Abstract
Biomedical imaging has become an important tool in the study of “-omics” fields by allowing the noninvasive visualization of functional and molecular events using in vivo staining and reporter gene approaches. This capacity can go beyond the understanding of the genetic basis and phenotype of such respiratory conditions as acute bronchitis, adult respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), and asthma and investigate the development of disease and of therapeutic events longitudinally and in unperturbed environments. Herein, we show how the application of novel quantitative optical imaging methods, using transillumination and fluorescence molecular tomography (FMT), can allow visualization of pulmonary inflammation in small animals in vivo. The results confirm prior observations using a protease-sensitive probe. We discuss how this approach enables in vivo insights at the system level as to the dynamic role of proteases in respiratory pathophysiology and their potential as therapeutic targets. Overall, the proposed imaging method can be used with a significantly wider range of possible targets and applications in lung imaging.
Keywords: fluorescence; tomography; proteases; lung; inflammation, in vivo
Investigation of the respiratory system at the cellular, biochemical, and molecular level is critical in understanding lung disease and designing effective and novel strategies for prevention and intervention. Our knowledge of the cells and mediators that are involved in lung diseases has increased immensely during the last decade (1–3). This knowledge provides the basis for improving the rational development of therapeutic strategies that may relieve or prevent the development of symptoms.
Coupled to the importance of understanding the genetic basis and phenotype of the disease is the ability to visualize the associated molecular processes in vivo. Fluorescence molecular tomography (FMT) is a technology developed to quantitatively image the up-regulation and function of tumoral proteases, cellular receptors, and other proteins (4). Recently this technology has been applied to in vivo imaging of brain, mammary, and lung tumors and for studying tumor angiogenesis and chemotherapeutic effects in entire animals (5, 6). The technique may be ideally also suited for studying lung diseases and responses to environmental stimuli noninvasively and in vivo in small animals, especially if multi-projection approaches are implemented (5). This is in contrast to the mainstay of fluorescence imaging techniques currently available, such as microscopy, which does not penetrate under the tissue surface for more than about 500 μm, or photographic methods that penetrate to only superficial depths (∼2–3 mm) and yield single projection qualitative images. The FMT application to lung imaging is foreseen to play a markedly different role than microscopy, since the method achieves macroscopic resolutions similar to the ones of nuclear imaging. The benefits of FMT are associated with its ability for three-dimensional imaging and importantly the use of physical models of photon propagation that can account for the nonlinear signal variations in tissues as a function of (1) fluorochrome depth and (2) tissue optical properties and optical property heterogeneity. Therefore FMT can bring a new dimension in lung small animal research by allowing true quantification of optical signals and volumetric in vivo imaging of proteases using activatable probes, of receptors and other proteins by targeted probes or transgenic fluorescent protein gene-reporter technology, and of functional characteristics with more conventional agents tracking vascular changes and permeability. By imaging at different wavelengths, many of these targets may be concurrently resolved. By further using safe, nonionizing radiation, FMT may be well suited to study disease progression and longitudinally characterize drug efficacy.
The ability of imaging pulmonary inflammation has been recently corroborated by normalized transillumination data demonstrating marked changes between healthy and lipopolysaccharide (LPS)-treated animals with confirmed lung inflammation (7), as also presented as part of the 2009 Transantlantic Airway Conference. Herein we provide reconstructed data from this study that further support the FMT ability to visualize through the mouse lung. This showcased capacity opens the possibility for accurate and volumetric quantification of pulmonary inflammation based on fluorescence signals. While we have limited this study to imaging proteases within the cathepsin family, a significantly larger number of molecules and processes can be potentially targeted. Examples include matrix metalloproteinases, other proteases such as airway trypsin and mast cell tryptase, integrins, or physiologic responses associated with vascularization, blood flow, and blood oxygenation. We discuss how FMT can offer a new potent research imaging tool for lung disease and associated drug discovery and can become the method of choice for small animal molecular imaging of asthma and overall lung disease.
METHODS
Mouse Model
Female BALB/c mice from 8 to 10 weeks of age were used. LPS (Escherichia coli, serotype O55:B5; Sigma, St. Louis, MO) dissolved in 50 ml sterile 0.9% NaCl was instilled intratracheally via a cannula followed by 150 ml of air, using a high-pressure syringe attached to a microspray needle. LPS dosage ranged from 10 to 20 mg/mouse. Mice were left in an upright position for 10 minutes after instillation to allow for thorough distribution of LPS throughout the lungs. Instillation was performed under isoflurane anesthesia. Overall, nine experimental animals and three control animals (no LPS treatment) were studied herein.
Fluorescent Probes
Six hours after LPS instillation and 24 hours before FMT imaging, 2 nmol of the cathepsin-activatable probe Prosense (excitation wavelength = 680 nm; emission wavelength ∼700–740 nm; Visen Medical, Woburn, MA) were injected via tail vein in all animals (experimental and control). This probe is optically silent until it interacts with cathepsins, after which it becomes brightly fluorescent.
Fluorescence Molecular Tomography
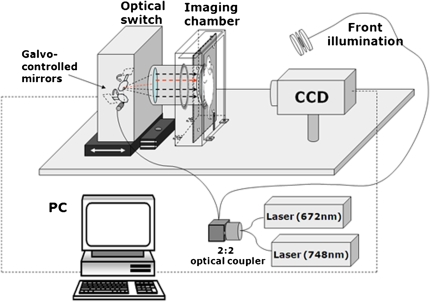
The system employed in this study was a home-built FMT scanner that employed noncontact approaches to avoid the use of fibers and to use direct coupling of highly sensitive CCD cameras onto tissue (8). This technology yields high-quality datasets and can offer high spatial sampling of diffusive photon fields that have propagated through the animal. Data sets obtained at high spatial sampling (for example, by assuming a photon measurement every 1 or 0.5 mm) have been shown necessary to improve imaging quality and performance (9). In the FMT prototype employed, a light beam was focused at the back of the animal, as seen in Figure 1, and sequentially scanned forming two-dimensional grids. Each light spot implements different diffusive projections through the animal. These projections are captured with a CCD camera through the glass window against which animals are very mildly pressed (< 300 Pa) and combined into a mathematical inversion model to obtain image reconstruction. This design achieved ∼millimeter resolution in the corresponding FMT reconstructions (10), at the center of the animal (worst case scenario), along the plane parallel to glass window but only 3 to 4 mm along the axis perpendicular to the glass window due to the limited view projections used along this dimension.
Figure 1.
Experimental fluorescence molecular tomography (FMT) setup employed in the experiments. This home-made system allows for tomographic fluorescence reconstructions in mice, employing limited view angle projections in analogy to X-ray tomosynthesis.
MRI and Image Co-Registration
Before FMT, mice were placed in a 1.3-cm-thick holder constructed of Delrin and designed to maintain the mouse in a fixed positioning and orientation in order to ensure identical positioning in both MRI and FMT. Mice were imaged on a 4.7-T Bruker imaging system (Pharmascan, Karlsruhe, Germany) using a T1-weighted sequence. Immediately after MRI, mice were imaged in the FMT system using the Delrin holder for proper positioning. In this manner, accurate registration between MRI and FMT data could be obtained for anatomical guidance of the FMT data.
Lung Histology
After thoracotomy, the lungs were removed and the trachea was cannulated with an 18 G × 1/2 in. needle. The lungs were slowly filled with 1 ml of O.C.T. compound, after which they were embedded in O.C.T. and frozen at −80°C. Five-micron sections were stained with hematoxylin and eosin (H&E) for histologic analysis.
RESULTS
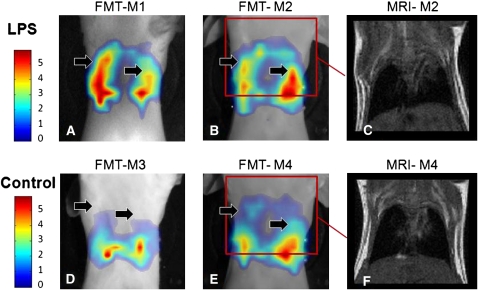
Figure 2 contains FMT and MRI images from four mice (two experimental [M1, M2] and two control [M3, M4]). Coronal MRI slices, passing from the center of the animal, are shown for mice M2, M4 for anatomical guidance. Color FMT signals are superimposed on mouse photographs, obtained under identical placement conditions, after a threshold was applied to allow co-visualization of the reconstructed data and their x-y position in relation to the animal imaged. The FMT images showcase a marked difference in fluorescence signals, indicated by the black solid arrows, compared with control. These signal differences are indicative of higher activation of the probe, due to protease up-regulation in the inflamed lungs. These are tomographically processed data of previously shown transillumination data (7), and confirm the observations obtained in transillumination mode. In both the transillumination and the FMT data, it is important to note that the data have significantly reduced sensitivity to the background optical properties, since they are corrected for tissue attenuation (11). This is achieved by obtaining attenuation measurements through the mouse immediately after the acquisition of fluorescence measurements. For practical purposes these measurements are captured at the excitation wavelength and take only a fraction of the acquisition time (∼1 min) that it takes for the fluorescence measurements (∼6 min). They therefore impart more accuracy compared with simple, single-wavelength observations as in the case of acquiring only the fluorescence wavelength. The particular methodology then employed to capitalize on the presence of a measurement that tracks intrinsic tissue attenuation is the use of the normalized Born (nBorn) approximation (12)—that is, a tomographic scheme that divides the fluorescence measurements with corresponding measurements after tissue illumination at the excitation (and possibly the emission) wavelength and similarly normalizes the theoretical model used for photon propagation. Photon attenuation measurements in this case were based on analytical solutions of the diffusion approximation, as previously described (12), whereas boundary conditions were implemented using the Kirchhoff approximation method (13). The optical properties utilized in this calculation were obtained from previous measurements under similar placement conditions (14), but due to the use of the nBorn method, the final images are not sensitive to their exact knowledge. In fact, in a previous study we have studied the nBorn accuracy, as a function of background optical heterogeneity, and found that up to 4× background attenuation changes (due to scattering or absorption), with size and spatial heterogeneity similar to that seen in tissues (due to the heart, rib cage, etc.), leads to less than 15% errors on the intensity of the reconstructed fluorescence activity on the FMT image and less than 7% on the reconstructed size of objects (11). This performance is essential in rendering optical tomography as a useful imaging method.
Figure 2.
Fluorescence molecular tomography (FMT) of lung inflammation in mice, compared with imaging of control mice. (A, B) FMT slices (color) from two experimental animals (M1, M2) superimposed onto mouse photographs (grayscale). (C) MRI corresponding to the red rectangle of B. (D, E) FMT slices from control animals. While the fluorescence probe can be seen accumulating in the liver, no significant activity is visualized in the lung, as marked by the black arrows. (F) MRI of the animal in e corresponding to the red rectangle drawn on E. All images are shown in the same colorscale, herein in relative increase units over baseline.
The FMT images provided in Figure 2 further depict activity on the lower part of the lung, coming from probe activation in the liver. This signal is common when using enzyme-activatable probes. As this signal is in principle similar in all mice, it can be used as a reference measurement to further substantiate the differences seen between experimental and control measurements.
Optical methods offer a number of practical advantages for the study of asthma or related lung disease in small animal models. The easy implementation of multi-spectral measurements offers a straightforward method for differentiating multiple targets. This feature can be used to monitor different molecular pathways or a combination of physiologic and cellular responses. It can also facilitate the use of internal controls on the same animal by co-injecting appropriate probes. Furthermore, fluorochromes are stable and can be easily stored, avoiding time-sensitive production techniques and high-investment facilities as in the case of radioisotopes. While the utility and powerful molecular imaging applications of nuclear imaging is unquestionable, this study supports evidence that FMT can resolve molecular-based signatures from the lung, offering a potent approach for small animal–based research.
Supported in part by NIBIB R01EB004382 and by the European Union FP7 FMT-XCT research grant.
Conflict of Interest Statement: V.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jones H, Marino P, Shakur B, Morrell N. In vivo assessment of lung inflammatory cell activity in patients with COPD and asthma. Eur Respir J 2003;21:567–573. [DOI] [PubMed] [Google Scholar]
- 2.Cataldo D, Gueders M, Rocks N, Sounni N, Evrard B, Bartsch P, Louis R, Noel A, Foidart J. Pathogenic role of matrix metalloproteases and their inhibitors in asthma and chronic obstructive pulmonary disease and therapeutic relevance of matrix metalloproteases inhibitors. Cell Mol Biol 2003;49:875–884. [PubMed] [Google Scholar]
- 3.Barnes PJ. Molecular basis for corticosteroid action in asthma. Chem Immunol 2000;78:72–80. [DOI] [PubMed] [Google Scholar]
- 4.Ntziachristos V, Tung C, Bremer C, Weissleder R. Fluorescence-mediated tomography resolves protease activity in vivo. Nat Med 2002;8:757–760. [DOI] [PubMed] [Google Scholar]
- 5.Ntziachristos V, Ripoll J, Wang LHV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol 2005;23:313–320. [DOI] [PubMed] [Google Scholar]
- 6.Sosnovik D, Nahrendorf M, Deliolanis N, Novikov M, Aikawa E, Josephson L, Rosenzweig A, Weissleder R, Ntziachristos V. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation 2007;115:1384–1391. [DOI] [PubMed] [Google Scholar]
- 7.Haller J, Hyde D, Deliolanis N, de Kleine R, Niedre M, Ntziachristos V. Visualization of pulmonary inflammation using noninvasive fluorescence molecular imaging. J Appl Physiol 2008;104:795–802. [DOI] [PubMed] [Google Scholar]
- 8.Schultz R, Ripoll J, Ntziachristos V. Fluorescence tomography of tissues with non-contact measurements. IEEE Trans Med Imaging 2004;23:492–500. [DOI] [PubMed] [Google Scholar]
- 9.Graves E, Culver J, Ripoll J, Weissleder R, Ntziachristos V. Singular value analysis and optimization of experimental parameters in fluorescence molecular tomography. J Opt Soc Am A Opt Image Sci Vis 2004;21:231–241. [DOI] [PubMed] [Google Scholar]
- 10.Graves E, Ripoll J, Weissleder R, Ntziachristos V. A sub-millimeter resolution fluorescence molecular imaging system for small animal imaging. Med Phys 2003;30:901–911. [DOI] [PubMed] [Google Scholar]
- 11.Soubret A, Ripoll J, Ntziachristos V. Accuracy of fluorescent tomography in the presence of heterogeneities: study of the normalized Born ratio. IEEE Trans Med Imaging 2005;24:1377–1386. [DOI] [PubMed] [Google Scholar]
- 12.Ntziachristos V, Weissleder R. Experimental three-dimensional fluorescence reconstruction of diffuse media using a normalized Born approximation. Opt Lett 2001;26:893–895. [DOI] [PubMed] [Google Scholar]
- 13.Ripoll J, Ntziachristos V. From finite to infinite volumes: removal of boundaries in diffuse wave imaging. Phys Rev Letters 2006;96(17):173909 (and references therein). [DOI] [PubMed] [Google Scholar]
- 14.Niedre MJ, Turner GM, Ntziachristos V. Time-resolved imaging of optical coefficients through murine chest cavities. Biomed Optics 2006;11(6):064017-1-7. [DOI] [PubMed]