Abstract
This mini-review highlights developments that have been made over the past year to advance the construction of well-defined nanoscale objects to serve as devices for cell transfection. Design of the nanoscale objects originated from biomimicry concepts, using histones as the model, to afford cationic shell crosslinked knedel-like (cSCK) nanoparticles. Packaging and delivery of plasmid DNA, oligonucleotides, and peptide nucleic acids were studied by dynamic light scattering, transmission electron microscopy, gel electrophoresis, biological activity assays, RT-PCR measurements, flow cytometry, and confocal fluorescence microscopy. With the demonstration of more efficient cell transfection in vitro than that achieved using commercially-available transfection agents, together with the other features offered by the robust nanostructural framework, work continues toward the application of these cSCKs for in vivo molecular recognition of genetic material, for imaging and therapy targeted specifically to pulmonary injury and disease.
Keywords: biomimicry, transfection, cationic nanoparticle, gene delivery
BACKGROUND, MOTIVATION, AND INTRODUCTION
Using nature as inspiration is a common and longstanding approach to design functional devices. Often, those objects are of macroscopic dimensions, such as the development of airplanes from study of the flight of birds (1), but more recently, biomimicry has been applied increasingly toward the design, preparation, and study of nanoscopic materials. Advances that have been made with synthetic chemistry provide opportunities for the construction of well-defined synthetic materials having nanoscopic dimensions and features resembling biological nanomachines in structure, with the ultimate goal of attaining similar, yet controllable, functions. Nanoscience is the design, preparation, and study of nanoscale systems, and nanotechnology is the application of such systems. There is great promise for nanoscience to translate to medically relevant nanotechnologies.
The three inherent features of nanoscale materials that make them attractive for probing and manipulating biology, as nanomedicine devices, are their multi-functionality, capacity, and physical characteristics. Being smaller than microscopic particles, nanoscopic objects have a larger surface area–to-volume ratio for presentation of multiple types and numbers of active elements (e.g., imaging agents and tissue-selective targeting ligands). Being larger than small molecules, nanoscopic objects have a greater internal capacity for packaging, transport, and delivery of active elements (e.g., imaging agents and therapeutic agents). The nanoscopic dimension, therefore, takes advantage of both the surface area and internal volume of three-dimensional structures. In addition, the physical characteristics of being nanoscopic leads to different pharmacokinetics (2), simply based upon their size, avoiding rapid renal clearance as occurs for small molecules and passing into tissues that cannot be accessed by larger particles. Together, these features provide promise for nanomaterials to realize noninvasive detection, diagnosis, treatment and monitoring of disease progression, regression, or recurrence, because they are capable of carrying cargo and redirecting it to regions of interest.
Although there are many biological nanostructures from which to choose, the Wooley laboratory has focused for the past decade on the construction of organic polymer-based nanomaterials that resemble lipoproteins, histones, and viral capsids, using a biomimicry approach for their production (3). Each of these biological nanostructures involves a complex assembly of proteins to achieve nanoscopic sizes, a core-shell morphology, and specific surface chemistries. Analogous synthetic materials derive from polymers that are programmed for assembly. Well-defined amphiphilic block copolymers are prepared through covalent linkages (analogous to the primary structure of proteins). They are then assembled in water into multi-molecular micellar aggregates (analogous to protein secondary, tertiary, and quaternary structures) and, finally, stabilized through covalent crosslinking reactions between side chain functionalities along the hydrophilic block segment constituting the micellar corona (analogous to the disulfide crosslinks that reinforce protein assemblies) to afford shell crosslinked knedel-like (SCK) nanoparticles (4) having tunable sizes (5) and compositions (6). The SCKs, as prepared, have an amphiphilic core shell morphology for the packaging and transport of guests, as occurs with the packaging and transport of insoluble cholesteryl esters by lipoproteins. Past studies have shown that the core domain of the SCKs can be predesigned for selective degradation and removal, to give an excavated nanostructure, called a nanocage (illustrated schematically in Figure 1) (7, 8). The nanocage framework resembles viral capsids, in terms of the dimensions and also the ability to differentiate the internal and external surface chemistries to promote packaging of guests inside and interactions with other substrates from the outside (9). Our initial intention was to use the nanocages for the packaging of genes and gene delivery. However, we have not yet identified a process for the insertion of large DNA or other macromolecules into the nanocages. Rather, we turned our attention instead to the similarities of SCKs with histone core proteins, which are responsible for the DNA packaging of nucleosomes. Unlike the various cationic linear or branched polymers (10–13) or the dynamic micellar assemblies (14, 15) that have been studied for gene delivery, it was expected that a rigid and robust nanostructure that could organize DNA in a well-defined manner, modeled from components of the natural system, chromatin, would provide for enhanced packaging, transport, and release of DNA. Such synthetic systems would be of great value in the treatment of many human diseases, including those that are currently considered incurable, by facilitating intracellular delivery of therapeutic gene-based materials, without posing the risks of viral-based delivery vectors (16–18). This mini-review highlights our efforts over the past 12 years with emphasis on advances made during the past year, borrowing concepts from biology to address various aspects of DNA packaging and cell entry, to produce synthetic nanomaterials for delivery of plasmid DNA, oligonucleotides (ON), and also peptide nucleic acids (PNA).
Figure 1.
Schematic illustrations of several synthetic nanomaterials, designed as mimics of biological nanostructures—viruses and histones—for packaging, transport, and delivery of genetic materials. The evolution of the development of synthetic nanoparticles for gene delivery proceeds from left to right.
DESIGN OF CATIONIC SHELL CROSSLINKED NANOPARTICLES FOR DELIVERY OF GENETIC MATERIALS
The design of our nanoscale device for packaging, transport, and delivery of DNA, ON, and PNA builds from nucleosome structure and also borrows from viral-based cell entry. Consideration of the octameric histone cores of nucleosomes, in terms of their structural features, indicates that they assemble into a disc-like shape of approximately 10 nm diameter, around which 146 base pairs of DNA organize into a superhelix with assistance by the presentation of cationic arginine groups about their circumference for binding into the DNA minor groove (19). The earliest SCKs produced possessed an average diameter of approximately 15 nm, a persistent spherical shape, and cationic pyridyl functionalities on the surface and throughout the shell, providing immediate similarities to histones (20). Investigations of mixtures of those pyridyl-based shell SCKs (illustrated schematically in Figure 1) with plasmid DNA (pBR322, 4,361 bp) demonstrated their ability to compact DNA into complexes having mean volume-averaged hydrodynamic diameters of 40 to 120 nm, depending upon the molar ratio of SCK:DNA (20). The compaction was presumably through electrostatic interactions and, although protection against enzymatic digestion was shown, the complexes were incapable of cell transfection. With this initial failure to facilitate cell entry, our attention was then turned toward the incorporation of cell transducing peptide components onto the SCK surface (illustrated schematically in Figure 1). The viral HIV-I Tat protein transduction domain (PTD) sequence, YGRKKRRQRRR, was coupled onto the SCKs and was shown to then provide for cell transduction (21). Advanced synthetic methodologies were developed (22–24) and rigorous characterization studies were performed to determine the amount of PTD that was required to effect cell entry while maintaining biocompatibility (25). The role of nanoparticle shape was also explored, and it was found that small spherical particles entered cells to a greater extent than did elongated cylindrical particles (26). Returning to the issue of gene delivery, more recently, we hypothesized that the smaller, approximately 10-nm spherical particles would allow for more effective DNA packaging while also exposing cationic cell transducing units to accomplish intracellular delivery. Therefore, within the past year, a new cationic SCK (cSCK) has been designed, developed and studied for intracellular DNA, ON, and PNA delivery (Figure 2).
Figure 2.
Overall strategies for intracellular delivery of plasmid DNA (pDNA, pEGFP-N1), oligonucleotides (ps-MeON) and peptide nucleic acids (PNA).
As with the earlier nanocages and SCKs, the cSCKs originate from the self assembly of amphiphilic block copolymers in water, followed by their stabilization through the establishment of crosslinks throughout the shell layer. Access to well-defined, functional block copolymers has been dramatically improved over the past decade, with the advancement of controlled radical polymerization techniques (27–33). Although polyamides having primary amino side chain substituents have been prepared directly (34) by reversible addition-fragmentation chain transfer (35, 36) radical polymerization, the amphiphilic block copolymer, poly(acrylamidoethylamine)-b-polystyrene (PAEA-b-PS) used in our studies, was obtained by amidation of a common block copolymer precursor for SCK construction, poly(acrylic acid)-b-polystyrene, by reaction with mono-Boc-protected 1,2-ethylene diamine and subsequent deprotection under standard conditions. This chemical transformation afforded an amphiphilic block copolymer bearing large quantities of primary amines, which after micellar assembly were used partially for shell crosslinking reactions and remained largely to provide cationic character to the final cSCK nanostructures (Scheme 1). The zeta potential, as measured by electrophoretic light scattering, was 21.3 mV and the diameter, as measured by transmission electron microscopy, was 9 ± 1 nm (Figure 3a). The cSCKs exhibited lower cytotoxicity against CHO cells than did Polyfect and slightly higher cytotoxic effects against HeLa cells (37). The cSCKs possess an amine-rich shell for electrostatic interactions with DNA, a robust framework, and dimensions to allow for DNA packaging into complexes for protection, transport, and delivery.
Scheme 1.
Synthetic route for the preparation of well-defined cationic shell crosslinked nanoparticles (cSCKs).
Figure 3.
Complexation of pDNA by electrostatic interactions with cSCKs afforded uniform nanoscale packages of the synthetic delivery vectors and genes. (a) The cSCKs gave a dry-state diameter of 9 ± 1 nm as measured by transmission electron microscopy (TEM image captured after drop deposition onto a carbon-coated copper grid, negative-staining with phosphotungstic acid and drying under ambient conditions) and a volume-averaged hydrodynamic diameter of 14 ± 2 nm in water as measured by dynamic light scattering (514.5 nm argon-ion laser, histogram shown in inset). (b) cSCK complexation with pEGFP-N1 at a cSCK N to pEGFP-N1 P ratio of 6:1 gave a majority of aggregates having diameters of 50 to 100 nm.
As outlined in Figure 2, the cSCKs have been studied for the intracellular delivery of plasmid DNA (pDNA, pEGFP-N1), oligonucleotides (ON, ps-MeON) (37), and PNA (38). Delivery of pEGFP-N1 by cSCK/pEGFP-N1 complexes was evaluated by an EGFP expression assay. ps-MeON and PNA delivery were each measured by a luciferase splice correction assay. Although DNA and ONs could be packaged electrostatically by the cSCKs, PNAs lack negative charges, so their packaging was facilitated either noncovalently after hybridization with oligodeoxynucleotides (ODNs) or covalently through a bioreductively-cleavable linkage.
cSCK PACKAGING AND DELIVERY OF DNA
Plasmid DNA
Packaging of plasmid DNA (pDNA) (pEGFP-N1) by the cSCKs was monitored by a gel retardation assay, dynamic light scattering (DLS), and transmission electron microscopy (TEM). Gel electrophoresis on an agarose gel demonstrated that pEGFP-N1 underwent complete complexation with cSCK at cSCK/pEGFP-N1 amine-to-phosphate (N/P) ratios of 2:1 (mol:mol) and above. This critical N/P ratio for complexation is lower than those observed for poly(amido amine)s (39) or the earlier pyridyl-based SCK system (20), and is desirable to limit the amount of nanoparticle material required and limit the cytotoxic effects, while still allowing for an excess of cSCKs to provide a cationic outer layer to facilitate cell uptake. At an N/P ratio of 6:1, complex cSCK/pEGFP-N1 aggregates of approximately 50- to 100-nm diameters were observed by DLS and TEM (Figure 3), whereas at lower N/P values, larger and more heterogeneous aggregates were observed. As seen by the TEM image of Figure 3b, the cSCKs remain intact during the complexation with the pDNA, serving as a robust template for the assembly and packaging of the DNA, in analogy with histone core particles.
Cell transfection studies were performed in vitro as a function of the cSCK/pEGFP-N1 N/P ratio and were compared against Lipofectamine2000 and Polyfect. Flow cytometry and confocal fluorescence microscopy were used to observe the delivery of pEGFP-N1 and its expression of the GFPmut1 variant. Flow cytometry data indicated that the 6:1 N/P ratio gave the highest cell transfection, relative to other N/P ratios with the cSCKs, and that the cSCKs out-performed Polyfect (Figure 4). There remains opportunity for further improvements to achieve the transfection efficiencies of Lipofectamine2000. The comparative transfection efficiencies can be seen further by the confocal fluorescence microscopy images of Figure 5.
Figure 4.
Quantification of pEGFP-N1 transfection for HeLa cells by flow cytometry. The % cells transfected is a measure of the transfection efficiency, and is the ratio of the number of cells producing fluorescent signal to the number of total cells. N/P = cSCK/pEGFP-N1 amine-to-phosphate.
Figure 5.
Confocal laser scanning microscopy of HeLa cells (A–C), transfected with pEGFP-N1. In A, cSCK was used as the transfection agent with pEGFP-N1 at an N/P ratio of 6:1. B and C were positive controls using Polyfect and Lipofectamine 2000 as the transfection agent, respectively, at N/P ratios recommended by the manufacturers.
Oligomeric Nucleic Acids
Delivery of short nucleic acid sequences by cSCKs relied upon a luciferase splice correction assay (40), using ps-MeON, an 18-mer 2'-O-methyl phosphorothioate oligoribonucleotide that corrects luciferase pre-mRNA splicing in an engineered HeLa cell line, pLuc705. Two different approaches were employed to vary the N/P ratio from 16:1 to 1:1 or 1.6:1. One approach held constant the ps-MeON concentration at 100 pmol with variation in the cSCK amounts from 4.8 μg (N/P = 16:1) to 0.3 μg (N/P = 1:1), whereas the other held constant the amount of cSCK at 2.4 μg with variation in the ps-MeON concentration from 50 pmol (N/P = 16:1) to 500 pmol (N/P = 1.6:1). Incubations were performed for 24 and 48 hours, to allow for luciferase expression, which was then quantified by measurement of luminescence after administration of the Steady-Flo luciferase assay reagent. After 48 hours, the cSCK/ps-MeON complexes prepared via the first approach outperformed both Oligofectamine and Polyfect, with the optimum N/P ratio of only 1:1, which included 0.3 μg of cSCK, far less than the 1.0 μg of Oligofectamine and 2.0 μg of Polyfect (Figure 6). For the samples prepared according to the second approach, the highest luciferase expression was observed for the 16:1 N/P ratio after 24 hours. Additional studies are needed to determine the effects of cell internalization rate, rate of cSCK/ps-MeON dissociation, cytotoxic effects, and so on, and to optimize the cSCK to accommodate the multiple roles of DNA packaging, cell entry and DNA release.
Figure 6.
Luciferase activity assay of cSCK at different cSCK and ON (ps-MeON) concentrations, compared with commercially available transfection agents (Oligofectamine and Polyfect), after 24-hour and 48-hour incubations. (A) Oligofectamine 1.0 μg, ps-MeON 100 pmol. (B) Polyfect 2.0 μg, ps-MeON 100 pmol. (C) cSCK 0.3 μg, ps-MeON 100 pmol, N/P = 1:1. (D) cSCK 0.6 μg, ps-MeON 100 pmol, N/P = 2:1. (E) cSCK 1.2 μg, ps-MeON: 100 pmol, N/P = 4:1. (F) cSCK 2.4 μg, ps-MeON: 100 pmol, N/P = 8:1. (G) cSCK 4.8 μg, ps-MeON 100 pmol, N/P = 16:1. (H) cSCK 2.4 μg, ps-MeON 50 pmol, N/P = 16:1. (I) cSCK 2.4 μg, ps-MeON 100 pmol, N/P = 8:1. (J) cSCK 2.4 μg, ps-MeON 200 pmol, N/P = 4:1. (K) cSCK 2.4 μg, ps-MeON 500 pmol, N/P = 1.6:1. (L) ps-MeON 100 pmol. (M) ps-MeON 500 pmol. (N) Cells only.
cSCK PACKAGING AND DELIVERY OF PNA
PNAs are excellent units for molecular recognition of DNA or RNA in cells because their peptide-based backbone provides for chemical stability of the PNA sequence and the lack of anionically charged phosphodiester linkages allows for high binding affinity with DNA or RNA. Key challenges for their application, however, include ineffective transport of PNAs into cells and the limited cargo ability for PNAs. The cSCKs address both of these challenges, by exhibiting effective cell uptake and having the capacity for transport of large quantities of other active agents—for example, imaging agents (41) and therapeutics (42). Unlike the simple electrostatic attraction–based packaging that was possible for pDNA and ON, the lack of anionic character for PNAs required additional design strategies for complexation of PNAs with cSCKs.
Two approaches were developed for the association of PNA with the cSCKs. Each employed PNA of the sequence CCTCTTACCTCAGTTACA, which is complementary to the aberrant splice site on a luciferase gene (pLuc705) to correct the mRNA transcription and lead to an active luciferase. In the first approach, noncovalent electrostatic interactions were used—the anionic charges were provided by ODNs that were partially complementary to the PNA. This approach, therefore, is quite similar to the pDNA and ON systems (see above). The second strategy involved covalent attachment of the PNAs to the cSCKs, by amidation reactions between carboxy-terminated PNA and amino functionalities distributed throughout the shells of the cSCKs. To investigate the ability of PNA–cSCK conjugates versus PNA as a small molecule to undergo transport to the cell nucleus and accomplish the luciferase gene splice correction, comparisons were made between the covalent PNAs attached to the cSCK through a stable, noncleavable linker versus those connected to the cSCK with a bioreductively cleavable disulfide linker (Scheme 2). Quantification of PNA transfection was made by light output as a measure of luciferase activity and by RT-PCR as a direct measure of the extent of splice correction. Cytotoxicity was studied as cell viability measurements based on quantification of the amount of ATP produced by metabolically active cells.
Scheme 2.
Synthetic routes for the preparation of noncovalently and covalently linked peptide nucleic acid (PNA)–cSCK complexes. Micellization of the block copolymer (PAEA128-b-PS40) by dialysis of its DMSO solution against water followed by crosslinking with an activated diester (see Scheme 1) affords the cSCKs, having cationic character throughout the shell layer (left reaction sequence). Subsequent electrostatic association with PNA/DNA heteroduplex (PNA·ODN) gives the cSCK carrying PNA via noncovalent PNA absorption throughout the shell and the surface of the cSCK (lower left). The cSCKs having PNAs covalently linked were prepared by first coupling carboxy-terminated PNAs to the block copolymer (upper reaction sequence), followed by micellization and crosslinking (right reaction sequence). The covalently linked PNAs were of three types (central box): those having a stable linkage, those having a bioreductively cleavable disulfide linkage, and those having the disulfide linkage and also labeled with the fluorescent probe FITC.
Via ODN Hybridization and Electrostatic Association
The noncovalently associated PNA-cSCK complexes were found to be highly effective at PNA transfection. Hybridization of PNA with partially complementary ODN provided negative charges for electrostatic-based complexation with the cSCKs. Administration of the PNA·ODN-cSCK complexes to pLuc705 HeLa cells at variable concentrations of PNA·ODN while holding constant the cSCK amount at 20 μg/ml indicated that a sevenfold increase in luciferase bioactivity occurred upon a fivefold increase in PNA·ODN from 0.2 to 1.0 μM (Figure 7). The cell viability was constant over this PNA·ODN concentration range for the cSCK system. The threefold increase in luciferase bioactivity on doubling the PNA·ODN concentration from 0.5 to 1 μM coincided with a 2.6-fold increase in splice correction as measured by RT-PCR. The luciferase bioactivity for the PNA·ODN-cSCK complexes at 1 μM was approximately fivefold greater than that observed for Lipofectamine2000, which experienced a slight reduction in cell viability at 1 μM PNA·ODN. Polyfect was ineffective for PNA delivery over all of the concentrations evaluated and, similarly, PNA·ODN alone did not accomplish transfection.
Figure 7.
Bioactivity of electrostatically mediated PNA·ODN delivery by cSCKs and conventional agents. Splice-correcting PNA was hybridized to an equimolar amount of partially complementary ODN and then was mixed with 10 μg/ml of Lipofectamine 2000, 20 μg/ml Polyfect, or 20 μg/ml cSCK to give 0.2, 0.5, 1 μM final concentrations of PNA. pLuc705 HeLa cells were incubated with the transfection agents for 24 hours, and then assayed for PNA bioactivity via luciferase activity.
Via Reductively Cleavable Covalent Linkage
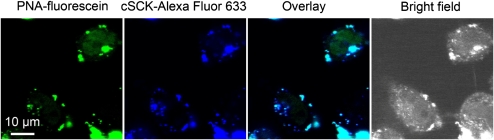
Covalent attachment of the PNAs to the cSCKs resulted in effective cell transfection and bioactivity when a bioreductively cleavable linker was used. cSCKs were synthesized, having an average of either one or two PNAs per block copolymer chain, with an average of about 60 chains per cSCK. There was not a significant difference between the luciferase bioactivity for these cSCKs when administered at 0.2 μM or 0.5 μM PNA concentrations (Figure 8), but at 1 μM, the cSCK having 2 PNAs per polymer chain, cSCK-SS-PNA2, gave higher transfection efficiency (data not shown), likely due to less cytotoxicity by delivering higher amounts of PNA with lower required levels of the cationic nanoparticles. The cSCKs were much more effective than was a nine-mer arginine, a cationic cell-penetrating peptide, when conjugated to PNA (Figure 8). The addition of chloroquine, an endosome-disrupting agent, improved the performance of the R9-PNA, but had no effect on the cSCK-SS-PNA system, suggesting that the cSCKs themselves were capable of endosome disruption to allow for endosomal escape by the PNA. When the PNAs were conjugated to the cSCKs via a noncleavable linker (cSCK-PNA2 of Figure 8), the bioactivity was very low. Dual labeling of the cSCK with Alexa Fluor 633 and the SS-PNA with fluorescein revealed PNA within the cell nucleus while the cSCK was trapped in endosomal or lysosomal bodies (Figure 9).
Figure 8.
Effect of endosomal disrupting agents on PNA bioactivity as measured by luciferase activity. Cells were treated with 1 μM Arg9-PNA, with 0.2 or 0.5 μM PNA from cSCK-SS-PNA2, or with cSCK-PNA in the presence or absence of 100 μM chloroquine.
Figure 9.
Cell localization of cSCK-SS-PNA and products. pLuc705 HeLa cells were incubated for 24 hours with a dual fluorescently labeled cSCK (Alexa Fluor 633)-SS-PNA(FITC)2, having Alexa Fluor 633 (excitation 633 nm, emission 650 nm) on the cSCK and FITC (excitation 488 nm, emission 510 nm) on the PNA.
CONCLUSIONS
By mimicking histone core proteins, robust, spherical nanoparticles having an approximately 10-nm diameter and cationic surface charge were prepared and shown to provide for excellent cell transfection efficiencies for plasmid DNA, oligonucleotides, and peptide nucleic acids. These cSCKs appear to be generally applicable nanotechnological devices in vitro and efforts are underway to translate their performance to primary cells in vitro and, ultimately, to in vivo delivery of genetic molecular recognition elements for imaging and therapy. Current targets include inducible nitric oxide synthase (iNOS) for imaging and treatment of acute lung injury.
Acknowledgments
The authors thank G. Michael Veith of the Washington University Department of Biology Microscopy Facility for providing technical support with transmission electron microscopy and fluorescence confocal microscopy. They also thank Dr. R. Kole (University of North Carolina, Chapel Hill, NC) for the pLuc705 HeLa cell line.
Supported by the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HL080729).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dorneanu L. Mobile wing plane flies like a bird: Leonardo da Vinci inspired engineers [Accessed February 19, 2009]. Available from: http://news.softpedia.com/news/Mobile-Wing-Plane-Flies-Like-a-Bird-52672.shtml.
- 2.Li S-D, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 2008;5:496–504. [DOI] [PubMed] [Google Scholar]
- 3.Wooley KL. Shell crosslinked polymer assemblies: nanoscale constructs inspired from biological systems. J Polym Sci Part Polym Chem 2000;38:1397–1407. [Google Scholar]
- 4.Thurmond KB II, Kowalewski T, Wooley KL. Water-soluble knedel-like structures: the preparation of shell-crosslinked small particles. J Am Chem Soc 1996;118:7239–7240. [Google Scholar]
- 5.Thurmond KB II, Kowalewski T, Wooley KL. Shell cross-linked knedels: a synthetic study of the factors affecting the dimensions and properties of amphiphilic core-shell nanospheres. J Am Chem Soc 1997;119:6656–6665. [Google Scholar]
- 6.Huang H, Remsen EE, Wooley KL. Amphiphilic core-shell nanospheres obtained by intramicellar shell crosslinking of polymer micelles with poly(ethylene oxide) linkers. Chem Commun (Camb) 1998;1415–1416.
- 7.Huang H, Remsen EE, Kowalewski T, Wooley KL. Nanocages derived from shell cross-linked micelle templates. J Am Chem Soc 1999;121:3805–3806. [Google Scholar]
- 8.Zhang Q, Remsen EE, Wooley KL. Shell crosslinked nanoparticles containing hydrolytically-degradable crystalline core domains. J Am Chem Soc 2000;122:3642–3651. [Google Scholar]
- 9.Turner JL, Chen Z, Wooley KL. Regiochemical functionalization of a nanoscale cage-like structure: robust core-shell nanostructures crafted as vessels for selective uptake and release of small and large guests. J Control Release 2005;109:189–202. [DOI] [PubMed] [Google Scholar]
- 10.Intra J, Salem AK. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J Control Release 2008;130:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko IK, Ziady A, Lu S, Kwon YJ. Acid-degradable cationic methacrylamide polymerized in the presence of plasmid DNA as tunable non-viral gene carrier. Biomaterials 2008;29:3872–3881. [DOI] [PubMed] [Google Scholar]
- 12.Prevette LE, Lynch ML, Kizjakina K, Reineke TM. Correlation of amine number and pdna binding mechanism for trehalose-based polycations. Langmuir 2008;24:8090–8101. [DOI] [PubMed] [Google Scholar]
- 13.Xiong MP, Bae Y, Fukushima S, Forrest ML, Nishiyama N, Kataoka K, Kwon GS. Ph-responsive multi-pegylated dual cationic nanoparticles enable charge modulations for safe gene delivery. ChemMedChem 2007;2:1321–1327. [DOI] [PubMed] [Google Scholar]
- 14.Sun T-M, Du J-Z, Yan L-F, Mao H-Q, Wang J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials 2008;29:4348–4355. [DOI] [PubMed] [Google Scholar]
- 15.Akagi D, Oba M, Koyama H, Nishiyama N, Fukushima S, Miyata T, Nagawa H, Kataoka K. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther 2007;14:1029–1038. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther 2000;7:31–34. [DOI] [PubMed] [Google Scholar]
- 17.Raper SE, Yadkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, Furth EE, Propert KJ, Robinson MB, Magosin S, et al. A pilot study of in vivo liver-directed gene transfer with adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther 2002;13:163–175. [DOI] [PubMed] [Google Scholar]
- 18.Verma IM, Somia N. Gene therapy - promises, problems and prospects. Nature 1997;389:239–242. [DOI] [PubMed] [Google Scholar]
- 19.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997;389:251–260. [DOI] [PubMed] [Google Scholar]
- 20.Thurmond KB II, Remsen EE, Kowalewski T, Wooley KL. Packaging of DNA by shell crosslinked nanoparticles. Nucleic Acids Res 1999;27:2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Zhang Q, Remsen EE, Wooley KL. Bioconjugates of protein transduction domain (PTD) and shell crosslinked nanoparticles: nanostructured materials designed for delivery into cells. Biomacromolecules 2001;2:362–368. [DOI] [PubMed] [Google Scholar]
- 22.Becker ML, Liu J, Wooley KL. Peptide-polymer bioconjugates: hybrid block copolymers generated via living radical polymerizations from resin-supported peptides. Chem Commun (Camb) 2003;802–803. [DOI] [PubMed]
- 23.Becker ML, Liu J, Wooley KL. Functionalized micellar assemblies prepared via block copolymers synthesized by living free radical polymerization upon peptide-loaded resins. Biomacromolecules 2005;6:220–228. [DOI] [PubMed] [Google Scholar]
- 24.Becker ML, Remsen EE, Pan D, Wooley KL. Peptide-derivatized shell crosslinked (SCK) nanoparticles: 1. Synthesis and characterization. Bioconjug Chem 2004;15:699–709. [DOI] [PubMed] [Google Scholar]
- 25.Becker ML, Bailey LO, Wooley KL. Peptide-derivatized shell crosslinked (SCK) nanoparticles: 2. Biocompatibility evaluation. Bioconjug Chem 2004;15:710–717. [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Fang H, Chen Z, Taylor J-SA, Wooley KL. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjug Chem 2008;19:1880–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawker CJ, Bosman AW, Harth E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev 2001;101:3661–3688. [DOI] [PubMed] [Google Scholar]
- 28.Matyjaszewski K, Xia J. Atom transfer radical polymerization. Chem Rev 2001;101:2921–2990. [DOI] [PubMed] [Google Scholar]
- 29.Barner-Kowollik C, Davis TP, Heuts JPA, Stenzel MH, Vana P, Whittaker M. Rafting down under: tales of missing radicals, fancy architectures, and mysterious holes. J Polym Sci Part Polym Chem 2003;41:365–375. [Google Scholar]
- 30.Lutz J-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: toward new generations of smart biocompatible materials. J Polym Sci Part Polym Chem 2008;2008:3459–3470. [Google Scholar]
- 31.de Cuendias A, Ibarboure E, Lecommandoux S, Cloutet E, Cramail H. Synthesis and self-assembly in water of coil-rod-coil amphiphilic block copolymers with central pi-conjugated sequence. J Polym Sci Part Polym Chem 2008;46:4602–4616. [Google Scholar]
- 32.Holder SJ, Durand GG, Yeoh C-T, Illi E, Hardy NJ, Richardson TH. The synthesis and self-assembly of aba amphiphilic block copolymers containing styrene and oligo(ethylene glycol) methyl ether methacrylate in dilute aqueous solutions: elevated cloud point temperatures for thermoresponsive micelles. J Polym Sci Part Polym Chem 2008;46:7739–7756. [Google Scholar]
- 33.Suriano F, Coulembier O, Degée P, DuBois P. Carbohydrate-based amphiphilic diblock copolymers: synthesis, characterization and aqueous properties. J Polym Sci Part Polym Chem 2008;46:3662–3672. [Google Scholar]
- 34.Deng Z, Bouchékif H, Babooram K, Housni A, Choytun N, Narain R. Facile synthesis of controlled-structure primary amine-based methacrylamide polymers via the reversible addition-fragmentation chain transfer process. J Polym Sci Part Polym Chem 2008;46:4984–4996. [Google Scholar]
- 35.Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: the raft process. Macromolecules 1998;31:5559–5562. [Google Scholar]
- 36.Barner-Kowollik C, Perrier S. The future of reversible addition fragmentation chain transfer polymerization. J Polym Sci Part Polym Chem 2008;46:5715–5723. [Google Scholar]
- 37.Zhang K, Fang H, Wang Z, Taylor J-SA, Wooley KL. Cationic shell-crosslinked knedel-like nanoparticles for highly efficient gene and oligonucleotide transfection of mammalian cells. Biomaterials 2009;30:968–977. [DOI] [PubMed] [Google Scholar]
- 38.Fang H, Zhang K, Shen G, Wooley KL, Taylor J-SA. Cationic shell-crosslinked knedel-like (cSCK) nanoparticles for highly efficient pna delivery. Mol Pharm 2009;6:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Novel bioreducible poly(amindo amine)s for highly efficient gene delivery. Bioconjug Chem 2007;18:138–145. [DOI] [PubMed] [Google Scholar]
- 40.Kang S, Cho M, Kole R. Up-regulation of luciferase gene expression with antisense oligonucleotides: Implications and applications in functional assay development. Biochemistry 1998;37:6235–6239. [DOI] [PubMed] [Google Scholar]
- 41.Sun G, Xu J, Hagooly A, Rossin R, Li Z, Moore DA, Hawker CJ, Welch MJ, Wooley KL. Strategies for optimized radiolabeling of nanoparticles for in vivo pet imaging. Adv Mater 2007;19:3157–3162. [Google Scholar]
- 42.Nyström AM, Xu Z, Xu J, Taylor S, Nittis T, Stewart SA, Leonard J, Wooley KL. SCKs as nanoparticle carriers of doxorubicin: investigation of core composition on the loading, release and cytotoxicity profiles. Chem Commun (Camb) 2008;3579–3581. [DOI] [PMC free article] [PubMed]