stage by the IASLC staging system included patients shifted from UICC-6 IB to IASLC IIA (n = 44, 60.2%) and IASLC IIB (n = 19, 26%) or UICC-6 IIB to IASLC IIIA (n = 10, 13.7%). Of the 73 patients who were assigned a higher stage by IASLC, none was assigned a stage that would preclude surgical resection. Application of the IASLC system resulted in downstaging patients from UICC-6 stages IIB, IIIB, and IV. Sixty-seven (53.2%) patients classified as being in UICC-6 IIB were downstaged to IASLC stage IIA. One hundred four (9%) patients were staged by the UICC-6 as having advanced locoregional disease precluding surgical resection. Of these 104 patients with stage IIIB or IV disease, 59 (56%) were restaged by the IASLC system as having potentially respectable disease. Of these, 59 patients in UICC-6 IIIB were downstaged with 51 (40.4%) patients shifted to IASLC stage IIIA and 8 (6.3%) patients to IASLC stage IIB. Three patients were downstaged from stage IV to IIIA (n = 2) and IIIB (n = 1). The 5-year and median survivals of the IIIA patients in the IASLC system, including those shifted from UICC-6 IIIB, were 37% and 35 months, respectively. Shifting of patients from UICC stage IIIB, usually considered unresectable, to IASLC stage IIIA, in which patients are frequently offered surgical resection, did not result in a decrease in median or overall survival for IASLC stage IIIA patients. The 5-year survival of IASLC stage IIA including those shifted from UICC-6 IB was 64.7% (Figure 2).
TABLE 3.
Patient characteristics
| Age, y (median, range) | 66 (32–90) |
| Sex (N, %) | |
| Male | 607 (52.6) |
| Female | 547 (47.4) |
| Histology | |
| Adenocarcinoma | 658 (57%) |
| Squamous cell | 388 (33.6%) |
| NSCLC (NOS) | 62 (5.4%) |
| Large cell | 24 (2.1%) |
| Adenosquamous | 22 (1.9%) |
| Procedure | |
| Lobectomy/bilobectomy | 947 (82.1%) |
| Pneumonectomy | 94 (8.1%) |
| Wedge resection | 65 (5.6%) |
| Segmentectomy | 48 (4.2%) |
NSCLC, Non–small cell lung cancer; NOS, not otherwise specified.
FIGURE 1.
Kaplan–Meier survival by overall stage for UICC-6 (A) and IASLC (B). HR, Hazard ratio; CI, confidence interval; MS, median survival; NR, not reached. UICC, Union Internationale Contre le Cancer; IASLC, International Association for the Study of Lung Cancer.
FIGURE 2.
Shifting of stage after application of IASLC system. IASLC, International Association for the Study of Lung Cancer.
Statistical Comparison of UICC6 and IASLC Staging Systems
We assessed each staging system’s ability to discriminate between lower stage and higher stage patients with respect to overall survival and monotonicity as assessed by strong inverse relationship between stage and overall survival using a permutation test described in the appendix. The IASLC staging system is significantly more effective with respect to discrimination and monotonicity than the UICC-6 system. Application of the permutation test on patients with operable disease showed that the IASLC staging system is more effective that the UICC-6 system at predicting overall survival of patients with operable disease on the basis of stage (P = .009).
DISCUSSION
Our study aim was to apply the IASLC T classification and overall stage groupings to a population of patients who underwent complete surgical resection for NSCLC at UTMDACC between 1998 and 2006. The proposed changes to the staging system represent a major change that will result in significant shifts of patients into higher or lower stages. We asked the question whether application of the IASLC staging system to a cancer center population replicates the findings of the IASLC International Staging Committee and whether the new IASLC staging system is an improvement over the UICC-6 staging system. Our findings confirmed the ordering of stages reported for the IASLC staging system. Furthermore, direct comparison of the IASLC and UICC-6 staging systems using the permutation test demonstrated that the IASLC system is more effective at differentiating high, mid, and low stage groupings than the current UICC-6 system at a high level of statistical significance. This increased effectiveness of the IASLC staging system may help identify those patients at higher risk for recurrence. The improved stratification of survival and limitation of heterogeneity among patients within a stage may have important implications with regard to clinical research and adjuvant treatment decisions.
Our data also demonstrate a significant shifting of patients between stages when the IASLC system was applied to their pathologic stage. Of the 1154 patients in the study, 202 (17.5%) changed stage when the IASLC system was applied. One hundred four (9%) patients were staged by the UICC-6 as having advanced locoregional disease precluding surgical resection. Of these 104 patients with stage IIIB or IV disease, 59 (56%) were restaged by the IASLC system as having potentially resectable disease. Of the 73 patients who were assigned a higher stage by IASLC, none was assigned a stage that would preclude resection.
There are several limitations to this analysis. The study was based on a single-institution experience with a relatively small number of patients. The data were entered into a database prospectively but the patients were not entered into this study on a protocol. The type of surgical resection and extent of nodal dissection were left to the discretion of the operating surgeon. Also, there was no centralized pathologic review, and each pathologic specimen was evaluated on a case-by-case basis. The strength of the study lies in the prospective data collection, uniformity of the staging procedures for this patient population, and the independent evaluation by our statistician using a novel application of the permutation test to evaluate the two staging systems at ordering and differentiating patients between stages.
Several controversies will undoubtedly arise with the adoption of the IASLC staging system.
In the current UICC-6 system, T4 lesions are staged as IIIB regardless of lymph node status and are considered unresectable except in special circumstances. In this study, shifting of stage with application of the IASLC may potentially alter the management of 134 (11.6%) patients. Sixty-three of these patients were upstaged from a stage where surgery alone is the recommended treatment to a stage where adjvant chemotherapy may be considered.13–15 Additionally, 10 patients were upstaged to a stage where neoadjuvant chemotherapy is frequently offered (stage II to IIIA). The role of adjuvant and neoadjuvant chemotherapy in these patient populations may need to be re-evaluated. The IASLC system T4 lesions would be considered as IIIA or IIIB and the designation is based on the presence of absence of mediastinal nodal metastases. Satellite nodules in the ipsilateral primary lobe are considered unresectable T4 (stage IIIB) disease by UICC-6 criteria but T3 (stage IIB or IIIA) and potentially resectable by IASLC. Additionally, a satellite nodule in the ipsilateral lung but outside the primary lobe is unresectable M1 (stage IV) in the UICC-6 system and potentially resectable T4 (stage IIIA or IIIB) by IASLC. The optimal treatment strategy for these stages needs to be re-evaluated. Further study and validation of IASLC staging system and its effects on patient care are warranted.
Acknowledgments
This work was partially supported by grants from the National Cancer Institute and the National Institute of Health: Specialized Program of Research Excellence (SPORE) in Lung Cancer (2P50-CA70907); by The University of Texas M. D. Anderson Cancer Center Support Core Grant (CA 16672); by a grant from the Tobacco Settlement Funds as appropriated by the Texas State Legislature (Project 8); by the W. M. Keck Foundation; and a sponsored research agreement with Introgen Therapeutics, Inc.
Abbreviations and Abstracts
- AJCC
American Joint Committee on Cancer
- IASLC
International Association for the Study of Lung Cancer
- NSCLC
non–small cell lung cancer
- TNM
tumor, node, metastasis
- UICC
Union Internationale Contre le Cancer
- UTMDACC
University of Texas M. D. Anderson Cancer Center
APPENDIX
The main purpose of this section is to provide a formal statistical test for comparing two staging systems (the standard AJCC nodal staging system for esophageal cancer and a modified AJCC nodal staging system) with respect to assessing the discrimination between lower and higher stage disease and assessing the monotonic relationship between stage and survival. Before we can compare these two staging systems, we must first define what makes a staging system effective. We also must define how to quantify this effectiveness, and last, we must have a way of statistically comparing the effectiveness of the two staging systems.
The characteristic that defines the effectiveness of any staging system is its ability to differentiate, within a given disease, between patients with low-stage patients (those patients who survive a long time), mid-stage patients (patients who survive a moderate amount of time, and high-stage patients (patients who survive a relatively short amount of time). Thus an effective staging system is characterized by (1) the ability to discriminate between lower and higher stage patients with respect to survival and (2) a monotone decreasing relationship between stage and survival; this monotone relationship is quantified graphically by Kaplan–Meier curves. Moreover, this type of monotone relationship between stage and survival may be quantified numerically by a log–rank trend test.A1 This statistic is used because it characterizes the effectiveness of a staging system as defined above: the more effective the staging system (ie, the stronger the relationship between stage and survival), the larger the value of the log–rank trend test statistic. Inasmuch as the log–rank trend test is available and can easily be calculated,A2 we chose this statistic as a metric for measuring the strength of the association between stage and survival.
Once we have quantified the strength of the relationship between stage and survival of each of the two staging systems (ie, assessed the effectiveness of each staging system) using the log–rank trend test statistic, we also need to assess whether one staging system has a stronger relationship between stage and survival than the other staging system. The complicating factor in assessing the difference in effectiveness of the two staging systems is that the same set of patients are categorized under both systems inducing correlation between the two log–rank trend test statistics. We address this complicating factor by assessing differences between staging systems in the strength of the relationship between stage and survival via a permutation (randomization) test. A permutation test is a type of hypothesis test in which the null distribution is obtained by calculating possible values of the test statistic under random rearrangements (ie, permutations) of the original labels assigned to the observed data.A3 By repeating this process many times (eg, 1000 times), we create a null distribution. Creation of a null distribution in this way only differs from null distributions derived from statistical theory (eg, standard normal, χ2, F) in how the null distribution is obtained but does not differ in how they are used or interpreted. An added benefit of using null distributions derived from permutation tests is that they can be used in situations in which the null distribution is difficult to construct analytically (as in this case).
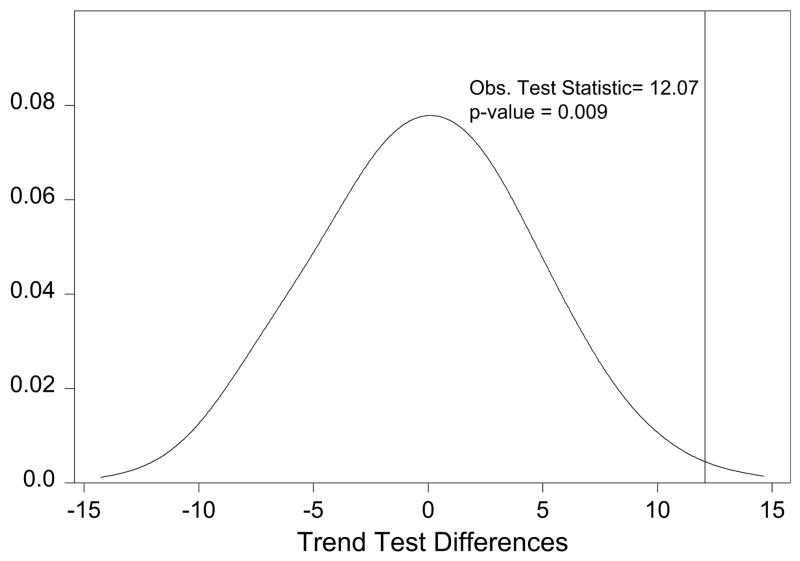
Each of the staging systems under consideration in this analysis has five staging categories. We call these five categories stage 1A, stage 1B, stage IIA, stage IIB, and stage IIIA. Under the null hypothesis for our permutation test, we assume that the two staging systems are exchangeable. This means that under the null hypothesis we assume that there is no difference between the two staging systems with respect to the strength of each staging system’s relationship between stage and survival. The alternative hypothesis is that there is difference in the two staging systems with respect to the strength of the relationship between stage and survival. To assess these hypotheses, we construct our test statistic, which is the difference in the two trend tests calculated under each staging system. For the observed data, the difference in the two staging system log–rank test statistics is 12.07 (53.88 for the UICC-6 staging system and 65.95 for the IASLC staging system). These log–rank trend tests tell us that both methods show a strong relationship between stage and survival. However, it appears that the IASLC staging system is better inasmuch as the observed test statistic is larger for this staging system and larger test statistics imply stronger evidence that the null hypothesis (ie, no relationship between stage and survival) should be rejected. To formally test whether the IASLC staging system is indeed statistically better (and assess whether the differences in test statistics may only be due to chance), we construct our null distribution to which this observed test statistic will be compared by performing the following steps:
For each patient with 50% probability, we randomly rearrange (ie, permute) the staging system labels originally assigned to that patient. That is, for a given patient the stage assigned under the standard staging system is switched and the stage assignment is now considered to have been assigned under the modified system and vice versa).
Once all patients have been permuted, we calculate the log–rank trend test statistic for the two staging systems and record the permuted difference in log–rank trend tests.
We repeat steps 1 and 2 a total of 1000 times.
The null distribution we construct using this method is given in Appendix Figure 1.
APPENDIX FIGURE 1.
Distribution of differences under the null distribution.
As shown, the differences in the trend statistics under the null distribution are centered around 0 as one would expect if there were no difference between staging systems in their ability to differentiate between low, middle, and high stage patients. Moreover, the probability of observing a difference in trend statistics is rare inasmuch as 12.07 is only 0.009 under the null hypothesis of no difference in the two staging systems.
Appendix References
- A1.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. Berlin, New York: Springer-Verlag; 1997. [Google Scholar]
- A2.Cantor A. Extending SAS survival analysis techniques for medical research. Cary (NC): SAS Institute; 1997. [Google Scholar]
- A3.Efron B, Tibshirani R. An introduction to the bootstrap. London, New York: Chapman & Hall Ltd; 1993. [Google Scholar]
Footnotes
Read at the Eighty-eighth Annual Meeting of The American Association for Thoracic Surgery, San Diego, Calif, May 10–14, 2008.
References
- 1.Naruke T, Goya T, Tsuchiya R, Suemasu K. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96:440–7. [PubMed] [Google Scholar]
- 2.Thomas PA, Piantadose S Lung Cancer Study Group. Postoperative T1 N0 non–small cell lung cancer. J Thorac Cardiovasc Surg. 1978;94:349–54. [PubMed] [Google Scholar]
- 3.Martini N, Burt ME, Bains MS, McCormack PM, Rusch VW, Ginsberg RJ. Survival after resection of stage II non–small cell lung cancer. Ann Thorac Surg. 1992;54:460–6. doi: 10.1016/0003-4975(92)90435-7. [DOI] [PubMed] [Google Scholar]
- 4.Burt ME, Pomerantz AH, Bains MS, McCormack PM, Kaiser LR, Hilaris BS, et al. Results of surgical treatment of stage III lung cancer invading the mediastinum. Surg Clin North Am. 1987;67:987–1000. doi: 10.1016/s0039-6109(16)44337-9. [DOI] [PubMed] [Google Scholar]
- 5.AJCC cancer staging handbook/American Joint Committee on Cancer. 6. Philadelphia: Lippincott-Raven; 2002. [Google Scholar]
- 6.Mountain CF. Revisions in the international staging for lung cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 7.Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 8.Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 9.Goldstraw P, Crowley J, Chansky K, Giroux D, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 10.Rusch VW, Crowley J, Giroux DJ, Goldstraw P, Im JG, Tsuboi M, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:603–12. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 11.Postmus PE, Brambilla E, Chansky K, Crowley J, Goldstraw P, Patz EF, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2007;2:686–93. doi: 10.1097/JTO.0b013e31811f4703. [DOI] [PubMed] [Google Scholar]
- 12.Hofstetter W, Correa AM, Bekele N, Ajani JA, Phan A, Komaki RR, et al. Proposed modification of nodal status in AJCC esophageal cancer staging system. Ann Thorac Surg. 2007;84:365–75. doi: 10.1016/j.athoracsur.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 13.The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 14.Douillard J, Rossel R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage 1B-111A non–small cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R, Gómez-Codina J, Camps C, Maestre J, Padille J, Cantó A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–8. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]