Abstract
Ever since the site and nature of airflow obstruction in chronic obstructive pulmonary disease was described by Hogg, Thurlbeck, and Macklem, investigators have been looking for methods to noninvasively measure the airway wall dimensions. Recent advances in computed tomography technology and new computer algorithms have made it possible to visualize and measure the airway wall and lumen without the need for tissue. However, while there is great hope for computed tomographic assessment of airways, it is well known that the spatial resolution does not allow small airways to be visualized and there are still concerns about the sensitivity of these measurements obtained from these airways. Optical coherence tomography is a new bronchoscopic imaging technique that has generated considerable interest because the spatial resolution is much higher than computed tomography. While relatively more invasive than computed tomography, it has the advantage of not exposing the patient to ionizing radiation. This review discusses some of the data surrounding these two imaging techniques in patients with chronic obstructive pulmonary disease. These imaging techniques are extremely important in the assessment of patients with chronic obstructive pulmonary disease because therapy that is designed to modulate the inflammation in airways may be contraindicated in subjects with the emphysema phenotype and visa versa. Therefore, these new imaging techniques are very likely to play a front-line role in the study of chronic obstructive pulmonary disease and will, hopefully, allow clinicians to phenotype individuals, thereby personalizing their treatment.
Keywords: computed tomography, airways, optical coherence tomography, chronic obstructive pulmonary disease
The last decade has seen monumental changes in the way that investigators study the lung in humans. Quantitative analysis of lung structure has always played a vital role in understanding the disease process because the underlying structure of the lung is vital to its function. With the advent of the computed tomography (CT) scanner and the ability to obtain “high-resolution images” of the lung without having to resort to biopsies or resection, investigators now had a method to study the lung “noninvasively.” This review will look at two noninvasive imaging techniques, CT and optical coherence tomography (OCT), used to measure airway structure.
COMPUTED TOMOGRAPHY
The initial high-resolution computed tomography (HRCT) images used 1 mm slice thickness and an edge-enhancing algorithm that made it possible to visualize the airway in a manner that was similar to gross pathology. This made the technique immediately popular with investigators, and there was a flurry of publications on how to best trace the airway wall to produce the best images (1–3). It was quickly realized that quantitative measurements of airway structure were very dependent on the display parameters of the image (4, 5), and that an automated computer approach that used the density attenuation values of the CT image was the correct method for analysis. Therefore, investigators turned their attention to developing algorithms that could measure the airway lumen and wall area on these CT scans. Some of the early approaches were very simple, using threshold techniques to find the lumen (6) and simple algorithms like the full-width at half maximum to assess the wall area (7, 8). It was quickly decided that while these simple approaches had merit, they were not always the most accurate at determining airway dimensions. More complex algorithms using model-based approaches to provide more reliable measurements were developed (9–13). Even though there is no consensus on the most appropriate method to measure airways, investigators have been using them to assess lung structure and function relationships in chronic obstructive pulmonary disease (COPD).
Early on, Nakano and coworkers used the full-width at half maximum method to measure the airway wall of the right apical segmental bronchus in a group of asymptomatic smokers and smokers with COPD (14). This was an important study, even though it is well known that the major site of airflow limitation is the small airway, which a segmental bronchus clearly is not. However, these investigators were still able to show that there was a correlation between the percent of the total airway (lumen plus wall) that was airway wall area (wall area percent [WA%]) and FEV1, FVC, and the residual volume (RV)/total lung capacity (TLC) (14). Other investigators have shown similar findings (15–18), and further work from Nakano and colleagues showed that there was a correlation between airway wall measurements obtained using CT and those obtained using histology (19). This is further backed up by other histologic data that show that in subjects with COPD, there is a thickening of the airway wall in the larger airways as well as the small airways (20). Other studies of large airways have shown that subjects with symptoms of chronic bronchitis have more airway wall thickening than those with airflow limitation but no symptoms (17, 18).
Because COPD is thought to be caused by airway wall remodeling, emphysema, or some combination of the two, it was hoped that CT could be used to differentiate the different COPD phenotypes. Since these phenotypes are likely to have a genetic component, Patel and coworkers used CT to quantify airway wall dimensions and emphysema in a cohort of subjects with COPD and their siblings (18). This study showed that there was a significant familial concordance of airway wall thickening such that if a subject with COPD has thickened airway walls, there is an increased odds ratio that their sibling would also have airway wall thickening (18). It has long been hypothesized that sex is responsible for differences in the airway wall dimensions. Using the NETT study, Martinez and colleagues showed that women had a higher airway wall area compared with lumen perimeter than men (21). However, this cohort is a highly selected group of subjects chosen to be candidates for lung volume reduction surgery. Recently, using a community sample of smoking control subjects and subjects with COPD, Grydeland and coworkers showed that the airway wall area was higher in subjects with COPD and higher in men regardless of disease status (22). These authors also showed that airway wall thickness decreased slightly with increasing age in subjects with and without COPD, and that it increased with smoking history only in the control group. These studies indicate that there are differences in the structure of the airway wall that are very likely under some form of genetic control.
What should be mentioned here is that most of the studies listed above were performed using traditional HRCT scans. During this time period, CT imaging took a leap forward in complexity with the introduction of the multi-detector row CT (MDCT) scanner. MDCT scanners have allowed the acquisition of sub-millimeter thickness images of the entire chest within a single breath-hold of 5 to 15 seconds. These new images can have the same resolution in the X, Y, and Z dimensions (isometric voxels), allowing images to be reconstructed in any orientation without loss of spatial resolution. This has greatly facilitated the visualization of airways and vessels that are oriented in a radial pattern around the pulmonary hila (Figure 1). Of course this now requires that a whole new group of airway algorithms be developed that take advantage of the new three-dimensional airway analysis (Figure 2). One of the first studies to compare the new three-dimensional airway analysis to lung function was by Hasegawa and colleagues, who examined airway wall area starting in the segmental bronchus (third generation), and successive generations of the airway tree out to the sixth generation (23). This study showed that as the airway became smaller (larger generation) the correlation with FEV1 increased, confirming what people have always known: that the small airways are the major site of airflow limitation.
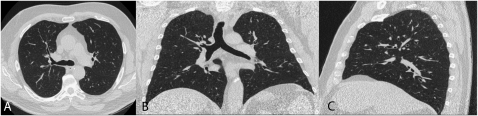
Figure 1.
This figure shows a thin slice (1-mm slice thickness) computed tomography (CT) scan acquired using a multi-detector CT scanner (A) in the traditional transverse plane and reformatted into (B) the coronal plane and (C) the sagittal plane.
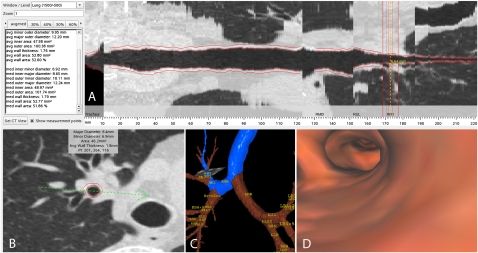
Figure 2.
A shows a multiplanar reformat of the right apical segmental bronchus (RB1). The cross-section of the airway at the point of measurement is shown in B, while the path from the trachea is outlined in blue on the three-dimensional airway tree reconstruction in C and internal view of the three-dimensional reconstructed airway lumen is shown in D. The airway tree was generated using Pulmonary Workstation 2.0 (VIDA Diagnostics Inc., Iowa City, IA).
As mentioned briefly above, there are limitations to the use of CT scanning to measure airways. The first and obvious limitation is the resolution of the CT scanner. In usual clinical CT scanning, the field of view limits the pixel size to approximately 0.5 mm in the X and Y dimension. Furthermore, until the recent advent of multi-slice CT scanners that can acquire images with 0.5-mm slice thickness; the CT slice thickness has limited the Z dimension to 1 mm. This means that the airways that are responsible for airflow limitation are below the resolution of the CT scanner. A CT method that is becoming quite popular to assess small airway disease is gas trapping using expiratory CT scans (Figure 3). One of the first studies to look at expiratory CT was performed by Gevenois and colleagues, who reported that expiratory CT scans did not accurately quantify emphysema but probably reflect air trapping (24). Expiratory CT scans have been used to quantify air trapping in post–lung transplant bronchiolitis (25, 26) and asthma (27–29), but have not been used extensively in COPD research. Lee and colleagues have shown that expiratory lung density correlates the BODE index and that the CT measurement of air trapping correlates with physiologic measurements of air trapping (30). Akira and coworkers also used expiratory CT scans in subjects with COPD and found that expiratory CT scans are associated with pulmonary function in subjects with severe obstruction, but not in subjects with less severe obstruction (31). One of the drawbacks of expiratory CT scans is that they expose the subject to the extra radiation associated with a second CT scan. However, it is becoming recognized that expiratory CT scans do provide very useful information, and it is very likely that more studies using this approach will be performed in the near future.
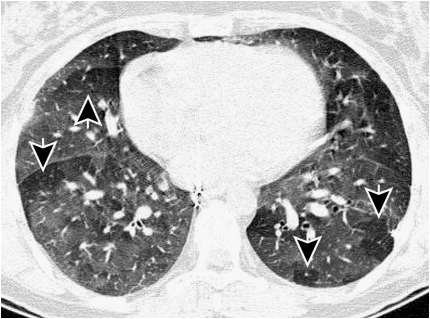
Figure 3.
This figure shows a thin slice (1 mm slice thickness) CT scan acquired after expiration. Most of the lung has increased density (less dark), but parts of the lung are exhibiting signs of gas trapping (arrows) and appear darker on the image. It is thought that these regions of gas trapping are caused by small airway narrowing.
A second limitation to the measurement of airways using CT scans is that there is no definitive data on the best algorithm to measure the airway wall. While a great deal of research has gone into airway wall algorithms (10, 11, 19, 23, 32–36), there is no clear indication that one algorithm provides more useful data than another one. Third, the analysis of airways using three-dimensional algorithms is still in its infancy, and while there is great hope that these data will provide more meaningful data than the simple sample of two-dimensional airways, the data are clearly lacking in this area. An obvious problem of the three-dimensional approach is that there are now many airways that can be “named” and measured, and because these data have never existed before, investigators do not know how many airways or how many airway paths to measure. It should also be noted that there are very few longitudinal studies of airways. Longitudinal analysis of airways is very problematic because the effect of CT image acquisition parameters such as X-ray dose, subject position, and volume of inspiration (to name a few) is completely unknown. It is very likely that the size of breath the subject takes will produce very different CT images of the airway tree, thereby affecting all of the data derived from the images. Finally, because airway measurements are still in their infancy and require specialized computer hardware and software, these analyses have a long way to go before they become practical in the clinical setting. As such they remain in the research domain and are limited in their applicability.
OPTICAL COHERENCE TOMOGRAPHY
Optical coherence tomography (OCT) is a new and promising relatively noninvasive imaging technique that can visualize cellular and extracellular structures at and up to 3 mm below the surface and has a spatial resolution of 3 to 16 μm (37–39). In principle, OCT is similar to B-type ultrasound, but instead of using sound waves, a low-coherence near-infrared light such as from a 1,300-nm superluminescent diode source is passed into the tissue. By detecting the back-scattered light as it interacts with tissue structures as a function of depth, a cross-sectional image is created through optical interferometry (Figure 4). The image contrast is the back-scattered light from interfaces at different depths in the tissue, due to the heterogeneity of optical refractive indices from different tissue compositions and densities. Changes in the extracellular matrix can be readily seen due to the strong back-scattering properties of collagen and elastin. The imaging procedure is performed using fiberoptic probes that can be miniaturized to enable imaging of airways down to the terminal bronchiole. Unlike ultrasound, light waves do not require liquid-based coupling medium and thus are more compatible with airway imaging. There are no associated risks to the subject from the weak near-infrared light. OCT has distinct advantages over CT for imaging small airways (the site of bronchiolitis) in vivo in that it has superior resolution approaching near-microscopic resolution and requires no ionizing radiation. While the procedure does involve local anesthesia and conscious sedation, it can be performed within minutes as part of a standard bronchoscopic examination. Furthermore, during the same procedure, bronchoalveolar lavage and bronchial brushing can be performed to retrieve bronchial fluid and cells for genomic and proteomic analyses. So, while being minimally invasive, the spatial information and collection of other information makes it very attractive as a tool for clinical investigation.
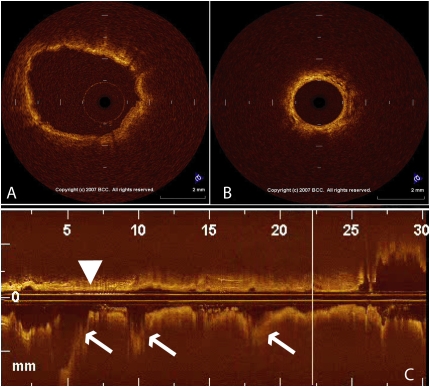
Figure 4.
This figure shows an optical coherence tomography (OCT) image of (A) a large airway and (B) a small airway, as well as (C) a reconstructed image of the bronchial path. The airway wall is indicated by the arrowhead and the airway branch points are indicated by the arrows.
In addition to morphometric information, functional OCT, such as spectral domain OCT and Doppler OCT, can also provide quantitative information on blood flow of blood vessels and microvasculature (40, 41). The physiologic state and biochemical composition of the bronchial tissues can also be determined using a combination of in vivo Raman spectroscopy and fluorescence spectroscopy. Raman spectroscopy uses the interaction of light with the vibrational modes of the major molecular components, while fluorescence spectroscopy uses the absorption and re-emission by fluorophors to identify compounds (42). Using a sheath catheter, OCT can be integrated with spectroscopic measurements to provide a map of the molecular structure of the airway tissue for molecules such as collagen and its cross links, elastin, and NADH/FAD (43). Development of these integrated tools will provide powerful tools to study the airway remodeling process and the effect of therapeutic intervention. To our knowledge, aside from our pilot study (see below), there have been no in vivo studies that have used OCT to examine airway remodeling in patients with COPD.
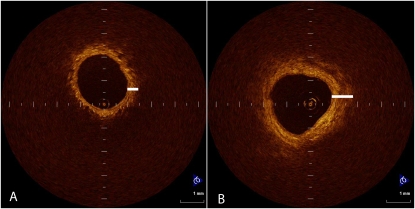
OCT imaging has been applied to study bronchial and lung tissues and has been shown to be promising (44–47). The first proof-of-principle study to apply OCT to study COPD used a small optical probe with an outer diameter of 1.5 mm which was inserted through the biopsy channel of the bronchoscope directly into a segmental bronchus of the lower lobe and advanced in 5-mm increments until the catheter fitted snugly into a small airway with a luminal diameter similar to the outer diameter of the OCT probe (1.5 mm) (48). The axial resolution of the OCT system was 16 ± 1 μm, and the lateral/transverse resolution was 20 to 30 μm. Axial profiles were digitized for each scan position to create a two-dimensional cross-section image; these were traced to measure the airway dimensions. These data were compared with CT measurements of the exact airway using a three-dimensional reconstruction of the airway tree (Pulmonary Workstation 2.0; VIDA Diagnostics, Inc., Iowa City, IA), and a strong correlation between CT and OCT measurements of lumen and wall area was observed. The correlation between FEV1% predicted and CT- and OCT-measured wall area (as percentage of the total area) of fifth-generation airways was very strong, but the slope of the relationship was much steeper using OCT than using CT, indicating greater sensitivity of OCT in detecting changes in wall measurements that relate to FEV1 (48). Furthermore, as FEV1% predicted decreased, the wall area as a percent of the total size of the airway increased, suggesting that airway remodeling of bronchioles contribute to airflow limitation of COPD. An example of the difference in airway structure between subjects with moderately severe COPD and a smoker of similar age but normal lung function is shown in Figure 5.
Figure 5.
This figure shows an optical coherence tomography (OCT) image from two different subjects. The subject in A has normal lung function and has a thinner airway wall, indicated by the short white line, while the subject in B has chronic obstructive pulmonary disease and a thicker airway wall. The airway lumen in each subject is the same size because the OCT probe just fits into each airway.
CONCLUSIONS
A thorough understanding of airway wall remodeling in COPD is vital to fully understand the pathogenesis of this devastating disease. This understanding of pathogenesis is especially important in the development of therapeutic interventions because the basic mechanisms behind the different phenotypes of this disease may dictate the type of treatment needed or contraindicated. Noninvasive imaging such as CT using both traditional inspiratory scans and expiratory scans and/or OCT may be two of the important techniques needed to understand the disease processes and phenotypes of the individual patients.
Funding: H.O.C. is a Canadian Institutes of Health Research/British Columbia Lung Association New Investigator and is funded in part by Pittsburgh COPD SCCOR NIH 1P50 HL084948 and R01 HL085096 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD to the University of Pittsburgh.
Conflict of Interest Statement: H.O.C. served as a consultant for GlaxoSmithKline (GSK) and AstraZeneca up to $1,000 and served on the Board or Advisory Board for GSK $1,001–$5,000. He received grant support from GSK $100,001 or more, Spiration Inc $50,001–$100,000, Wyeth Inc. $100,001 or more, NIH, and CIHR $50,001–$100,000. S.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.McNamara AE, Muller NL, Okazawa M, Arntorp J, Wiggs BR, Pare PD. Airway narrowing in excised canine lung measured by high-resolution computed tomography. J Appl Physiol 1992;73:307–316. [DOI] [PubMed] [Google Scholar]
- 2.Okazawa M, Muller NL, McNamara AE, Child S, Verburgt L, Pare PD. Human airway narrowing measured using high-resolution computed tomography. Am J Respir Crit Care Med 1996;154:1557–1562. [DOI] [PubMed] [Google Scholar]
- 3.Seneterre E, Paganin F, Bruel JM, Michel FB, Bousquet J. Measurement of the internal size of bronchi using high resolution computed tomography (HRCT). Eur Respir J 1994;7:596–600. [DOI] [PubMed] [Google Scholar]
- 4.Bankier AA, Fleischmann D, Mallek R, Windisch A, Winkelbauer FW, Kontrus M, Havelec L, Herold CJ, Hubsch P. Bronchial wall thickness: appropriate window settings for thin-section CT and radiologic-anatomic correlation. Radiology 1996;199:831–836. [DOI] [PubMed] [Google Scholar]
- 5.Webb WR, Gamsu G, Wall SD, Cann CE, Proctor E. CT of a bronchial phantom: factors affecting appearance and size measurements. Invest Radiol 1984;19:394–398. [DOI] [PubMed] [Google Scholar]
- 6.McNitt-Gray MF, Goldin JG, Johnson TD, Tashkin DP, Aberle DR. Development and testing of image-processing methods for the quantitative assessment of airway hyperresponsiveness from high-resolution CT images. J Comput Assist Tomogr 1997;21:939–947. [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y, Muller NL, King GG, Niimi A, Kalloger SE, Mishima M, Pare PD. Quantitative assessment of airway remodeling using high-resolution CT. Chest 2002;122:271S–275S. [PubMed] [Google Scholar]
- 8.Nakano Y, Whittall KP, Kalloger SE, Coxson HO, Pare PD. Development and validation of human airway analysis algorithm using multidetector row CT. Proc SPIE 2002;4683:460–469. [Google Scholar]
- 9.King GG, Muller NL, Whittall KP, Xiang QS, Pare PD. An analysis algorithm for measuring airway lumen and wall areas from high-resolution computed tomographic data. Am J Respir Crit Care Med 2000;161:574–580. [DOI] [PubMed] [Google Scholar]
- 10.Reinhardt JM, D'Souza ND, Hoffman EA. Accurate measurement of intrathoracic airways. IEEE Trans Med Imaging 1997;16:820–827. [DOI] [PubMed] [Google Scholar]
- 11.Saba OI, Hoffman EA, Reinhardt JM. Maximizing quantitative accuracy of lung airway lumen and wall measures obtained from X-ray CT imaging. J Appl Physiol 2003;95:1063–1075. [DOI] [PubMed] [Google Scholar]
- 12.San Jose Estepar R, Reilly JJ, Silverman EK, Washko GR. Three-dimensional airway measurements and algorithms. Proc Am Thorac Soc 2008;5:905–909. [DOI] [PubMed] [Google Scholar]
- 13.Leader JK, Zheng B, Rogers RM, Sciurba FC, Perez A, Chapman BE, Patel S, Fuhrman CR, Gur D. Automated lung segmentation in X-ray computed tomography: development and evaluaiton of a heuristic threshold-based scheme. Acad Radiol 2003;10:1224–1236. [DOI] [PubMed] [Google Scholar]
- 14.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med 2000;162:1102–1108. [DOI] [PubMed] [Google Scholar]
- 15.Berger P, Perot V, Desbarats P, Tunon-de-Lara JM, Marthan R, Laurent F. Airway wall thickness in cigarette smokers: quantitative thin-section CT assessment. Radiology 2005;235:1055–1064. [DOI] [PubMed] [Google Scholar]
- 16.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography: relation to clinical indices. Am J Respir Crit Care Med 2000;162:1518–1523. [DOI] [PubMed] [Google Scholar]
- 17.Orlandi I, Moroni C, Camiciottoli G, Bartolucci M, Pistolesi M, Villari N, Mascalchi M. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology 2005;234:604–610. [DOI] [PubMed] [Google Scholar]
- 18.Patel BD, Coxson HO, Pillai SG, Agusti AGN, Calverley PMA, Donner CF, Make BJ, Müller NL, Rennard SI, Vestbo J, et al. Airway wall thickening and emphysema show independent familial aggregation in COPD. Am J Respir Crit Care Med 2008;178:500–505. [DOI] [PubMed] [Google Scholar]
- 19.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005;171:142–146. [DOI] [PubMed] [Google Scholar]
- 20.Tiddens HA, Pare PD, Hogg JC, Hop WC, Lambert R, de Jongste JC. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med 1995;152:260–266. [DOI] [PubMed] [Google Scholar]
- 21.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 2007;176:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grydeland TB, Dirksen A, Coxson HO, Pillai SG, Sharma S, Eide GE, Gulsvik A, Bakke PS. Quantitative CT: Emphysema and airway wall area by gender, age and smoking. Eur Respir J (In press) [DOI] [PubMed]
- 23.Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:1309–1315. [DOI] [PubMed] [Google Scholar]
- 24.Gevenois PA, De Vuyst P, Sy M, Scillia P, Chaminade L, de Maertelaer V, Zanen J, Yernault J-C. Pulmonary emphysema: quantitative CT during expiration. Radiology 1996;199:825–829. [DOI] [PubMed] [Google Scholar]
- 25.de Jong PA, Dodd JD, Coxson HO, Storness-Bliss C, Pare PD, Mayo JR, Levy RD. Bronchiolitis obliterans following lung transplantation: early detection using computed tomographic scanning. Thorax 2006;61:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodd JD, de Jong PA, Levy RD, Coxson HO, Mayo JR. Conventional high-resolution CT versus contiguous multidetector CT in the detection of bronchiolitis obliterans syndrome in lung transplant recipients. J Thorac Imaging 2008;23:235–243. [DOI] [PubMed] [Google Scholar]
- 27.Busacker A, Newell JD Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009;135:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KY, Park SJ, Kim SR, Min KH, Choe YH, Jin GY, Lee YC. Low attenuation area is associated with airflow limitation and airway hyperresponsiveness. J Asthma 2008;45:774–779. [DOI] [PubMed] [Google Scholar]
- 29.Zeidler MR, Kleerup EC, Goldin JG, Kim HJ, Truong DA, Simmons MD, Sayre JW, Liu W, Elashoff R, Tashkin DP. Montelukast improves regional air-trapping due to small airways obstruction in asthma. Eur Respir J 2006;27:307–315. [DOI] [PubMed] [Google Scholar]
- 30.Lee YK, Oh YM, Lee JH, Kim EK, Kim N, Seo JB, Lee SD. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung 2008;186:157–165. [DOI] [PubMed] [Google Scholar]
- 31.Akira M, Toyokawa K, Inoue Y, Arai T. Quantitative CT in chronic obstructive pulmonary disease: inspiratory and expiratory assessment. AJR Am J Roentgenol 2009;192:267–272. [DOI] [PubMed] [Google Scholar]
- 32.Brown MS, Goldin JG, McNitt-Gray MF, Greaser LE, Sapra A, Li KT, Sayre JW, Martin K, Aberle DR. Knowledge-based segmentation of thoracic computed tomography images for assessment of split lung function. Med Phys 2000;27:592–598. [DOI] [PubMed] [Google Scholar]
- 33.King GG, Muller NL, Pare PD. Evaluation of airways in obstructive pulmonary disease using high-resolution computed tomography. Am J Respir Crit Care Med 1999;159:992–1004. [DOI] [PubMed] [Google Scholar]
- 34.Montaudon M, Berger P, de Dietrich G, Braquelaire A, Marthan R, Tunon-de-Lara JM, Laurent F. Assessment of airways with three-dimensional quantitative thin-section CT: in vitro and in vivo validation. Radiology 2007;242:563–572. [DOI] [PubMed] [Google Scholar]
- 35.Aykac D, Hoffman EA, McLennan G, Reinhardt JM. Segmentation and analysis of the human airway tree from three-dimensional X-ray CT images. IEEE Trans Med Imaging 2003;22:940–950. [DOI] [PubMed] [Google Scholar]
- 36.Tschirren J, Hoffman EA, McLennan G, Sonka M. Intrathoracic airway trees: segmentation and airway morphology analysis from low-dose CT scans. IEEE Trans Med Imaging 2005;24:1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimoto JG, Brezinski ME, Tearney GJ, Boppart SA, Bouma B, Hee MR, Southern JF, Swanson EA. Optical biopsy and imaging using optical coherence tomography. Nat Med 1995;1:970–972. [DOI] [PubMed] [Google Scholar]
- 38.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science 1991;254:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, Fujimoto JG. In vivo endoscopic optical biopsy with optical coherence tomography. Science 1997;276:2037–2039. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Milner TE, Srinivas S, Wang X, Malekafzali A, van Gemert MJ, Nelson JS. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Opt Lett 1997;22:1119–1121. [DOI] [PubMed] [Google Scholar]
- 41.Yang VX, Tang SJ, Gordon ML, Qi B, Gardiner G, Cirocco M, Kortan P, Haber GB, Kandel G, Vitkin IA, et al. Endoscopic Doppler optical coherence tomography in the human GI tract: initial experience. Gastrointest Endosc 2005;61:879–890. [DOI] [PubMed] [Google Scholar]
- 42.Short MA, Lam S, McWilliams A, Zhao J, Lui H, Zeng H. Development and preliminary results of an endoscopic Raman probe for potential in vivo diagnosis of lung cancers. Opt Lett 2008;33:711–713. [DOI] [PubMed] [Google Scholar]
- 43.Wagnieres G, McWilliams A, Lam S. Lung cancer imaging with fluorescence endoscopy. In: Mycek M, Pogue B, editors. Handbook of biomedical fluorescence. New York: Marcel Dekker; 2003. pp. 361–396.
- 44.Hanna N, Saltzman D, Mukai D, Chen Z, Sasse S, Milliken J, Guo S, Jung W, Colt H, Brenner M. Two-dimensional and 3-dimensional optical coherence tomographic imaging of the airway, lung, and pleura. J Thorac Cardiovasc Surg 2005;129:615–622. [DOI] [PubMed] [Google Scholar]
- 45.Lam S, Standish B, Baldwin C, McWilliams A, leRiche J, Gazdar A, Vitkin AI, Yang V, Ikeda N, MacAulay C. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res 2008;14:2006–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuboi M, Hayashi A, Ikeda N, Honda H, Kato Y, Ichinose S, Kato H. Optical coherence tomography in the diagnosis of bronchial lesions. Lung Cancer 2005;49:387–394. [DOI] [PubMed] [Google Scholar]
- 47.Whiteman SC, Yang Y, Gey van Pittius D, Stephens M, Parmer J, Spiteri MA. Optical coherence tomography: real-time imaging of bronchial airways microstructure and detection of inflammatory/neoplastic morphologic changes. Clin Cancer Res 2006;12:813–818. [DOI] [PubMed] [Google Scholar]
- 48.Coxson HO, Quiney B, Sin DD, McWilliams AM, Mayo JR, Lam S. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med 2008;177:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]