Abstract
Rationale: Fluoroquinolones are the most commonly prescribed antibiotic class in the United States. They have the potential to become first-line antituberculosis therapy, but the effect of fluoroquinolone use on fluoroquinolone resistance in Mycobacterium tuberculosis is not well characterized.
Objectives: To determine the prevalence of and risk factors for fluoroquinolone-resistant tuberculosis in a large United States population.
Methods: We identified all people with culture-confirmed tuberculosis enrolled in TennCare (Medicaid) and reported to the Tennessee Department of Health from January 2002 to December 2006. People with fluoroquinolone-resistant M. tuberculosis isolates (cases) were compared with those with susceptible isolates (control subjects). Fluoroquinolone resistance was determined by agar proportion using ofloxacin 2 μg/ml. Outpatient fluoroquinolone exposure in the 12 months before tuberculosis diagnosis was ascertained from TennCare pharmacy data.
Measurements and Main Results: Of 640 study patients, 116 (18%) had fluoroquinolone exposure in the 12 months before diagnosis, and 16 (2.5%; 95% confidence interval [CI], 1.4–4.0%) M. tuberculosis isolates were fluoroquinolone resistant. Among the 54 patients with more than 10 days of fluoroquinolone exposure, 7 (13%) had fluoroquinolone resistance. In multivariable logistic regression analyses using propensity score to control for age, sex, race, HIV serostatus, and site of disease, more than 10 days of fluoroquinolone exposure before tuberculosis diagnosis was associated with fluoroquinolone resistance (odds ratio 7.0; 95% CI, 2.3–20.6; P = 0.001). Fluoroquinolone exposure for more than 10 days that occurred more than 60 days before tuberculosis diagnosis was associated with the highest risk of resistance (20.8%; odds ratio 17.0; 95% CI, 5.1–56.8; P < 0.001 compared with no exposure).
Conclusions: Overall, fluoroquinolone resistance was relatively low. However, receipt of fluoroquinolones for more than 10 days, particularly more than 60 days before tuberculosis diagnosis, was associated with a high risk of fluoroquinolone-resistant tuberculosis.
Keywords: drug resistance, mycobacteria, antibacterial
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The effect of fluoroquinolone use on its resistance in Mycobacterium tuberculosis is not well characterized.
What This Study Adds to the Field
Although overall prevalence of fluoroquinolone-resistant tuberculosis was low, receipt of fluoroquinolones for more than 10 days, particularly more than 60 days before the diagnosis of tuberculosis, was associated with a high risk of fluoroquinolone-resistant tuberculosis.
Treatment of drug-susceptible tuberculosis requires 6 to 9 months of combination therapy. Poor adherence to treatment results in suboptimal treatment completion rates, which has necessitated the use of directly observed therapy (1). Shorter treatment duration could improve adherence and treatment outcomes.
The fluoroquinolones, particularly later-generation agents, such as levofloxacin, gatifloxacin, and moxifloxacin, have excellent in vitro and in vivo activity against Mycobacterium tuberculosis (2, 3). Clinical trials in humans suggest that fluoroquinolones, such as ofloxacin, gatifloxacin, and moxifloxacin, might shorten treatment duration (4–6). Thus, fluoroquinolones can potentially improve current tuberculosis treatment and become first-line antituberculosis therapy.
Fluoroquinolone use for routine bacterial infections is widespread; they are the most commonly prescribed antibiotic class in the United States (7). Current Infectious Diseases Society of America/American Thoracic Society guidelines for community-acquired pneumonia recommend that fluoroquinolones be used for both inpatient and outpatient treatment of pneumonia (8). As a result, fluoroquinolones are frequently prescribed to people who are subsequently diagnosed with tuberculosis. Among a cohort of patients with tuberculosis in Tennessee from 2000 to 2004, 23% received fluoroquinolone monotherapy before diagnosis. The proportion of exposed people increased from 9% in 2000 to 41% in 2004 (P < 0.001) (9).
Fluoroquinolone resistance in M. tuberculosis can develop after as little as 13 days of fluoroquinolone therapy (10). Although fluoroquinolone resistance in M. tuberculosis is not routinely assessed, the proportion of newly diagnosed (i.e., previously untreated) patients with tuberculosis with fluoroquinolone resistance has ranged from 0.15 to 3.6% in previous reports (11–15). However, the risk factors for fluoroquinolone resistance in M. tuberculosis have not been fully elucidated. It is important to characterize the extent of fluoroquinolone resistance in M. tuberculosis, as well as the risk factors for resistance, so that this potent class of antimicrobial agents can be used rationally to treat both routine bacterial infections and tuberculosis. Some of the results of this study have been previously reported in the form of an abstract (15).
METHODS
Additional detail on methods is provided in the online supplement.
Study Population
We conducted a retrospective case-control study among all newly diagnosed patients with culture-confirmed tuberculosis reported to the Tennessee Department of Health between January 2002 and December 2006 who were also enrolled in Tennessee's Medicaid Program, TennCare. TennCare is a managed health care program that insures Tennessee residents who are eligible for federal Medicaid benefits and other low-income groups. The TennCare pharmacy database consists of all outpatient and emergency department prescriptions filled and includes the drug dispensed, fill date, dose, and days of supply. The Tennessee tuberculosis case registry was linked to the TennCare database using demographic information included in both databases. Data on fluoroquinolone prescriptions filled in the 12 months before tuberculosis diagnosis were obtained from the TennCare pharmacy database. TennCare prescription insurance from January 2002 to July 2005 covered prescriptions for all formulary medications. Starting in August 2005 prescription coverage for TennCare enrollees covered two nongeneric and three generic drugs per month.
Cases were defined as patients with fluoroquinolone-resistant tuberculosis. Control subjects were patients with fluoroquinolone-susceptible tuberculosis; all such patients were included in the study. The study was approved by the Institutional Review Boards of Vanderbilt University, the Tennessee Department of Health, and the Davidson County Metro Public Health Department. The study was also reviewed by the Bureau of TennCare.
Laboratory Methods
The first M. tuberculosis isolate obtained for each patient was used in the study. Patients were excluded from the study if the M. tuberculosis isolate was unavailable. A 1.0 McFarland inoculum prepared from colonies on Lowenstein-Jensen slants served as the standard inoculum for all susceptibility testing. Screening for fluoroquinolone resistance was performed via agar proportion using ofloxacin (16). A minimum inhibitory concentration of 2 μg/ml was used to identify ofloxacin resistance in Middlebrook 7H9 medium. Spoligotyping and mycobacterial interspersed repetitive units testing of fluoroquinolone-resistant M. tuberculosis isolates were performed at the Michigan Department of Community Health Laboratory according to standard methods (17).
Statistical Analysis
Demographic and clinical characteristics were compared using the Wilcoxon rank-sum test for continuous variables and the χ2 or Fisher exact tests for categorical variables. Fluoroquinolone exposure was measured as a categorical (i.e., any versus none), continuous (i.e., number of days of exposure), and dichotomous (i.e., ≤10 d vs. >10 d; cutoff determined preanalysis) variable. Trends in the proportion of patients with fluoroquinolone exposure and resistance from 2002 to 2006 were assessed using the χ2 test for trend.
Factors associated with fluoroquinolone resistance were assessed using univariate and multivariate logistic regression analysis. Risk factors included age, sex, race (black vs. non-black), HIV serostatus, site of disease (extrapulmonary vs. pulmonary), and fluoroquinolone exposure. Because of the relatively few fluoroquinolone-resistant tuberculosis cases, for multivariable modeling we used propensity score adjustment for each of the above risk factors.
Propensity score adjustment preserved statistical power by reducing covariates into a single variable. For example, when the adjusted effect of fluoroquinolone exposure was evaluated, the propensity score was created through a binary logistic regression providing the predicted probability of having fluoroquinolone exposure as a function of the other candidate risk factors (age, sex, race, HIV serostatus, and site of disease). For continuous variables (i.e., age) the proportional odds logistic regression model derived the propensity score. Propensity score was computed separately for each candidate risk factor and was then used as a covariate in the model evaluating the adjusted effect of each factor. A locally weighted scatterplot smoothing (LOWESS) was used to assess the association between fluoroquinolone exposure and fluoroquinolone-resistant tuberculosis.
The statistical package STATA, version 9 (STATA Corporation, College Station, TX) and R 2.6.0 (The R Project for Statistical Computing) were used for all analyses. All P values were two-sided. P values less than 0.05 were considered statistically significant.
RESULTS
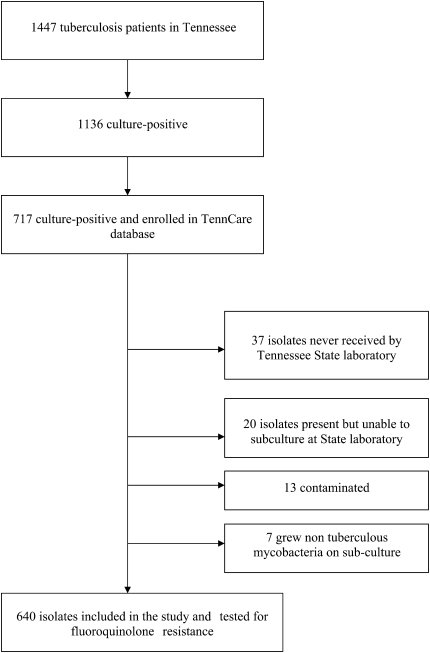
There were 1,136 culture-confirmed tuberculosis cases reported to the Tennessee Department of Health during the study period, of whom 717 (63%) were linked to the TennCare enrollment file (Figure 1). The 419 (37%) culture-positive patients not enrolled in TennCare were of similar sex compared with the 717 TennCare patients, but were older (median age 58 yr vs. 48 yr; P < 0.001) and less likely to be black (25% vs. 54%; P < 0.001) and HIV-infected (4% vs. 13%; P < 0.001). Of the 717 patients, 640 (89%) had isolates available for fluoroquinolone susceptibility testing and composed the study population. Median duration of TennCare enrollment in the year before diagnosis was 159 days; 284 (45%) were enrolled more than 300 days.
Figure 1.
Patients with tuberculosis during the study period: January 2002 to December 2006.
The 640 study patients were predominantly male (68%), black (53%), and HIV-seronegative (88%), and had pulmonary tuberculosis disease (78%). The clinical and demographic characteristics of the study population according to fluoroquinolone-susceptible and -resistant tuberculosis are shown in Table 1. Age, sex, race, HIV serostatus, and foreign-born status were not significantly different between the two groups. Patients with fluoroquinolone-resistant tuberculosis were more likely to have extrapulmonary disease and received more than 10 days of outpatient fluoroquinolones before tuberculosis diagnosis. There was not a significant difference between the 356 patients in TennCare for less than 300 days and the 284 patients in TennCare for more than 300 days regarding race, HIV serostatus, and extrapulmonary disease. Patients in TennCare for more than 300 days were more likely to be female, foreign-born, and older (median age 53 yr vs. 45 yr).
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF THE 640 STUDY PATIENTS
| Variable | Fluoroquinolone Susceptible (n = 624) n (%) | Fluoroquinolone Resistant (n = 16) n (%) | P Value* |
|---|---|---|---|
| Median age, years (IQR) | 47 (35–62) | 57 (36–74) | 0.6 |
| Male sex | 426 (68) | 8 (50) | 0.1 |
| Black race | 336 (54) | 5 (31) | 0.1 |
| HIV seropositive | 73 (12) | 2 (13) | 1.0 |
| Foreign born | 72 (12) | 2 (13) | 0.9 |
| Site of disease | 0.03 | ||
| Pulmonary only | 489 (78) | 11 (69) | |
| Extrapulmonary only | 72 (12) | 5 (31) | |
| Both pulmonary/extrapulmonary | 63 (10) | 0 (0) | |
| Sputum smear positive | 337 (54) | 9 (56) | 0.9 |
| Cavitary lesion present on chest radiograph | 226 (37) | 6 (38) | 0.9 |
| >10 d of outpatient fluoroquinolone exposure before tuberculosis diagnosis | 47 (8) | 7 (44) | <0.001 |
Definition of abbreviation: IQR = interquartile range.
Values are n (%) unless otherwise indicated.
Wilcoxon rank sum test was used for comparison of continuous variables. The χ2 test compared categorical variables.
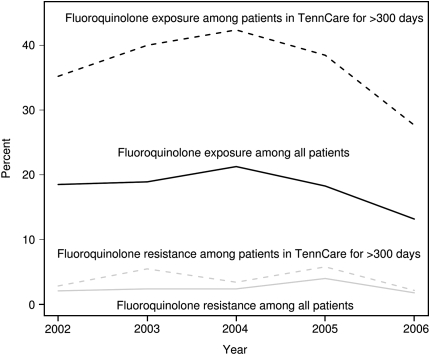
Of the 640 study patients, 116 (18%) received outpatient fluoroquinolones before tuberculosis diagnosis and 54 (47%) of these received more than 10 days of fluoroquinolones. Of the 116 fluoroquinolone-exposed patients, 264 courses of fluoroquinolones were prescribed, of which 147 (56%) were levofloxacin, 78 (30%) were ciprofloxacin, and 20 (8%) were moxifloxacin. There was no trend in fluoroquinolone exposure from 2002 to 2006 (χ2 test for trend = 0.4) (Figure 2). Among the 284 patients in TennCare for more than 300 days, 105 (37%) received a fluoroquinolone and 49 (47%) of these patients received more than 10 days of fluoroquinolones. There was no trend in fluoroquinolone exposure between 2002 and 2006 (χ2 test for trend P = 0.7) (Figure 2).
Figure 2.
Percent of study patients with fluoroquinolone exposure and resistance by year, from 2002 to 2006. χ2 test for trend for fluoroquinolone exposure among patients in TennCare more than 300 days; P = 0.7. χ2 test for trend for fluoroquinolone exposure among all patients; P = 0.4. χ2 test for trend for fluoroquinolone resistance among patients in TennCare more than 300 days; P = 1.0. χ2 test for trend for fluoroquinolone resistance among all patients; P = 0.8.
Of the 640 study patients, 16 (2.5%; 95% CI, 1.4–4.0%) had fluoroquinolone-resistant M. tuberculosis isolates. None of the 16 fluoroquinolone-resistant isolates were resistant to isoniazid, rifampin, pyrazinamide, or ethambutol. There was no trend in fluoroquinolone resistance over time (χ2 test for trend P = 0.8) (Figure 2). Of the 16 patients with fluoroquinolone-resistant tuberculosis, 8 (50%) had documented outpatient fluoroquinolone exposure. Of the 284 patients in TennCare for more than 300 days, 11 (3.9%; 95% CI, 1.6–6.1) had fluoroquinolone-resistant M. tuberculosis isolates. There was also no trend in fluoroquinolone resistance (χ2 test for trend = 1.0) (Figure 2).
In univariate logistic regression analyses, more than 10 days of fluoroquinolone exposure before tuberculosis diagnosis and extrapulmonary disease was significantly associated with fluoroquinolone resistance (Table 2). Any fluoroquinolone exposure was also significantly associated with fluoroquinolone resistance (odds ratio [OR] 4.8; 95% CI, 1.8–13.0; P = 0.002). Age, sex, and HIV serostatus were not associated with fluoroquinolone resistance. Black race trended toward a decreased risk of resistance, but did not achieve statistical significance.
TABLE 2.
FACTORS ASSOCIATED WITH FLUOROQUINOLONE RESISTANCE IN MYCOBACTERIUM TUBERCULOSIS, UNIVARIATE AND MULTIVARIABLE LOGISTIC REGRESSION ANALYSIS
| Risk Factor | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Age* | 1.01 (0.99–1.04) | 0.4 | 1.0 (0.97–1.02) | 0.1 |
| Male sex | 0.5 (0.2–1.3) | 0.1 | 0.6 (0.2–1.6) | 0.3 |
| Black race | 0.4 (0.1–1.1) | 0.08 | 0.5 (0.2–1.5) | 0.2 |
| HIV seropositive | 1.1 (0.2–4.8) | 0.9 | 1.5 (0.3–9.2) | 0.8 |
| Extrapulmonary disease only | 3.5 (1.2–10.3) | 0.02 | 2.9 (0.9–9.1) | 0.1 |
| >10 d of outpatient fluoroquinolone exposure† | 9.6 (3.4–26.8) | <0.001 | 7.0 (2.3–20.6) | 0.001 |
Definition of abbreviations: CI = confidence interval; OR= odds ratio.
OR was derived from multivariable logistic regression. Propensity score was used as an adjustment covariate for each factor separately derived as a function of the other candidate risk factors. Reference groups: female sex, non-black race, HIV seronegative, only pulmonary disease, 10 days or less of fluoroquinolone exposure.
Per 1-year increase.
Before tuberculosis diagnosis.
In multivariable logistic regression analyses using propensity score to control for age, sex, race, HIV serostatus, and site of disease, more than 10 days of fluoroquinolone exposure before tuberculosis diagnosis remained associated with fluoroquinolone resistance (Table 2). Multivariable logistic regression analysis of patients in TennCare for more than 300 days was consistent with the findings in the larger group, with more than 10 days of fluoroquinolone exposure associated with fluoroquinolone-resistant tuberculosis (OR 8.0; 95% CI, 2.1–31.1; P = 0.003). Age, sex, race, HIV serostatus, and site of disease were not associated with fluoroquinolone resistance.
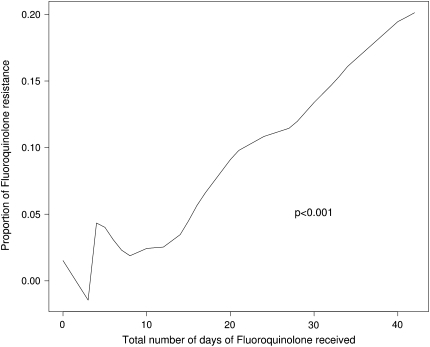
Overall, as the number of days of fluoroquinolones received increased, the proportion of tuberculosis cases with fluoroquinolone resistance increased (Figure 3). In the same multivariable model as above, the odds of fluoroquinolone resistance increased 51% for every 10-day increase in fluoroquinolone exposure (OR 1.5; 95% CI, 1.2–1.9; P < 0.001). People with fluoroquinolone exposure but fluoroquinolone-susceptible tuberculosis had a median of 10 days (interquartile range [IQR] 7–20 d) of fluoroquinolone exposure compared with 30.5 (IQR 19–56 d) days of exposure in fluoroquinolone-resistant patients (P = 0.01).
Figure 3.
Association between number of days of fluoroquinolones received and fluoroquinolone-resistant tuberculosis.
Among the 116 fluoroquinolone-exposed patients, the median number of fluoroquinolone courses received was one (IQR 1–3). Among those who received more than one course of fluoroquinolones, the median total duration of drug exposure was 16 days (IQR 27–40 d). Among the fluoroquinolone-exposed patients who received only one course, the median total duration of fluoroquinolone exposure was 10 days (IQR 5–10 d). Patients who received more than one course of fluoroquinolone were more likely to have fluoroquinolone-resistant tuberculosis, compared with those who received only one course (P = 0.007). In univariate analysis more than one course of fluoroquinolone use remained significantly associated with fluoroquinolone resistance (OR 11; 95% CI, 1.3–92.6).
In further analysis we compared patients according to duration of fluoroquinolone exposure (>10 d vs. ≤10 d). Of the 54 patients with more than 10 days of fluoroquinolone exposure, 7 (13%) had fluoroquinolone-resistant tuberculosis compared with 1 of 62 (1.6%) patients with 10 days or less of fluoroquinolone exposure (P = 0.02). In univariate and multivariate analysis the association between fluoroquinolone exposure and fluoroquinolone resistance was not found when the 54 patients with more than 10 days of fluoroquinolone exposure were excluded.
We also assessed risk factors for fluoroquinolone resistance among only the 116 people who had received fluoroquinolones before tuberculosis diagnosis. Of the 116, 8 (6.9%) had fluoroquinolone-resistant disease. In univariate logistic regression analyses, extrapulmonary disease (OR 6.2; 95% CI, 1.4–27.5; P = 0.02) and more than 10 days of fluoroquinolone exposure (OR 9.1; 95% CI, 1.1–76.4; P = 0.04) were associated with fluoroquinolone resistance. These variables were also associated with fluoroquinolone resistance in multivariate logistic regression analyses, but they were of borderline statistical significance, likely due to small sample size (extrapulmonary disease OR 4.5; 95% CI, 1.0–20.9; P = 0.06; >10 d of fluoroquinolone exposure OR 7.1; 95% CI, 0.8–61.8; P = 0.08). Among those who were fluoroquinolone exposed, patients with extrapulmonary disease had a median of 21 days (IQR 10–48 d) of fluoroquinolone exposure compared with patients with pulmonary disease who had a median of 10 days (IQR 7–20 d) of fluoroquinolone exposure (P = 0.03).
One hundred (93%) of the 116 patients with fluoroquinolone exposure before tuberculosis diagnosis had data available regarding the indication for the first fluoroquinolone prescription. A respiratory diagnosis was documented in 52 (52%) patients and sixteen (16%) patients had a genitourinary diagnosis. Neither respiratory nor genitourinary indications were associated with fluoroquinolone resistance (data not shown).
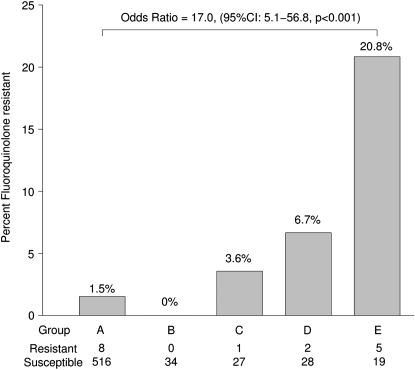
In an assessment of the duration (≤10 d vs. >10 d) and timing of the last fluoroquinolone exposure before tuberculosis diagnosis (≤60 d vs. >60 d prior), patients receiving more than 10 days of fluoroquinolones more than 60 days before diagnosis had the highest proportion (20.8%) of fluoroquinolone-resistant tuberculosis (OR 17.0; 95% CI, 5.1–56.8; P < 0.001 compared with patients not exposed to fluoroquinolone) (Figure 4). Patients in TennCare for more than 300 days receiving more than 10 days of fluoroquinolones more than 60 days before diagnosis had the highest proportion (23.8%) of fluoroquinolone-resistant tuberculosis (OR 18.3; 95% CI, 4.0–83.8; P < 0.001 compared with patients not exposed to fluoroquinolone).
Figure 4.
Percent fluoroquinolone resistance according to duration of fluoroquinolone exposure (≤10 d vs. >10 d) and timing of last exposure (≤60 d vs. >60 d) before tuberculosis diagnosis. (A) No outpatient fluoroquinolone exposure. (B) ≤10 days of fluoroquinolones and last fluoroquinolone exposure ≤60 days before tuberculosis diagnosis. (C) ≤10 days of fluoroquinolones and last fluoroquinolone exposure >60 days before tuberculosis diagnosis. (D) >10 days of fluoroquinolones and last fluoroquinolone exposure ≤60 days before tuberculosis diagnosis. (E) >10 days of fluoroquinolones and last fluoroquinolone exposure >60 days before tuberculosis diagnosis.
Spoligotyping and mycobacterial interspersed repetitive units results were available for 14 of 16 fluoroquinolone-resistant M. tuberculosis isolates. Five isolates (36%) had a unique genotype, eight (57%) matched with clusters that were not otherwise present among the 14 cases, and two (14%) isolates matched one another. There were no known epidemiologic links between these two cases.
DISCUSSION
There were several important findings of this study. First, the proportion of people who received fluoroquinolones before tuberculosis diagnosis was high, consistent with our previous study in the same population over a different time period (9). In the subset of our study population that was in TennCare for more than 300 days before tuberculosis diagnosis (and therefore for whom ascertainment of outpatient fluoroquinolone exposure was most complete), 37% received a fluoroquinolone before diagnosis. Second, the overall proportion of M. tuberculosis isolates with fluoroquinolone resistance was 2.5% (95% CI, 1.4–4.0%) and among those in TennCare for more than 300 days the fluoroquinolone resistance rate was 3.9% (95% CI, 1.6–6.1). These rates are comparable to previous reports of fluoroquinolone resistance among patients newly diagnosed with tuberculosis, which has ranged from 0.15 to 3.6% (11–14). They are also comparable to the proportion of M. tuberculosis isolates with isoniazid resistance in patients with tuberculosis in Tennessee (3.4%). However, the fluoroquinolone resistance rate was higher among patients with a history of fluoroquinolone exposure before tuberculosis diagnosis (6.9% among the 116 patients exposed to fluoroquinolone). Not surprisingly, the primary risk factor for fluoroquinolone resistance in this study was exposure to fluoroquinolones before tuberculosis diagnosis. The relationship between the duration of fluoroquinolone exposure and fluoroquinolone resistance was almost linear (Figure 3). Among people who received more than 10 days of fluoroquinolones, a substantial proportion (13%) had fluoroquinolone-resistant tuberculosis. The timing of fluoroquinolone exposure in relation to tuberculosis diagnosis was also important. Although one might hypothesize that fluoroquinolone exposure shortly before tuberculosis diagnosis might increase the risk of resistance, we found that the rate of fluoroquinolone resistance was highest among people who received more than 10 days of fluoroquinolones and received their most recent course of fluoroquinolones more than 60 days before diagnosis. Among such people, 20.8% had fluoroquinolone-resistant tuberculosis. Exposure to fluoroquinolones early in the course of disease may select for and allow a fluoroquinolone-resistant strain to predominate. Of note, exposure to less than 10 days of fluoroquinolones (the duration of a single course for indications such as pneumonia, sinusitis, and urinary tract infection) was associated with a relatively low rate of resistance (1.6%).
We are unaware of previous studies that have assessed outpatient fluoroquinolone exposure in people with tuberculosis. However, two studies that assessed inpatient fluoroquinolone exposure support our findings that earlier exposure for longer periods of time is a key determinant of fluoroquinolone-resistant disease. In a study of 2,788 M. tuberculosis isolates in Korea, there was no relationship between inpatient fluoroquinolone exposure in the 3 months to 5 days before tuberculosis diagnosis and fluoroquinolone resistance. The median duration of fluoroquinolone exposure was 7 days and the duration from initial exposure to tuberculosis diagnosis was 20 days (14). A Taiwanese study evaluated 420 randomly selected M. tuberculosis isolates and assessed previous fluoroquinolone exposure in these patients over an 18-month period. The mean duration of fluoroquinolone exposure was 9.5 days (timing in relation to tuberculosis diagnosis was not provided). Again, there was no association between fluoroquinolone exposure and resistance (18). Thus, inpatient fluoroquinolone exposure, which generally occurs close to the time of tuberculosis diagnosis and is of relatively short duration (perhaps as empiric therapy for pneumonia before the tuberculosis diagnosis is established), does not appear to increase the risk of fluoroquinolone resistance. In contrast, repeated and early exposure of fluoroquinolones is more likely to result in fluoroquinolone-resistant tuberculosis.
Our study was limited by the lack of inpatient fluoroquinolone exposure data so that a direct comparison of the effect of outpatient and inpatient fluoroquinolone exposure could be made in the same cohort; such future studies are warranted. A second limitation was that not all patients were in TennCare for the full 12 months before tuberculosis diagnosis, which could decrease ascertainment of fluoroquinolone exposure. However, when the analysis was limited to patients in TennCare for more than 300 days before tuberculosis diagnosis, the results were the same as for the entire group. Third, TennCare prescription coverage changed in August 2005, covering up to five medications per month. Because patients had to pay for additional medications, they may have had decreased access to fluoroquinolones after August 2005. Finally, the study population differed slightly compared with Tennessee patients with tuberculosis who were not in TennCare. However, the age, sex, race, and HIV prevalence of the study population was comparable to that of patients with tuberculosis throughout the United States suggesting that the study results are generalizable to the United States tuberculosis population (19).
In this study, fluoroquinolones were frequently prescribed to people who were subsequently diagnosed with tuberculosis. The proportion of patients with tuberculosis with fluoroquinolone resistance was 2.5%, but it was substantially higher among people who received more than 10 days of fluoroquinolones, particularly if it occurred more than 60 days before tuberculosis diagnosis. This highlights the importance of considering the diagnosis of tuberculosis in people with symptoms suggestive of this disease and limiting the use of prolonged or repeated courses of fluoroquinolones in such patients.
Supplementary Material
Acknowledgments
Data to conduct the study were obtained from the Tennessee Department of Health and the TennCare Bureau. The authors thank the Michigan Department of Community Health for genotyping M. tuberculosis isolates.
Supported by National Institutes of Health grants NIAID R01 AI 063200, NIAID K24 AI 65298, and KL2 RR024977-02.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200901-0146OC on May 29, 2009
Conflict of Interest Statement: R.A.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.G. has participated as a speaker for meetings sponsored by Wyeth and received unrestricted grant funding ($300,000) from Pfizer from 2005–2007. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003;167:603–662. [DOI] [PubMed] [Google Scholar]
- 2.Hoffner SE, Gezelius L, Olsson-Liljequist B. In-vitro activity of fluorinated quinolones and macrolides against drug-resistant Mycobacterium tuberculosis. J Antimicrob Chemother 1997;40:885–888. [DOI] [PubMed] [Google Scholar]
- 3.Berlin OG, Young LS, Bruckner DA. In-vitro activity of six fluorinated quinolones against Mycobacterium tuberculosis. J Antimicrob Chemother 1987;19:611–615. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan P. Shortening short course chemotherapy: a randomised clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Indian J Tuberc 2002;49:27–38. [Google Scholar]
- 5.Chaisson RE, Conde M, Efron A, Loredo C. A randomized placebo-controlled trial of moxifloxacin vs. Ethambutol in the initial phase of tuberculosis therapy in Brazil. Interscience Conference of Antimicrobial Agents and Chemotherapy. Sept. 17–20, 2007, Chicago, IL. Abstract L-736-A.
- 6.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, Reddy C, Sturm AW, Sirgel FA, Allen J, et al. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008;12:128–138. [PubMed] [Google Scholar]
- 7.Linder J, Huang E, Steinman M, Gonzales R, Stafford R. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med 2005;118:259–268. [DOI] [PubMed] [Google Scholar]
- 8.Mandell L, Wunderink R, Anzueto A, Bartlett J, Campbell G, Dean N, Dowell S, File T Jr, Musher D, Niederman M, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44:S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaba PD, Haley C, Griffin MR, Mitchel E, Warkentin J, Holt E, Baggett P, Sterling TR. Increasing outpatient fluoroquinolone exposure before tuberculosis diagnosis and impact on culture-negative disease. Arch Intern Med 2007;167:2317–2322. [DOI] [PubMed] [Google Scholar]
- 10.Ginsburg AS, Woolwine SC, Hooper N, Benjamin WH Jr, Bishai WR, Dorman SE, Sterling TR. The rapid development of fluoroquinolone resistance in M. Tuberculosis. N Engl J Med 2003;349:1977–1978. [DOI] [PubMed] [Google Scholar]
- 11.Bozeman L, Burman W, Metchok B, Welch L, Weiner M, Tuberculosis Trials Consortium. Fluoroquinolone susceptibility among mycobacterium tuberculosis isolates from the United States and Canada. Clin Infect Dis 2002;40:386–391. [DOI] [PubMed] [Google Scholar]
- 12.Ginsburg A, Hoope N, Parrish N, Dooley K, Dorman S, Booth J, Diener-West M, Merz W, Bishai W, Sterling T. Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin Infect Dis 2003;37:1448–1452. [DOI] [PubMed] [Google Scholar]
- 13.Umubyeyi A, Rigouts L, Shamputa I. Limited fluoroquinolone resistance among mycobacterium tuberculosis isolates from Rwanda: results of a national survey. J Antimicrob Chemother 2007;59:1031–1033. [DOI] [PubMed] [Google Scholar]
- 14.Park I, Hong S, Oh Y, Lim C, Lee S, Lew W. Impact of short-term exposure to fluoroquinolones on ofloxacin resistance in HIV-negative patients with tuberculosis. Int J Tuberc Lung Dis 2007;11:319–323. [PubMed] [Google Scholar]
- 15.Devasia R, Griffin M, Blackman A, May S, Holt E, Smith T, Warkentin J, Mitchel E. Trends in fluoroquinolone resistance and exposure in newly diagnosed tuberculosis: 2002–2006 [abstract]. Am J Respir Crit Care Med 2008;177:A19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. CLSI document M24-A [ISBN 1–56238–500–3] Vol. 23. Wayne, PA: The Institute; 2003. [PubMed]
- 17.Cowan LS, Diem L, Monson T, Wand P, Temporado D, Oemig TV, Crawford JT. Evaluation of a two-step approach for large-scale, prospective genotyping of mycobacterium tuberculosis isolates in the United States. J Clin Microbiol 2005;43:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JY, Lee LN, Lai HC, Wang SK, Jan IS, Yu CJ, Hsueh PR, Yang PC. Fluoroquinolone resistance in mycobacterium tuberculosis isolates: associated genetic mutations and relationship to antimicrobial exposure. J Antimicrob Chemother 2007;59:860–865. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Trends in tuberculosis. MMWR Morb Mortal Wkly Rep 2007;57:245–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.