Abstract
Rationale: Angiogenesis is a defining pathologic feature of airway remodeling and contributes to asthma severity. Women experience changes in asthma control over the menstrual cycle, a time when vessels routinely form and regress under the control of angiogenic factors. One vital function modulated over the menstrual cycle in healthy women is gas transfer, and this has been related to angiogenesis and cyclic expansion of the pulmonary vascular bed.
Objectives: We hypothesized that changes in gas transfer and the pulmonary vascular bed occur in women with asthma over the menstrual cycle and are associated with worsening airflow obstruction.
Methods: Twenty-three women, 13 with asthma and 10 healthy control subjects, were evaluated over the menstrual cycle with weekly measures of spirometry, gas transfer, nitric oxide, hemoglobin, factors affecting hemoglobin binding affinity, and proangiogenic factors.
Measurements and Main Results: Airflow and lung diffusing capacity varied over the menstrual cycle with peak levels during menses that subsequently declined to nadir in early luteal phase. In contrast to healthy women, changes in lung diffusing capacity (DlCO) were associated with changes in membrane diffusing capacity and DlCO was not related to proangiogenic factors. DlCO did not differ between the two groups, although methemoglobin and carboxyhemoglobin were higher in women with asthma than in healthy women.
Conclusions: Women with asthma experience cyclic changes in airflow as well as gas transfer and membrane diffusing capacity supportive of a hormonal effect on lung function.
Keywords: gas transfer, angiogenesis, asthma, menstrual cycle, proangiogenic progenitor cell
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
One of the functions modulated over the menstrual cycle in the healthy woman is gas transfer, and this has been related to angiogenesis and cyclic expansion of the pulmonary vascular bed. However, gas transfer has never been evaluated in women with asthma over the menstrual cycle.
What This Study Adds to the Field
Airflow and gas transfer vary over the menstrual cycle, but the cyclic respiratory changes occur by different physiologic mechanisms in women with asthma compared with healthy women.
The sex distribution of asthma changes at puberty, at which time there is an increase in the ratio of women to men (1–4). The female preponderance is maintained into adult life, and asthma morbidity is greater in women than men (5). Sex hormones have been suggested to play a role in mediating the sex differences in asthma, and particularly in the asthma exacerbation that many women experience around the premenstrual period (6). The menstrual cycle is divided into two phases, follicular and luteal, typified by specific hormonal fluctuations and separated by ovulation at midcycle. The follicular phase starts with the first day of menstruation and is followed by the luteal phase from ovulation until menstruation restarts. Menstrual-related adverse effects on asthma have been recognized since 1931, but the causes are poorly understood (7–9). Studies investigating sex hormone/female sex effects in asthma have evaluated airflow, airway responsiveness, and/or allergic-inflammatory parameters over the menstrual cycle (10–14). Diffusing capacity has not been evaluated, even though gas transfer varies in healthy women over the menstrual cycle (15, 16). Lung diffusing capacity measured by the single breath carbon monoxide diffusing capacity (DlCO) peaks at the end of the luteal phase, then rapidly drops over the time of menses to reach a nadir during the follicular phase in healthy women (16). Circulating proangiogenic progenitor cells, which are biomarkers of angiogenesis, change in concert with lung diffusing capacity and pulmonary capillary blood volume, suggesting cyclic changes may be due to neovascularization in the pulmonary vascular bed (16).
Proangiogenic progenitor cells are bone marrow–derived cells, which are identified by expression of CD34, a common cell surface marker for hematopoietic stem cells and endothelial cells, and the coexpression of the stem cell marker CD133. They are essential for the formation of new blood vessels (17, 18). A recent study identified that circulating proangiogenic progenitors are increased in asthma and related to the pathologic expansion of submucosal vessels that typifies airway remodeling (19). Increased amounts and sizes of submucosal vessels were the most striking features reported in early detailed histopathologic studies of asthmatic lungs (20) and have been repeatedly confirmed in recent reports (21–25). The expanded airway vascular bed likely contributes to the airflow limitation of asthma either through vascular tissue increasing the airway wall thickness and/or through edema formation. Proangiogenic progenitors as well as Th2 polarized lymphocytes and mast cells secrete vascular endothelial growth factor (VEGF), which is found at high levels in sputum and in the bronchoalveolar lavage fluid of individuals with asthma (19, 26, 27). As a key proangiogenic factor in neovascularization, VEGF contributes to the angiogenic milieu in the asthmatic lung (26, 27), and its effects are mediated in part by nitric oxide (NO), production of which is also increased in the asthmatic lung (28). Thus, asthma is characterized by a highly proangiogenic lung environment (i.e., high levels of proangiogenic progenitors, VEGF, and NO).
In the context of cyclic changes in DlCO and pulmonary vascular bed in healthy women, we hypothesized that these physiologic changes may be amplified in women with asthma and adversely be associated with airflow obstruction. To test this, women with asthma were evaluated over the menstrual cycle with weekly measures of airflow, fraction of NO in the exhaled breath (FeNO), and lung diffusing capacity along with its components, Vc, membrane diffusing capacity (Dm), Hb, and the factors affecting Hb binding affinity. Simultaneously, parameters of angiogenesis were evaluated over the menstrual cycle, including circulating bone marrow–derived CD34+CD133+ cells, VEGF, and stem cell factor (SCF). Some of the results of these studies have been previously reported in the form of abstracts and as a publication (16, 29, 30).
METHODS
Thirteen nonsmoking women with asthma (aged 31 ± 2 yr) with regular menstrual cycles were enrolled in the study. Four were on contraceptive pills or patch. Women with asthma were on inhaled bronchodilators as needed; seven were on inhaled corticosteroid, six were on long-acting β-agonist, five were on leukotriene receptor antagonists, and none were on oral corticosteroid. Two women with asthma reported a history of second-hand smoking history and three were former smokers. Ten healthy, nonsmoking women were enrolled in the study as well; three were on contraceptives. They were on no other medications. Hormonal contraceptives were permitted, as previous studies have shown that changes in DlCO were detectable in menstruating women, whether or not they were on hormonal contraceptives (15, 16). Healthy volunteers were used as a comparative control group and some of their data have been previously published (16). All volunteers were longitudinally assessed over 4 to 5 weeks with one visit each week. The menstrual cycle was divided into weeks. Week 1 was defined by the first day of menstruation. Menstrual cycles varied from 28 to 31 days long. The phases of the cycle were verified by changes in hormonal levels (progesterone and estrogen). Asthma was verified based on a positive methacholine challenge test and/or reversible airway obstruction by documentation of change in FEV1 or FVC by 12% and greater than 200 ml after two puffs of inhaled bronchodilator except for two subjects, who were unavailable for testing (Table 1). Healthy subjects had no history of any lung symptoms, and all had normal spirometry. Nine of the 10 also had documentation of negative methacholine challenge and/or negative bronchodilator response. The Institutional Review Board at the Cleveland Clinic approved this study and all subjects gave written informed consent. Lung function and diffusing capacity for carbon monoxide were performed, and inspired oxygen concentrations of 21 and 42% were used to determine Dm and Vc as previously described (16) (Detailed methods are available in the online supplement.) Single-breath on-line measurement of fractional NO concentration in expired breath (FeNO) was measured using the NIOX (Aerocrine, New Providence, NJ). The ABL 700 radiometer (Radiometer America Inc., Westlake, OH) was used to measure oxygen, carbon dioxide, pH, Hb, methemoglobin (MetHb), carboxyhemoglobin (COHb), and to get a one-point determination of oxygen tension at half saturation (p50) using Hill's equation in venous blood collected in heparin tubes (31). Progesterone, VEGF, and SCF were measured in serum using quantikine ELISA (R&D Systems, Minneapolis, MN), and estrogen in serum using ELISA (Cayman Chemical Company, Ann Arbor, MI). The sensitivity and lower limit of detection for the variable assays were as follows: progesterone (<8.57 pg/ml, 15.6 pg/ml), VEGF (<9 pg/ml, 31.2 pg/ml), SCF (<9 pg/ml, 31.2 pg/ml), and estrogen (129 pg/ml, 19 pg/ml).
TABLE 1.
CHARACTERISTICS OF SUBJECTS WITH ASTHMA
| Study Subject | PC20 (mg/ml) | FEV1% | Medications Used Regularly* |
|---|---|---|---|
| 1 | 0.554 | 123 | |
| 2 | 0.192 | 97 | ICS+LABA |
| 3 | Positive BD† | 44 | |
| 4 | 0.109 | 101 | ICS+LABA, Montelukast |
| 5 | —‡ | 92 | ICS+LABA |
| 6 | 2.5 | 97 | Montelukast |
| 7 | 10 | 99 | ICS+LABA |
| 8 | 5.572 | 97 | Montelukast |
| 9 | 3.548 | 85 | |
| 10 | —‡ | 102 | Montelukast |
| 11 | 0.261 | 72 | ICS+LABA |
| 12 | 4.475 | 92 | ICS+LABA, Montelukast |
| 13 | 0.437 | 108 |
Definition of abbreviations: ICS+LABA = combination inhaled corticosteroid and long-acting β-agonist; PC20 = provocative concentration of methacholine causing a 20% decrease in FEV1.
Albuterol used as needed by all subjects.
Positive BD, bronchodilator response = 12% and 200 ml.
Data not available.
Flow Cytometry Evaluation of CD34+CD133+ Progenitor Cells
Mononuclear cells (2 × 106) isolated from peripheral blood were labeled with anti-human CD34-FITC (Becton Dickinson, Franklin Lakes, NJ) and anti-human CD133-PE (Miltenyi Biotec, Auburn, CA) monoclonal antibodies to quantify CD34+CD133+ cells. To control for nonspecific antibody binding, isotype-matched irrelevant antibodies were used. After incubation, cell suspensions were washed with phosphate-buffered saline/1% bovine serum albumin/0.02% sodium azide and suspended in FACS flow (Becton Dickinson). The FACScan flow cytometer (Becton Dickinson) was used to count 0.5 × 106 events. Data were analyzed using Cell-Quest 3.3 Software (Becton Dickinson).
Statistical Analysis
Descriptive measures for quantitative variables consist of means with appropriately derived standard errors in the form mean ± SE. Comparisons of subjects with and without asthma were performed. For quantitative measures observed only once per patient, comparisons were performed using the Wilcoxon rank sum test. Comparisons with respect to quantitative measures observed over multiple weeks or phases were performed using linear models with parameters estimated using generalized estimating equations (GEE) to account for a correlation among observations within a subject. An exchangeable correlation structure, common to all subjects, was applied. Linear models with GEE were also fit with covariate adjustments for week or phase. Within subjects with asthma, comparisons of weeks or phases were also performed using linear models with GEE and exchangeable correlation structure. Linear regression was used to identify and describe relationships among pairs of quantitative variables when data were normally distributed, and Spearman correlation coefficients were used to describe relationships among pairs of quantitative variables in a manner free of the normality assumption. Pearson correlations derived through linear regression are denoted as “R,” whereas Spearman correlations are denoted as “Spearman R.” Analyses were performed using R version 2.3.1 (R Foundation, Vienna, Austria) (32).
RESULTS
Women with asthma were similar to healthy women in terms of age, height, race, and use of contraceptives but had greater body mass and lower FEV1% and FEV1/FVC, and tended to have higher FeNO over the time of measures (Table 2).
TABLE 2.
CHARACTERISTICS OF STUDY SUBJECTS
| Variable | Healthy (N = 10) | Asthma (N = 13) | Mean Difference ± SE (Adjusted for Multiple Observations) | P Value |
|---|---|---|---|---|
| Age, years | 31 ± 1 | 31 ± 1 | —* | 0.9 |
| Race, white/African American/Asian | 9/1/0 | 12/1/0 | — | 1 |
| Height, cm | 149 ± 16 | 166 ± 2 | — | 0.5 |
| Weight, kg | 64 ± 2 | 81 ± 5 | — | 0.03 |
| OCP use, yes/no | 3/7 | 4/9 | — | 1 |
| Pulse, beats/min | 73 ± 2 | 78 ± 2 | 5 ± 4 | 0.3 |
| FVC% | 102 ± 2 | 100 ± 2 | 2 ± 5 | 0.7 |
| FEV1% | 106 ± 2 | 95 ± 3 | 11 ± 6 | 0.07 |
| FEV1/FVC | 83 ± 1 | 77 ± 2 | 6 ± 3 | 0.08 |
| FeNO, ppb | 15 ± 1 | 31 ± 7 | 17 ± 14 | 0.2 |
| O2 saturation, % of Hb | 98.7 ± 0.1 | 98.2 ± 0.3 | 0.5 ± 0.5 | 0.3 |
| Venous blood gas | ||||
| pH | 7.39 ± 0.005 | 7.42 ± 0.005 | 0.03 ± 0.008 | <0.001 |
| Po2 | 36 ± 2 | 46 ± 2 | 11 ± 5 | 0.02 |
| Pco2 | 44.0 ± 0.8 | 38.1 ± 0.7 | 6 ± 1 | <0.001 |
| p50 | 26.7 ± 0.3 | 25.4 ± 0.2 | 1.3 ± 0.5 | <0.01 |
| COHb, % | 0.5 ± 0.04 | 1.4 ± 0.09 | 1 ± 0.2 | <0.001 |
| MetHb, % | 0.5 ± 0.02 | 1.1 ± 0.06 | 0.6 ± 0.1 | <0.001 |
| Hb, gm/dl | 13.5 ± 0.1 | 13.5 ± 0.2 | 0.08 ± 0.5 | 0.9 |
| Va, L | 5.5 ± 0.07 | 5.2 ± 0.1 | 0.3 ± 0.3 | 0.3 |
| DlCO, ml/min/mm Hg | 24.0 ± 0.4 | 25.3 ± 0.6 | 1.2 ± 1.5 | 0.4 |
| DlCO /Va, L/min/mm Hg | 4.4 ± 0.1 | 4.8 ± 0.1 | 0.5 ± 0.2 | 0.05 |
| Dm, ml/min/mm Hg | 62 ± 6 | 80 ± 8 | 19 ± 13 | 0.1 |
| Vc, ml | 55 ± 3 | 50 ± 3 | 4 ± 4 | 0.3 |
Definition of abbreviations: COHb = carboxyhemoglobin; DlCO = diffusing capacity of carbon monoxide; Dm = membrane diffusing capacity; MetHb = methemoglobin; OCP = oral contraceptive pills; p50 = oxygen tension at half saturation. * Nonapplicable.
Airflow and FeNO over the Menstrual Cycle
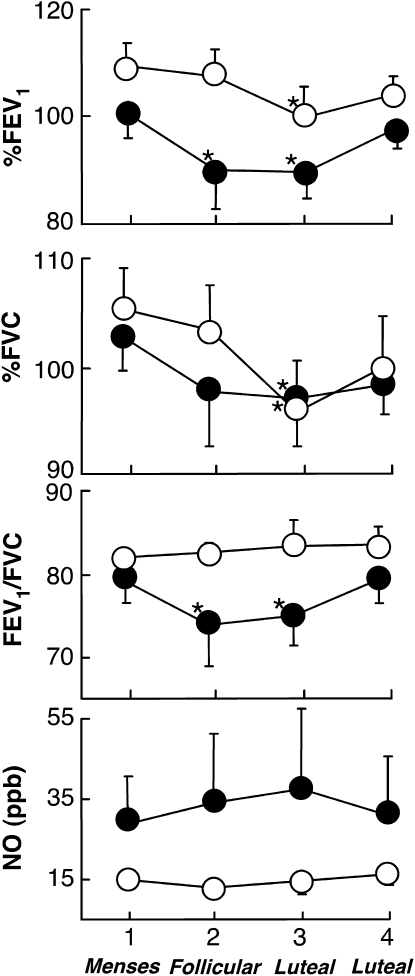
Pattern of airflow of women with asthma over the weeks of the cycle was evaluated and compared with the healthy women. None of the participants experienced an asthma exacerbation during the time of the study. Healthy women had significant decreases in both FVC and FEV1% from Week 1 to Week 3 (P = 0.01 and P = 0.03, respectively) and from Week 2 to Week 3 (P = 0.01 and P < 0.01, respectively), but no significant change in FEV1/FVC (all P > 0.1). Similarly, FVC and FEV1% varied over the menstrual cycle in women with asthma with significant decline from Week 1 to Week 2 (P = 0.07 and P = 0.01, respectively) and Week 3 (P = 0.02 and P = 0.01, respectively) (Figure 1). In contrast to the healthy women, FEV1/FVC in women with asthma decreased from Week 1 to Week 2 (P = 0.08) and Week 3 (P = 0.04) (Figure 1). FeNO did not vary over the time of the study (all P > 0.1). FeNO was inversely related to airflow as measured by FEV1/FVC (R = −0.6, P < 0.001) and directly related to FVC% (R = 0.4, P = 0.02). Although the mean FeNO of women with asthma appears greater than healthy controls, the P value adjusted to multiple observations per subject did not achieve significance.
Figure 1.
Airflow and fraction of nitric oxide (NO) in the exhaled breath measures over the menstrual cycle in women with asthma and healthy women. Airflow changes significantly in healthy women and women with asthma over the menstrual cycle. Closed circles represent women with asthma and open circles represent healthy control subjects. Asterisks represent significant changes from Week 1 (all P < 0.05).
Gas Transfer in Women with Asthma over the Menstrual Cycle
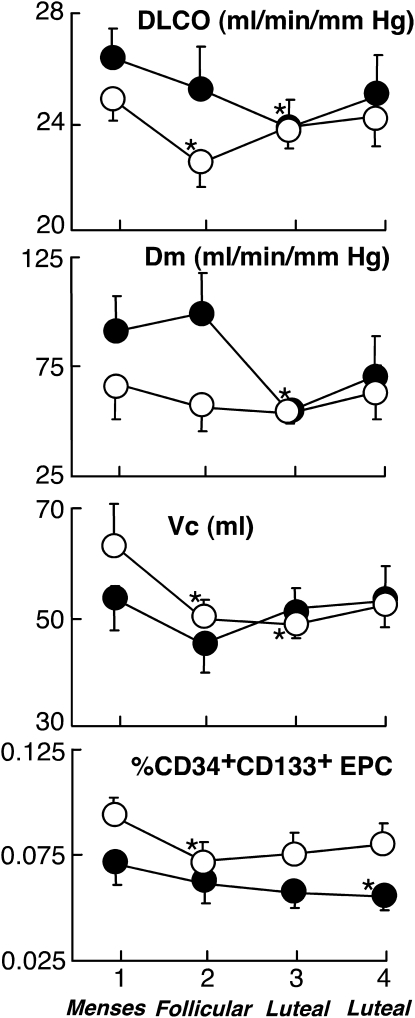
In general, women with asthma had greater single breath DlCO/Va than healthy women (Table 2). DlCO varied significantly over the menstrual cycle in women with asthma with the highest values at Week 1 and the lowest values at Week 3 (P = 0.03) (Figure 2). Parallel to DlCO, Dm changed over the menstrual cycle reaching a nadir on Week 3 with a significant drop from Weeks 1 and 2 to Week 3 (P = 0.03 and P = 0.01). Overall, Dm was lower in the luteal phase than in the follicular phase (Dm [ml/mmHg/min]: follicular phase 92 ± 12; luteal phase 63 ± 10; P = 0.07). In contrast, Vc did not vary significantly over time of weeks or from follicular to luteal phase (Vc [ml]: follicular phase 49 ± 4; luteal phase 52 ± 4; P = 0.5). We subgrouped the women with asthma based on use of contraceptives. As a group, women with asthma who are on contraceptives have higher FEV1 and FEV1/FVC (P < 0.01 for both) compared with women with asthma who are not on contraceptives. Regardless of contraceptive use, changes over the menstrual cycle were comparable (i.e., the highest DlCO and CD34+CD133+ cells were at Week 1). On the other hand, when we divided women with asthma into two subgroups according to use of inhaled corticosteroid, there were no differences in lung function (all P > 0.1), and these subgroups had similar CD34+CD133+ cells in circulation (P > 0.1). The changes over the menstrual cycle were also comparable (i.e., the highest DlCO, FEV1, FEV1/FVC, and CD34+CD133+ cells at Week 1 in both subgroups).
Figure 2.
Lung diffusing capacity for carbon monoxide (DlCO) and its components, the alveolar capillary membrane diffusing capacity (Dm), and lung capillary blood volume (Vc), and circulating CD34+CD133+ progenitor cells over the menstrual cycle in women with asthma and healthy women. Circulating levels of CD34+CD133+ progenitor cells also change over time in women with asthma similar to healthy women. Closed circles represent women with asthma and open circles represent healthy control subjects. Asterisks represent significant changes from Week 1 (all P < 0.05).
Other factors that influence DlCO were also evaluated. Estimated Hb affinity (θ) and measures of Va were unchanged over the menstrual cycle (P = 0.8 and 0.4, respectively). Furthermore, Hb concentration did not change over the menstrual period (all P > 0.5) (Hb [gm/dl]: follicular phase 13.3 ± 0.3; luteal phase 13.5 ± 0.3; P = 0.6). Similarly, venous MetHb and COHb, which would diminish DlCO, did not change over the cycle (P = 0.9 and P = 0.6, respectively). However, COHb and MetHb were significantly higher in women with asthma compared with healthy control subjects (Table 2). Given the high affinity of carbon monoxide toward Hb and its low plasma solubility, cardiac output effect on DlCO is typically considered trivial and was not taken into consideration as a significant determinant (33).
Angiogenic Factors in Women with Asthma
Although a previous study has shown increased circulating CD34+CD133+ progenitor cells in men and women with asthma compared with healthy individuals (19), women with asthma in this study had circulating CD34+CD133+ progenitor cell levels similar to healthy women (% CD34+CD133+ EPC: asthma 0.06 ± 0.004, control subjects 0.08 ± 0.005; P = 0.1). Serum VEGF was lower in women with asthma compared with control subjects (VEGF [pg/ml]: asthma 163 ± 15, control subjects 513 ± 47; P < 0.01), whereas serum SCF was higher (SCF [pg/ml]: asthma 1,234 ± 65, control subjects 1,024 ± 28; P = 0.06). Women with asthma had changes in CD34+CD133+ progenitor cells in the circulation over the menstrual cycle (P = 0.01) (Figure 2). However, VEGF and SCF did not change significantly over the menstrual cycle of women with asthma (P = 0.2 and P = 0.8, respectively), and CD34+CD133+ progenitor cells were unrelated to serum VEGF or SCF (all P > 0.3).
The use of inhaled corticosteroids or contraceptives did not affect the changes in CD34+CD133+ progenitor cells over the menstrual cycle in women with asthma and the highest value was consistently noted at Week 1.
Relation of Angiogenic Factors to Airflow and Diffusion
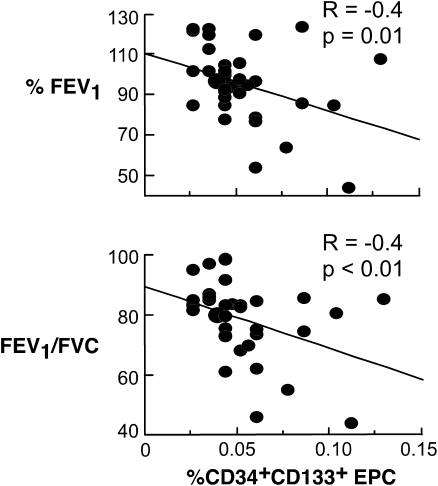
A previous study identified that CD34+CD133+ progenitor cells are related to changes in Vc and DlCO over the menstrual cycle of healthy women (16). In women with asthma, the circulating numbers of CD34+CD133+ progenitor cells were unrelated to DlCO (P = 0.5), Vc, or Dm (all P > 0.3). Rather, EPC were related to FeNO (R = 0.5, P < 0.01) and inversely related to airflow (FEV1%, R = −0.4, P = 0.01; FEV1/FVC, R = −0.4, P < 0.01) (Figure 3). Serum VEGF and SCF were unrelated to circulating numbers of EPC or airflow (all P > 0.3).
Figure 3.
Relation of CD34+CD133+ progenitor cells to airflow (%FEV1 and FEV1/FVC) in women with asthma.
Greater DlCO in Asthma: Relationship to FeNO, pH, Po2, Pco2, and Hb
The changes in DlCO over the menstrual cycle in asthma were accounted for by changes in Dm (Figure 2). Although there were no variations of pH, Po2, or Pco2 over the menstrual cycle (Pco2 [mm Hg]: Week 1, 39.7 ± 1.0; Week 2, 37.4 ± 1.3; Week 3, 38.1 ± 1.3; Week 4, 37.6 ± 1.8; P = 0.4), venous blood gases revealed differences in pH, Po2, and Pco2 among asthma and controls (Table 2). The findings showed lower Pco2 in asthma, with resultant increases in pH. This led to predictable pH-related changes in the venous blood p50; p50 was lower in asthma as compared with healthy controls (Table 2). FeNO was unrelated to p50, Po2, or Pco2 (all P > 0.2).
Venous COHb was higher in asthma than control subjects (Table 2). Similarly, MetHb was greater in asthma than control subjects (Table 2). The greater MetHb and COHb may also contribute to the lower p50 of women with asthma as compared with control subjects. As expected, DlCO values of women with asthma were inversely related to MetHb (R = −0.3, P = 0.04) and COHb (R = −0.3, P = 0.03). Despite the greater MetHb and COHb in asthma, women with asthma had comparable DlCO and Dm to healthy women. MetHb or COHb levels in asthma were not related to FeNO or airflow (all P > 0.1).
Relationships of DlCO to Airflow Limitation
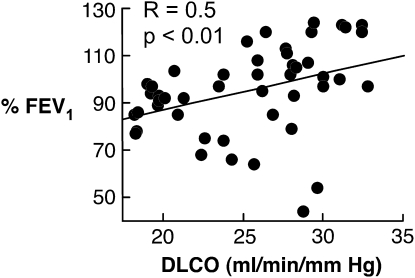
Air trapping and thinning of the alveoli–capillary membrane might produce faster diffusion. However, better airflow was actually related to faster diffusion; that is, FEV1% was directly related to DlCO among women with asthma (R = 0.5, P < 0.01) (Figure 4). The higher levels of NO in asthma might be predicted to reduce DlCO due to its greater affinity for Hb than CO, but higher values of NO were associated with greater DlCO (R = 0.4, P = 0.03), which implies that both CO and NO diffusion may be faster in subjects with asthma. Furthermore, the greater DlCO in asthma was not related to larger overall lungs, as the Va was similar among the groups, and DlCO/Va still tended to be greater in asthma than in control subjects (Table 2). Overall, it appears that peak DlCO occurs at the same time that there is the best airflow in women with asthma, at the end of luteal phase and start of menses.
Figure 4.
Airflow as determined by %FEV1 is related to the lung diffusing capacity (DlCO).
DISCUSSION
In contrast to prior studies that did not find changes in spirometry over the menstrual cycle (10, 11, 34), airflow varied significantly in this study. Women with asthma achieved the best airflow at the end of the luteal phase through the beginning of menstruation followed by a decline over the subsequent 2 weeks. Although there was some variation in FEV1 in healthy women, the ratio of FEV1/FVC did not vary among the healthy women over the cycle. Lung diffusing capacity changed in parallel to airflow in women with asthma. These findings were consistent in all women with asthma regardless of use of contraceptives or inhaled corticosteroids. In healthy women, the changes in DlCO have been attributed to changes in the pulmonary vascular bed capacity (16). However, variation of DlCO in women with asthma was due to changes in membrane diffusion. In further contrast to healthy women (16), circulating proangiogenic progenitor cells of women with asthma were unrelated to lung diffusion. Unlike our prior report (19), the women with asthma in this study did not have higher levels of proangiogenic progenitors than control women. Failure to show a difference between women with asthma and control subjects regarding CD34+CD133+ cells could be related to the relatively mild characteristics of the asthma sample in this study, the low sample numbers, the fact that the prior study evaluated men and women, and/or the effects of inhaled corticosteroids. Our prior report, which identified higher CD34+CD133+ cells in asthma, included subjects with more severe asthma than in the current study, which evaluated very mild asymptomatic women with asthma. The finding of an inverse relationship among CD34+CD133+ cells and airflow in this study supports the possibility that subjects with asthma with more severe airflow limitation have higher circulating levels of CD34+CD133+ cells. The relationship of CD34+CD133+ progenitor cells to airflow obstruction in this study, and in a murine model of asthma in prior study (19), also suggests that angiogenesis may contribute to airflow obstruction, and/or inflammation, over the time of the menstrual cycle in women with asthma. In fact, the CD34+CD133+ cells are pluripotent and can give rise to multiple lineages, including mast cells and fibroblasts, both known to play a role in airway inflammation and remodeling (35).
In general, individuals with asthma have greater gas transfer capacity as compared with healthy individuals (36–40). Although several studies have investigated the causes for higher DlCO in individuals with asthma, the findings are conflicting and the physiologic mechanisms underlying the increased levels are uncertain (36, 38, 40). Stewart found that subjects with asthma had higher DlCO and Vc when corrected for alveolar volume in comparison with healthy control subjects, and concluded that the greater gas transfer was due to an increase in lung capillary blood volume (40). Collard and colleagues showed that greater DlCO in asthma was associated with an increased perfusion of the lung apices (36). On the other hand, in a study of children with asthma with different degrees of overinflation, Pecora and colleagues showed that DlCO was increased due to an increase in Dm, which they attributed to an increased surface area and/or thinner membrane secondary to overinflation (38). The finding of changes in Dm over the menstrual cycle suggests that diffusion across the alveolar–capillary membrane varies in women with asthma over the menstrual cycle, but not in healthy women. This might occur secondary to cyclic expansion of the available surface for gas exchange (i.e., gas exchange may occur more proximally than the level of the respiratory bronchioles in asthma). Alternatively, it has been proposed that children with asthma have greater growth of the lungs, which results in an increased total lung capacity and higher DlCO (33). Although this might contribute to overall greater DlCO and Dm, women with asthma in this study had similar levels of alveolar volume as healthy women and alveolar volume did not change over the menstrual cycle. In the context of new studies that suggest a role for membrane channels, such as aquaporin in gas transfer (41, 42), the cyclic changes of gas transfer in asthma may also be due to alterations of an active transporter that modifies alveolar–capillary cell membrane permeability and/or channels. Finally, the changes of Dm over the menstrual cycle may be related to hyperinflation and/or air trapping, even though the best DlCO and Dm occurred at the times of the best airflow. In this context, a limitation of this study is that measurement of lung volumes was not available to evaluate air trapping, and imaging of vascular changes was not done. More subtle measurements of small airway function and emptying, as well as the measure of ventilation and perfusion over the menstrual cycle, would have been helpful in understanding the findings. Similarly, direct assessment of the pulmonary vasculature through nuclear imaging might have provided insight into changes in DlCO, although spatial resolution of even the best imaging modalities for blood distribution in the lungs, such as single photon emission computed tomography, is only approximately 15 mm, which is relatively poor compared with other imaging modalities.
The higher level of venous COHb in women with asthma was similar to findings from other studies that investigated arterial COHb (43, 44). The greater COHb has been attributed to inflammation-mediated up-regulation of heme oxygenases, the enzymes responsible for CO production (43, 44). Nevertheless, COHb did not vary over the menstrual cycle, which indicates that it did not contribute to the changes in lung functions observed in women with asthma, and that inflammation did not vary substantially over the menstrual cycle.
Although data indicate that FeNO serves as a surrogate marker of eosinophilic airway inflammation and airflow obstruction in asthma (45), NO has also been proposed as a method for measure of gas transfer (46). Borland and coworkers originally proposed inhaled NO as a substitute for CO; NO and CO similarly diffuse and have high reactivities with hemoglobin (46). In this context, NO values are associated with DlCO over the time of the menstrual cycle in this study. The data suggest that endogenously produced NO, as measured by FeNO, may also reflect overall gas transfer across the alveoli in women with asthma. Alternatively, the American Thoracic Society (ATS) standardized flow rate to measure exhaled NO, which was used in this study, may not be optimal for evaluating the vascular and inflammation changes occurring over the menstrual cycle. Thus, future studies might perform measures of exhaled NO at varying flow rates as previously described (47) and/or use other specific biomarkers of inflammation, such as 3-bromotyrosine for eosinophil-related oxidation and F2-isopostanes for lipid peroxidation (48, 49).
In summary, FEV1 and gas transfer are at peak levels in all women at the end of the luteal phase through the start of menstruation. In contrast to healthy women, the cyclic changes in gas transfer of women with asthma are due to the changes in diffusion characteristics across the alveolar capillary membrane. The menstrual cycle is one of the most important biological rhythms that govern physiological processes of living beings. Here, airflow and gas transfer are shown to be among the many vital functions modulated over the menstrual cycle, but the cyclic respiratory changes occur by very different physiologic mechanisms in women with asthma as compared with healthy women. These findings begin to provide insight into understanding features of asthma unique to women, including perhaps the much greater prevalence of difficult-to-control and severe asthma among women and the phenomenon of perimenstrual asthma exacerbations.
Supplementary Material
Acknowledgments
The authors thank Jacqueline Sharp and Marcelle Baaklini for study coordination.
Supported by grants HL081064, HL69170, HL60917, AI70649, and M01 RR018390 from the National Institutes of Health, and the Cleveland Clinic Research Programs Council.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200904-0497OC on June 11, 2009
Conflict of Interest Statement: S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.L. receives less then $10,000 per year as a consultant for Aerocrine USA, received $10,001 to $50,000 as a consultant for Telecris, received $1,001 to $5,000 as a speaker for Novartis, and holds a patent on a nano sensor pending from BreathTech Inc. J.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.P.W. received $5,001 to $10,000 as the Chair of DSMB for Clinical Trial from Agennix, Inc. and $5,001 to $10,000 as the Chair of DSMB for Clinical Trial from Eisai, received $1,001 to $5,000 in royalties from UpToDate, and holds up to $1,000 in stock ownership or options from Advanced Life Sciences (options have no current value). S.C.E. received a grant for more than $100,001 from 2006 to 2011 from Asthmatx Bronchial Thermoplasty in Asthma.
References
- 1.Dawson B, Illsley R, Horobin G, Mitchell R. A survey of childhood asthma in Aberdeen. Lancet 1969;1:827–830. [DOI] [PubMed] [Google Scholar]
- 2.Venn A, Lewis S, Cooper M, Hill J, Britton J. Questionnaire study of effect of sex and age on the prevalence of wheeze and asthma in adolescence. BMJ 1998;316:1945–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerritsen J. Airway responsiveness in teenagers is becoming sexier. Am J Respir Crit Care Med 2008;178:321–322. [DOI] [PubMed] [Google Scholar]
- 4.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med 2008;178:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA 1992;268:3437–3440. [PubMed] [Google Scholar]
- 6.Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax 1999;54:1119–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank RT. The hormonal causes of premenstrual tension. Arch Neurol Psychiatry 1931;26:1053–1057. [Google Scholar]
- 8.Claude F, Vall RA. Asthme et menstruation. Press Medicale 1938;38:755–759. [In French.] [Google Scholar]
- 9.Haggerty CL, Ness RB, Kelsey S, Waterer GW. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol 2003;90:284–291. [Quiz pp. 291–283, 347.] [DOI] [PubMed] [Google Scholar]
- 10.Oguzulgen IK, Turktas H, Erbas D. Airway inflammation in premenstrual asthma. J Asthma 2002;39:517–522. [DOI] [PubMed] [Google Scholar]
- 11.Pauli BD, Reid RL, Munt PW, Wigle RD, Forkert L. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am Rev Respir Dis 1989;140:358–362. [DOI] [PubMed] [Google Scholar]
- 12.Shames RS, Heilbron DC, Janson SL, Kishiyama JL, Au DS, Adelman DC. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy Asthma Immunol 1998;81:65–72. [DOI] [PubMed] [Google Scholar]
- 13.Juniper EF, Kline PA, Roberts RS, Hargreave FE, Daniel EE. Airway responsiveness to methacholine during the natural menstrual cycle and the effect of oral contraceptives. Am Rev Respir Dis 1987;135:1039–1042. [DOI] [PubMed] [Google Scholar]
- 14.Weinmann GG, Zacur H, Fish JE. Absence of changes in airway responsiveness during the menstrual cycle. J Allergy Clin Immunol 1987;79:634–638. [DOI] [PubMed] [Google Scholar]
- 15.Sansores RH, Abboud RT, Kennell C, Haynes N. The effect of menstruation on the pulmonary carbon monoxide diffusing capacity. Am J Respir Crit Care Med 1995;152:381–384. [DOI] [PubMed] [Google Scholar]
- 16.Farha S, Asosingh K, Laskowski D, Licina L, Sekiguchi H, Losordo DW, Dweik RA, Wiedemann HP, Erzurum SC. Pulmonary gas transfer related to markers of angiogenesis during the menstrual cycle. J Appl Physiol 2007;103:1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 18.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000;95:952–958. [PubMed] [Google Scholar]
- 19.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1- and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol 2007;178:6482–6494. [DOI] [PubMed] [Google Scholar]
- 20.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960;13:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwano K, Bosken CH, Pare PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1993;148:1220–1225. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229–233. [DOI] [PubMed] [Google Scholar]
- 23.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 2005;116:477–486. [Quiz 487]. [DOI] [PubMed] [Google Scholar]
- 24.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax 2001;56:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrugt B, Wilson S, Bron A, Holgate ST, Djukanovic R, Aalbers R. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J 2000;15:1014–1021. [DOI] [PubMed] [Google Scholar]
- 26.Hossny E, El-Awady H, Bakr S, Labib A. Vascular endothelial growth factor overexpression in induced sputum of children with bronchial asthma. Pediatr Allergy Immunol 2008;20:89–96. [DOI] [PubMed] [Google Scholar]
- 27.Feltis BN, Wignarajah D, Zheng L, Ward C, Reid D, Harding R, Walters EH. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med 2006;173:1201–1207. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, et al. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci USA 2006;103:11021–11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farha SAK, Laskowski D, Licina L, Sekigushi H, Losordo D, Dweik R, Wiedemann HP, Erzurum SC. Enhanced gas transfer and neovascularization during the menstrual cycle [abstract]. Proc Am Thorac Soc 2007;A941:2007. [Google Scholar]
- 30.Farha SAK, Laskowski D, Hammel J, Dweik R, Wiedemann HP, Erzurum SC. Respiratory function in asthmatic women over time of the menstrual cycle [abstract]. Proc Am Thorac Soc 2009;A30:2009. [Google Scholar]
- 31.Wimberley PD, Burnett RW, Covington AK, Fogh-Andersen N, Maas AH, Muller-Plathe O, Siggaard-Andersen O, Zijlstra WG. Guidelines for routine measurement of blood hemoglobin oxygen affinity. International federation of clinical chemistry, scientific division, committee on pH, blood gases and electrolytes. Scand J Clin Lab Invest 1990;203:227–234. [PubMed] [Google Scholar]
- 32.R Development Core Team (2006). R: a language and environment for statistical computing [Internet]. (Accessed December 2006). R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0. Available at: http://www.R-project.org
- 33.Cotes J. Lung function. Oxford: Blackwell Scientific Publications; 1993.
- 34.da Silva SB, de Sousa Ramalho Viana E, de Sousa MB. Changes in peak expiratory flow and respiratory strength during the menstrual cycle. Respir Physiol Neurobiol 2006;150:211–219. [DOI] [PubMed] [Google Scholar]
- 35.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci USA 2006;103:13156–13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collard P, Njinou B, Nejadnik B, Keyeux A, Frans A. Single breath diffusing capacity for carbon monoxide in stable asthma. Chest 1994;105:1426–1429. [DOI] [PubMed] [Google Scholar]
- 37.Evans CC, Ogilvie CM. Transfer factor in asthma. Lancet 1970;1:891. [DOI] [PubMed] [Google Scholar]
- 38.Pecora LJ, Bernstein IL, Feldman DP. Pulmonary diffusing capacity, membrane diffusing capacity, and capillary blood volume in children with intractable asthma with and without chronic overinflation of the lungs. J Allergy 1966;37:204–215. [DOI] [PubMed] [Google Scholar]
- 39.Saydain G, Beck KC, Decker PA, Cowl CT, Scanlon PD. Clinical significance of elevated diffusing capacity. Chest 2004;125:446–452. [DOI] [PubMed] [Google Scholar]
- 40.Stewart RI. Carbon monoxide diffusing capacity in asthmatic patients with mild airflow limitation. Chest 1988;94:332–336. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Cohen J, Boron WF, Schulten K, Tajkhorshid E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J Struct Biol 2007;157:534–544. [DOI] [PubMed] [Google Scholar]
- 42.Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of xenopus oocytes expressing aquaporin 1 or its c189s mutant. Am J Physiol 1998;275:C1481–C1486. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda H, Yamaya M, Nakayama K, Ebihara S, Sasaki T, Okinaga S, Inoue D, Asada M, Nemoto M, Sasaki H. Increased arterial carboxyhemoglobin concentrations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:1246–1251. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda H, Yamaya M, Yanai M, Ohrui T, Sasaki H. Increased blood carboxyhaemoglobin concentrations in inflammatory pulmonary diseases. Thorax 2002;57:779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Thoracic Society. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children—1999. Am J Respir Crit Care Med 1999;160:2104–2117. [DOI] [PubMed] [Google Scholar]
- 46.Borland CD, Higenbottam TW. A simultaneous single breath measurement of pulmonary diffusing capacity with nitric oxide and carbon monoxide. Eur Respir J 1989;2:56–63. [PubMed] [Google Scholar]
- 47.Silkoff PE, McClean PA, Slutsky AS, Furlott HG, Hoffstein E, Wakita S, Chapman KR, Szalai JP, Zamel N. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am J Respir Crit Care Med 1997;155:260–267. [DOI] [PubMed] [Google Scholar]
- 48.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med 2003;35:213–225. [DOI] [PubMed] [Google Scholar]
- 49.Wedes SH, Khatri SB, Zhang R, Wu W, Comhair SAA, Wenzel S, Teague WG, Israel E, Erzurum SC, Hazen SL. Noninvasive markers of airway inflammation in asthma. Clin Transl Sci 2009;2:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.