Abstract
Polyvinylphosphonic acid (PVPA), a biomimetic analog of phosphoproteins, is crucial for recruiting polyacrylic acid (PAA)-stabilized amorphous calcium phosphate nanoprecursors during biomimetic remineralization of dentin collagen matrices. This study tested the null hypothesis that phosphoric acid esters of methacrylates in dentin adhesives cannot replace PVPA during bimimetic remineralization of resin-dentin interfaces. Human dentin specimens were bonded with: I) XP Bond, an etch-and-rinse adhesive using moist bonding; II) XP Bond using dry bonding; and III) Adper Prompt L-Pop, a self-etching adhesive. The control medium contained only set Portland cement and a simulated body fluid (SBF) without any biomimetic analog. Two experimental Portland cement/SBF remineralization media were evaluated: the first contained PAA as the sole biomimetic analog, the second contained PAA and PVPA as dual biomimetic analogs. No remineralization of the resin-dentin interfaces could be identified from specimens immersed in the control medium. After 2–4 months in the first experimental medium, specimens exhibited either no remineralization or large crystal formation within hybrid layers. Only specimens immersed in the second remineralization medium produced nanocrystals that accounted for intrafibrillar remineralization within hybrid layers. The null hypothesis could not be rejected; phosphoric acid esters in dentin adhesives cannot replace PVPA during biomimetic remineralization of adhesive-bonded dentin.
Keywords: Biomimetics, Phosphate esters, Dentin adhesive, Remineralization, Intrafibrillar, Interfibrillar
1. Introduction
The advent of new generations of dentin adhesives and bonding techniques has improved the quality of resin-dentin bonds [1,2]. However, degradation of collagen fibrils by endogenous matrix metalloproteinases (MMPs) [3] in hybrid layers of resin-bonded interface over time remains a vexing problem [4–6]. This probably accounts for the reduced longevity of clinically-applied adhesive restorations [7,8], which results in billions of dental care dollars spent annually on replacement of these restorations. Although the integrity of hybrid layers may be preserved by application of MMP inhibitors such as chlorhexidine as part of the dentin bonding procedures [3–6], a zone of resin-sparse, demineralized dentin inadvertently remains that is potentially susceptible to creep rupture [9] or cyclic fatigue rupture [10] after prolonged intraoral function. In addition, it remains to be determined whether MMP-inhibiting activity of chlorhexidine is provisional or permanent (i.e. delaying instead of arresting hybrid layer degradation) as it is water-soluble and may eventually leach from hybrid layers. Thus, there is a compelling need to pursue alternative methods for extending the longevity of resin-dentin bonds.
Biomimetic remineralization offers the potential for remineralizing incompletely-infiltrated hybrid layers [11]. A Portland cement-based [12] biomimetic remineralization protocol that involves the use of dual biomimetic analogs has succeeded in inducing interfibrillar and intrafibrillar remineralization of acid-etched dentin [13] and incompletely resin-infiltrated hybrid layers created by etch-and-rinse adhesives [11]. To promote the remineralization of the imperfectly-created resin-dentin interfaces, a low molecular weight polyacrylic acid (PAA) was used to mimic the function of dentin matrix protein-1 (DMP-1) on stabilizing amorphous calcium phosphate precursors [14] and reducing the dimension of these liquid phase precursors to a nanoscale [15]. The other biomimetic analog, polyvinylphosphonic acid (PVPA), is a polyanion that mimics the negative charges of phosphoproteins such as DMP-1, phosphophoryn or bone sialoprotein [16,17]. Similar to phosphoprotein molecules, PVPA is conjectured to bind to demineralized collagen fibrils, thereby guiding the deposition of PAA-stabilized amorphous calcium phosphate nanoprecursors within and along specific sites of the collagen fibrils.
In an effort to develop effective restorative materials which enable demineralized dentin to be remineralized, glass-ionomer cements were thought to accomplish this objective due to their long-term fluoride release [18,19]. However, remineralization sites were restricted to the dentin surface [20,21]. Moreover, their poor wear resistance, brittleness, and low bond strength limited their clinical use. Hashimoto et al. [22] recently reported that crystals were formed in 40-μm thick gaps between fluoride-releasing resin adhesives and dentin after long-term water storage. Hayakawa et al. [23] also reported deposition of carbonated apatite on polymer disks created from a phosphorylated dentin bonding agent (Clearfil Photo Bond, Kuraray Medical Inc., Tokyo, Japan) after these disks were immersed in an electrolyte solution. The authors opined that the phosphonic acid and phosphate groups in the phosphorylated dentin bonding agent had the capability to induce nucleation and growth of apatite crystals. The results of these two studies were reminiscent of dystrophic calcification that was recently reported in intraocular lens [24–26]. Nevertheless, the ability for phosphorylated dentin bonding agents to induce apatite formation may create a window of opportunity for utilizing phosphate ester resin monomers as substitutes for PVPA as biomimetic analogs for dentin remineralization. As these resin monomers are applied to dentin and polymerized in-situ, they are located directly adjacent to the demineralized collagen matrix so that it is not necessary for them to diffuse from the external environment into resin-sparse regions of the resin-infiltrated dentin. Polymerized adhesives containing hydrophilic resin derivatives are also permeable to calcium and hydroxyl ions which are necessary to remineralize dentin.
Thus, the objective of this study was to examine whether dentin adhesives containing phosphoric acid esters of methacrylates can replace the use of PVPA for inducing intrafibrillar and interfibrillar remineralization of collagen fibrils within hybrid layers. The null hypothesis tested was that phosphoric acid esters present in etch-and-rinse and self-etching adhesives cannot replace PVPA as biomimetic analogs during remineralization of resin-dentin interfaces.
2. Materials and methods
2.1 Dentin adhesives
Two phosphoric acid ester-containing adhesives were used in the present study. The first adhesive was XP Bond (Dentsply De Trey, Konstanz, Germany). It is a tertiary butanol-based, filled, etch-and-rinse adhesive. It contains dipentaerythritol penta-acrylate phosphate (PENTA) as an adhesion promoter. The second adhesive was Adper Prompt L-Pop (3M ESPE, St. Paul, MN, USA). It is a self-etching adhesive which contains methacrylated phosphoric acid mono-and diesters [27]. The phosphoric acid and methacrylate group in these acidic resin monomers are combined into one molecule that etches and primes simultaneously.
2.2 Specimen preparation
Forty-five recently extracted noncarious human third molars were collected after patients’ informed consents were obtained under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. A flat dentin bonding surface in mid-coronal portion of tooth was prepared perpendicular to the longitudinal axis of each tooth using a slow-speed Isomet diamond saw (Buehler Ltd, Lake Bluff, IL, USA) under copious water cooling. Each dentin surface was then polished with a 400-grit silicon carbide paper under running water. The specimens were randomly divided into three groups (N=15): I) XP Bond, the etch-and-rinse adhesive using a moist bonding technique; II) XP Bond using a dry bonding technique; and III) Adper Prompt L-Pop, the self-etching adhesive.
For XP Bond, each dentin surface was etched with 32% phosphoric acid gel (Uni-Etch, Bisco Inc. Schaumburg, IL, USA) for 15 sec and thoroughly rinsed with deionized water. For group I, a moist bonding technique was employed by keeping the acid-etched dentin visibly moist during bonding. For group II, the etched dentin surface was air-dried with 30 psi compressed air for 5 sec. The latter was applied at 5 mm away from the dentin surface. XP Bond was then applied to the dentin surface using two adhesive coats and light-cured for 20 sec using a light-curing unit (LEDemetron 1, Kerr Corp., Danbury, CT, USA).
For Adper Prompt L-Pop, the two components of this one-step self-etching adhesive were mixed according to manufacturer’s instruction, applied to the dentin surface, and agitated for 15 sec. The liquid was then gently air-dried and spread into a homogenous, slightly shiny film and light-cured for 20 sec.
Incremental placement of two 2-mm thick layers of a resin composite was done and light-cured separately for 40 sec each. The bonded teeth were stored at 100% relative humidity for 24 hrs. Each tooth was then sectioned occluso-gingivally into 1-mm thick slabs, each containing the resin-dentin interface.
2.3 Remineralization medium
Type I white Portland cement (Lehigh Cement Company, Allentown, PA, USA) was sieved and mixed with deionized water in a water-to-powder ratio of 0.35:1, placed in flexible silicone molds and allowed to set and age at 100% relative humidity for one week before use.
A simulated body fluid (SBF) was prepared by dissolving 136.8 mM NaCl, 4.2 mM NaHCO3, 3.0 mM KCl, 1.0 mM K2HPO4·3H2O, 1.5 mM MgCl2·6H2O, 2.5 mM CaCl2 and 0.5 mM Na2SO4 in deionized water [28] and adding 3.08 mM sodium azide to prevent bacterial growth. This SBF also served as the control remineralization medium, which contained no biomimetic analog. Two experimental remineralization media were prepared. For the first medium, 500 μg/mL of PAA (MW 1,800; Sigma-Aldrich, St. Louis, IL, USA) was added to the SBF as the sole biomimetic analog. For the second remineralization medium, the SBF was supplemented with 500 μg/mL of PAA and 200 μg/mL of PVPA (MW 24,000; Sigma-Aldrich) as dual biomimetic analogs. All solutions were buffered to pH 7.4 with 0.1 M Tris Base or 0.1 M HCl and filtered through 0.22 μm Millipore filters.
2.4 Biomimetic remineralization
Each specimen slab was placed over a set Portland cement block (ca. 1 g) inside a glass scintillation vial. The latter was filled with 15 mL of solution. The specimens of each group were randomly divided into three subgroups according to solution that was placed inside the vial: SBF (control medium), SBF with PAA (first experimental remineralization medium), and SBF with PAA and PVPA (second experimental remineralization medium). The glass vials were stored at ambient temperature (ca. 25°C.) The medium was changed every month. The pH of biomimetic remineralization system was monitored weekly and kept above 9.25 [29]. Specimens were retrieved after 2 and 4 months (N=5) for ultrastructural examination of the extent of remineralization.
2.5 Transmission Electron Microscopy (TEM)
The specimens were fixed in Karnovsky’s fixative and post-fixed in 1% osmium tetroxide. After fixation, each specimen was rinsed three times in sodium cacodylate buffer. The specimens were dehydrated in an ascending series of ethanol (50–100%), immersed in propylene oxide as a transitional fluid and subsequently embedded in epoxy resin. Non-demineralized epoxy resin-embedded, 90–110 nm thick sections were prepared and examined without further staining using a JEM-1230 transmission electron microscope (JEOL Inc., Peabody, MA, USA) operated at 110 kV.
3 Results
3.1 Control Medium
Control specimens of moist-bonded and dry-bonded XP Bond and those bonded with Adper Prompt L-pop examined after 2 and 4 months of immersion in SBF exhibited no remineralization within hybrid layers (not shown).
3.2 Polyacrylic acid-containing remineralization medium
Specimens that were immersed in the first remineralization medium exhibited different results among the three groups. No remineralization was apparent in the moist-bonded XP Bond specimens when PVPA was excluded from the remineralization medium (Fig. 1A). Dry-bonded XP Bond specimens had clusters of relatively large crystalline deposits within the hybrid layers (Fig. 1B). The majority of these crystalline platelets were more than 100 nm along their C-axes (Fig. 1C).
Fig. 1.
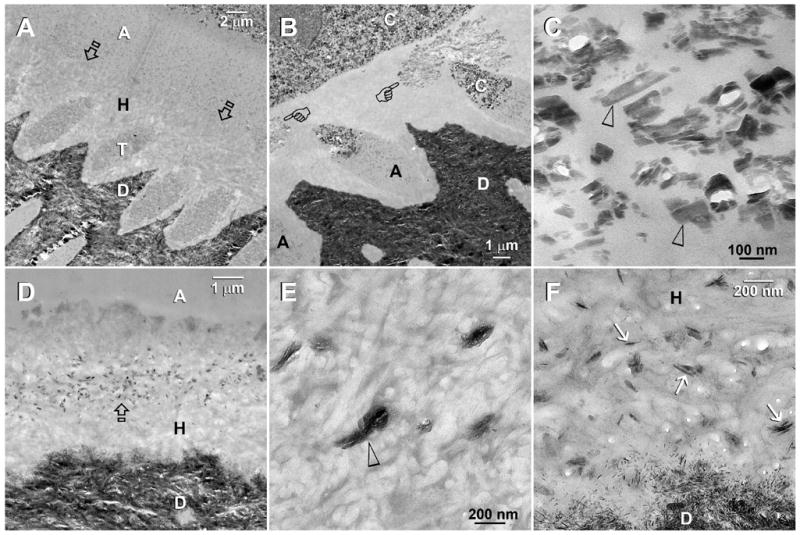
TEM images taken from unstained, undemineralized sections of resin-bonded dentin that were immersed in the first experimental Portland cement/SBF remineralization medium containing PAA as the only biomimetic analog. Abbreviations: C: composite; A: adhesive; H: hybrid layer; T: dentinal tubule; D: mineralized intertubular dentin. A. A representative 4-month old specimen that was bonded with XP Bond using the moist bonding technique. There was no remineralization when PVPA was absent. Presumably, the hybrid layer was better infiltrated than the dry bonding group [see Fig. 1B], and there were no spaces to accommodate the deposition of large crystals. Open arrows: top of the hybrid layer. B. A 4-month old specimen that was bonded with XP Bond using a dry bonding technique. A cluster of large crystals (pointers) could be seen within the hybrid layer. C. A high magnification view of Fig. 1B. Many of the crystals that were formed in the absence of PVPA were longer than 100 nm along their C-axes (arrowheads). They were too large to fit inside collagen fibrils (i.e. intrafibrillar remineralization) and were probably formed around the interfibrillar spaces. D. Adper Prompt L-Pop-bonded dentin that was retrieved after 4 months of immersion in the PAA-containing remineralization medium. A zone of electron-dense crystals appeared in the middle of the hybrid layer. E. A high magnification view of electron-dense amorphous structures (open arrowhead) that appeared within the hybrid layer of Adper Prompt L-Pop-bonded dentin after immersion in the same remineralization medium for 2 months (low magnification not shown). F. A high magnification of Figure 1D. At 4 months, the electron-dense amorphous structures depicted in Fig. 1E had been converted to crystalline plates (arrows) that were 100–150 nm in length along their C-axes. These remineralized crystals were considerably larger than the natural apatite crystallites in the mineralized dentin base.
Adper Prompt L-Pop bonded specimens exhibited electron-dense amorphous clusters in the interfibrillar spaces of the hybrid layers after 2 months (Fig. 1E). The amorphous clusters had converted into crystalline plates within the middle portion of the hybrid layers after 4 months (Fig. 1D). These crystalline plates were 100–150 nm along their C-axes (Fig. 1F), which were larger than the natural apatite crystallites in the underlying mineralized dentin base (Fig. 1F).
3.3 Polyacrylic acid and PVPA-containing remineralization medium
For Group I specimens bonded with XP Bond using the moist bonding technique, remineralization could be vaguely discerned from hybrid layers at 2 months (Figs. 2A,2B) and were clearly identified after 4 months (Fig. 2D) of immersion in the second remineralization medium. Regional intrafibrillar remineralization was observed in the resin-infiltrated collagen matrix without interfibrillar remineralization. The intrafibrillar minerals so formed were able to recapitulate the rope-like subfibrillar arrangement of the collagen fibrils (Figs 2C, 2E), but lacked the banded appearance that was characteristic of the mineralized fibrils from the underlying dentin base (Fig. 2E). Intrafibrillar mineral nanoplatelets in heavily remineralized collagen fibrils derived from the surface of the hybrid layer were 50–70 nm long along their C-axes (Fig. 2F).
Fig. 2.
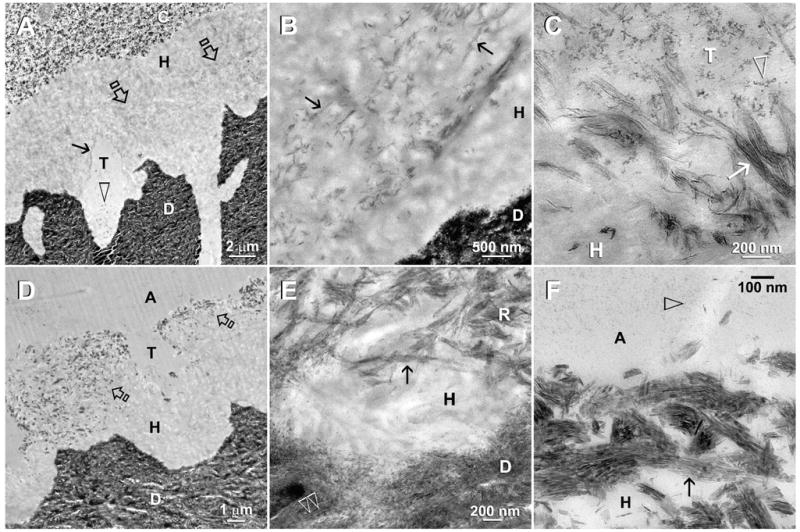
TEM of moist-bonded XP bond specimens that had been immersed in the second experimental PAA- and PVPA-containing remineralization medium. The specimens were examined after 2 months (A–C) and 4 months (D–F) of biomimetic remineralization. Abbreviations: C: composite; A: adhesive; H: hybrid layer; T: dentinal tubule; D: mineralized intertubular dentin. A. In this two-month old specimen, remineralization could be vaguely discerned from the middle of the hybrid layer (open arrows) and around the periphery of the dentinal tubule (arrow). Adhesive nanofillers (open arrowhead) were found within a dentinal tubule. B. A moderately high magnification view of Fig. 2A showing regional intrafibrillar remineralization (arrows) of the resin-infiltrated collagen matrix. Presumably, the adjacent interfibrillar spaces were well-infiltrated by resin and lacked spaces for mineral deposition. C. High magnification of Fig. 2A showing the close proximity between adhesive nanofillers (arrowhead) and the partially-remineralized collagen fibrils (arrow) along the periphery of a dentinal tubule. These fine intrafibrillar minerals recapitulated the rope-like subfibrillar architecture of the collagen fibrils. As these fibrils were probably swollen and partially-unraveled, no collagen banding was observed. D. At 4-months, remineralization within the hybrid layer (open arrows) could be identified even at a low magnification. E. A moderately high magnification view of Fig. 2D showing remineralized collagen fibrils (R) close to the base of the hybrid layer. Although the hierarchy of intrafibrillar mineralization had been recapitulated (arrow) in these fibrils, the crystallite arrangement lacked the banded characteristic of mineralized fibrils (open arrowheads) present in the underlying dentin. F. Remineralized collagen fibrils along the hybrid layer surface. Intrafibrillar mineral nanoplatelets (arrow) were 50–70 nm long and their arrangement reflected the pleaded, rope-like arrangement of the collagen microfibrils. Open arrow: adhesive nanofillers.
For dry-bonded XP Bond specimens which contained collapsed resin-sparse collagen matrices, extensive remineralization of the re-expanded matrices was readily apparent even at low magnifications after the specimens were immersed for 2 months in the aqueous PAA- and PVPA-containing remineralization medium (Figs. 3A, 3B). These remineralized crystallites consisted of 50–70 nm long needles and 25–35 nm long platelets (Fig. 3C). Heavy remineralization within segregated regions of the hybrid layers was readily observed in all 4 month old specimens (Fig 3D). In those specimens, the mineral density of the remineralized part of the hybrid layer was similar to that of the underlying dentin (Fig. 3E), with a dense arrangement of interfibrillar needles and intrafibrillar platelets (Fig. 3F).
Fig. 3.
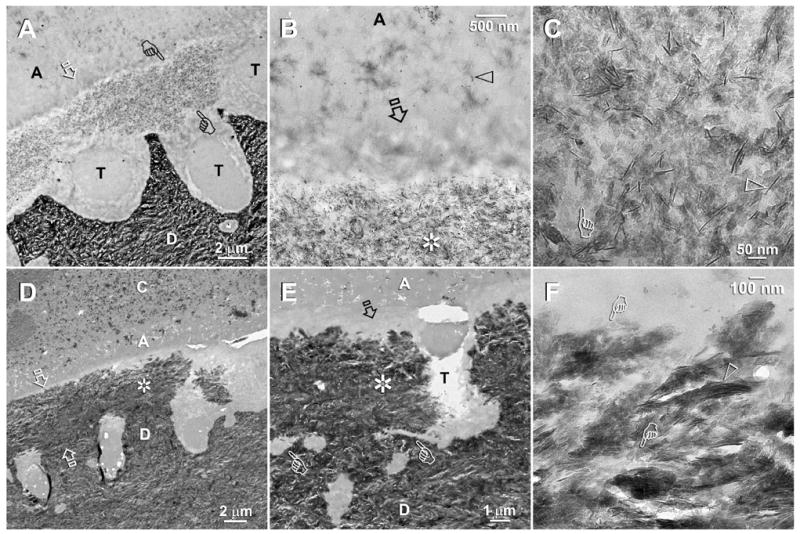
TEM of XP Bond-bonded, air-dried, collapsed acid-etched dentin that re-expanded after immersion in the second experimental PAA- and PVPA-containing remineralization medium. Specimens were examined after 2 months (A–C) and 4 month (D–F) of biomimetic remineralization. Abbreviations: C: composite; A: adhesive; H: hybrid layer; T: dentinal tubule; D: mineralized intertubular dentin. A. A low magnification view of a two-month old specimen showing extensive mineral deposition in the middle part of the hybrid layer (between pointers). The surface 1 μm (open arrow) and the base of the hybrid layer were better infiltrated by resin and did not remineralize. B. A moderately high magnification view of Fig. 3A showing the heavily mineral-filled, remineralized superficial part of hybrid layer (asterisk) beneath the well resin-infiltrated, non-remineralized basal part of the hybrid layer (open arrow). Open arrowhead: adhesive nanofillers. C. A high magnification view of the remineralized hybrid layer showing that the minerals exists as needles (open arrowhead; ca. 50–70 nm long) and plates (pointer; 25–35 nm long). D. A low magnification view of the resin-dentin interface after 4 months of biomimetic remineralization. The full thickness of the hybrid layer was depicted by two open arrows. Heavy remineralization could be identified from some parts of the hybrid layer (asterisk). E. A moderately high magnification view of Fig. 3D showing that the mineral density of the remineralized hybrid layer (asterisk) was similar to that of the underlying dentin. The top (open arrow) and the base (pointers) of the hybrid layer were better infiltrated and did not remineralize. F. A high magnification view of the heavily remineralized hybrid layer in Fig. 3D showing the presence of a dense arrangement of mineral platelets (pointers) and needles (open arrowhead).
At 2 months, the Adper Prompt L-Pop bonded specimens demonstrated remineralization that commenced either from the base (Fig. 4A) or the top of the hybrid layers (not shown). Interestingly, only intrafibrillar remineralization was observed (Fig. 4B), with an orderly arrangement of nanoplatelets that revealed the pleaded, rope-like subfibrillar architecture of the collagen fibrils (Fig. 4C). After four months, the 4–6 μm thick hybrid layers were almost completely remineralized (Fig. 4D). There was minimal remineralization of the interfibrillar spaces (Figs. 4E, 4F) despite the presence of heavy remineralization within the collagen fibrils (Fig. 4F).
Fig. 4.
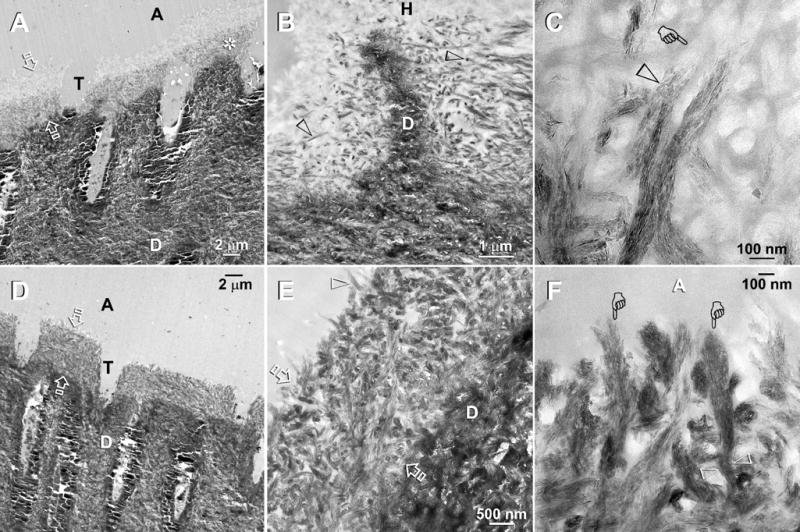
TEM of Adper Prompt L-Pop bonded specimens that that had been immersed in the second experimental PAA- and PVPA-containing remineralization medium. The specimens were examined after 2 months (A–C) and 4 months (D–F) of biomimetic remineralization. Abbreviations: C: composite; A: adhesive; H: hybrid layer; T: dentinal tubule; D: mineralized intertubular dentin. A. A low magnification view of a two-month old specimen showing a 4–6 μm thick hybrid layer (between open arrows) with remineralization of the bottom part the hybrid layer (asterisk). Other specimens revealed remineralization initiating from the top and the hybrid layer (not shown). B. A moderately high magnification view of a less heavily remineralized specimen from the same time period. Remineralization was seen exclusively as intrafibrillar remineralization within some collagen fibrils (open arrowheads). C. A high magnification view of the collagen matrix at the base of the hybrid layer showing part of a collagen fibril that had undergone intrafibrillar remineralization (open arrowhead) and another non-mineralized region within the same fibril (pointer). The remineralized part of these fibrils consisted of orderly arranged mineral nanoplatelets, revealing the rope-like subfibrillar architecture within these collagen fibrils. D. Low magnification of a four-month old specimen showing partial remineralization of the entire 4–6 μm thick hybrid layer (between open arrows). E. A moderately high magnification view of Fig. 4D showing remineralization within the hybrid layer (between open arrows) that is principally intrafibrillar in nature. Highly remineralized shag carpet-like fibrils (pointers) could be indentified along the dentin surface. Presumably, the adhesive resin-infiltrated interfibrillar spaces did not remineralize and appeared electron-lucent. F. A high magnification view of the heavily remineralized collagen fibrils along the hybrid layer surface. The extent of surface unraveling in these collagen fibrils (pointers) could be readily appreciated by comparing the width of these fibrils from a subsurface location (between open arrowheads).
4. Discussion
The results of the present study do not warrant rejection of the null hypothesis that phosphoric acid esters present in etch-and-rinse and self-etching adhesives cannot replace PVPA as biomimetic analogs during remineralization of resin-dentin interfaces. In the first experimental group with specimens immersed in PAA-containing SBF, moist-bonded XP Bond specimens showed no remineralization. This is probably because the demineralized collagen matrix was better infiltrated with resin compared with the dry-bonded specimens and did not permit the deposition of the large crystals that were seen in the latter. Although dry-bonded XP Bond and Adper Prompt L-Pop specimens exhibited some evidences of remineralization, the crystals formed in the absence of PVPA were too large to fit inside collagen fibrils. These large crystals are more likely to occupy interfibrillar spaces. Conversely, intrafibrillar remineralization was identified from all bonded specimens after immersion in the PAA- and PVPA-containing biomimetic remineralization medium. In addition, interfibrillar remineralization was seen in the dry-bonded XP Bond specimens. Presumably, the interfibrillar spaces in the moist-bonded XP Bond and the Adper Prompt L-Pop groups were still occupied by resin. As the remineralized minerals are unlikely to displace polymerized resins, this provided a plausible explanation for the observation of intrafibrillar remineralization only in these two groups. On the contrary, the collapsed air-dried demineralized collagen matrices in the dry-bonded XP Bond specimens were resin-sparse and that the interfibrillar spaces were likely to be devoid of adhesive resin. This probably explained why both intrafibrillar and interfibrillar remineralization were identified in this dry-bonded group. Collectively, the results of this study led us to conclude that polymerized phosphoric acid esters in XP Bond and Adper Prompt L-Pop cannot replace binding of PVPA molecules on demineralized collagen fibrils (Gu et al., unpublished results) as potential phosphoprotein analogs in a biomimetic remineralization scheme.
Biomineralization is the process by which living organisms secrete inorganic minerals in the form of biominerals (e.g. magnetite, silica, oxalates, various forms of calcium carbonates, carbonated apatites) within cytoplasms, shells, teeth and bony skeletons [30]. The processes exhibit a high level of spatial and hierarchical control as mineralization usually takes place in a confined reaction environment under ambient temperature and pressure conditions. It is generally accepted that type I collagen matrix does not have the capacity to induce matrix-specific mineral formation [31] from metastable calcium phosphate solutions [32]. Although noncollagenous proteins macromolecules are known to play crucial roles in the control of nucleation and growth of the mineral phase [33,34], it was only very recently that the “bottom-up” concept of particle-mediated nanoprecursor assembly and mesocrystalline transformation [30,35–37] was proposed as an alternative to the classical paradigm of “top-down” ion-mediated crystalline growth to account for the intricate biomineralization strategies identified in nature.
Noncollagenous proteins (NCPs) play critical roles in the mineralization of the predentin collagen network during dentinogenesis [17,38]. They possess carboxylic acid and phosphate functional groups that act as preferential sites for apatite nucleation [39,40]. Dentin matrix protein-1 (DMP-1) is a member of the highly acidic NCPs [41]. Its N-terminal domain is rich in aspartic acid and is thought to participate in stabilizing the amorphous calcium phosphate phase and inhibits apatite formation [17]. Its C-terminal is a glutamic acid- and serine-rich domain which has been proposed as a site that facilitates apatite nucleation. Serine molecules are potential sites for phosphorylation by casein kinases I and II [17,38]. In addition to its potent calcium binding capacity, DMP-1 has a high affinity to fibrillar collagen [42]. This complex NCP molecule has been shown to induce heterogeneous nucleation of calcium phosphate crystals and regulate crystal growth. Phosphophoryn, another phosphoprotein rich in aspartyl (Asp) and O-phosphoseryl (Ser(P)) residues, is involved in matrix-mediated biomineralization of dentin [43]. Its phosphorylation is crucial for its function as a mediator of biomineralization [44]. It is also believed to have a specific affinity for collagen [45,46] in-situ, especially with the ‘e’ band of collagen fibrils [46].
In the present study, the PVPA biomimetic molecules probably simulate the calcium phosphate binding sites of DMP-1 and act as templates for recruiting amorphous calcium phosphate nanoprecursors along the collagen microfibrils as well as on the surface of the collagen fibrils [44]. Thus, both PAA and PVPA are required for biomimetic mineralization through the particle-mediated non-classical crystallization pathway [47]. Previous studies on remineralization with analogs of dentin phosphoprotein-phosvitin [48] and casein phosphopeptides (CPP) [49] failed to demonstrate conclusively the presence of remineralized apatites in a dentin collagen matrix, in particular as intrafibrillar minerals within the collagen fibrils. When PAA was employed as the exclusive biomimetic analog in the first remineralization medium, large crystals appeared in the dry-bonded XP Bond and Adper Prompt L-Pop specimens. As there is no room inside a collagen fibril to accommodate these large crystals, the only place that they could have been deposited is either the interfibrillar spaces or the lateral branches of the dentinal tubules into which the interfibrillar spaces open. Therefore, the precipitation of large crystals either on the surface of, or within the hybrid layer is not consistent with the epitome of a biomimetic remineralization strategy. Phosphonate groups covalently linked to PEG-b-PMAA (polyethylene glycol-block-polymethacrylic acid) block copolymers resulted in the formation of barium chromate nanofibers, whereas doping of a PEG-b-PMAA solution with equal amounts of a phosphate salt (Na2HPO3) failed to reproduce such structures. This indicated that the phosphonate-functionalized block copolymer is required for morphogenic targeting instead of the simple adsorption of a single phosphoric acid functional group derived from an ionic analog [50]. In the context of the present study, although it is possible for phosphate esters of methacrylates to induce mineral precipitation, as elegantly demonstrated by Hayakawa et al. [23], these crystalline precipitates are at best conceived as dystrophic calcifications instead of true biomimetic remineralization. The results of our present work, while confirming our previous findings [13], provided new, important data demonstrating that phosphate esters from dentin adhesives cannot be used as substitutes for PVPA in biomimetic remineralization. Phosphonates are thought to be far more stable than are phosphate esters that are susceptible to the hydrolytic action of esterases. It remains to be seen whether binding of PVPA molecules to demineralized dentin and their subsequent entrapment with dentin adhesives will result in similar remineralization results. This important issue has to be investigated in future studies before the provision of a clinically-relevant remineralization scheme involving the in-situ application of biomimetic analogs can be realized.
The importance of self-assembly as a central theme was well demonstrated in our remineralization experiment. Although intrafibrillar remineralization was consistently identified, it is noteworthy that the mineral nanoplatelets were associated with the collagen microfibrils, allowing the rope-like subfibrillar architecture [51] of the collagen fibrils to be revealed. Unlike the naturally mineralized collagen fibrils from the underlying dentin base, collagen banding was not apparent in these remineralized collagen fibrils. It is tempting to attribute this phenomenon to the inability of biomimetic molecules to fully duplicate the natural biologic functions of structural phosphoprotein molecules [52]; one must admit that in comparison with the sophistication of biological self-assembly, synthetic self-assembly still operates at a relatively crude level [53,54]. However, arguing against these notions is that we were able to observe remineralized collagen fibrils with highly ordered banded appearance of the apatite platelets in experiments that involve thicker (ca. 300–1000 μm thick) layers of completely demineralized dentin (Tay, unpublished results). Presumably, the collagen fibrils were better preserved when these thick layers of dentin were demineralized in mild forms of demineralizing solutions. During the application of aggressive phosphoric acid or self-etching primers, often under continuous agitation, the collagen fibrils could have become swollen and partially-unraveled after treatments with the acid etchants and/or acidic monomers. In addition, the residual acidic monomers of the adhesives in the hybrid layer after polymerization may have an influence on the conformation of collagen. Conformational changes might involve a decrease in intermolecular hydrogen bonded amide I carbonyls associated with the α-helix structure and an enhancement of imide carbonyls hydrogen bonded to water [55], resulting in the unfolding of the α-helix structure [55,56]. These findings raise concerns on remineralization potential of caries-affected and caries-infected dentin as a minimally invasive strategy in restorative dentistry [57]. Reductions in antigenicity of type I collagen in caries-affected dentin [58] may have been caused by alterations in the collagen fibrillar conformation within the dentin organic matrix during the carious process [3,59]. Because of these subtle alterations in collagen ultrastructure, the order of apatite remineralization within hybrid layers of acid-etched normal dentin and caries-affected dentin may be different from that in natural mineralized sound dentin. This hypothesis requires validation in future studies that compare remineralization of natural caries-affected dentin with artificial carious lesions generated from sound dentin.
Based on the decline in the mechanical properties of teeth derived from patients with dentinogenesis imperfecta, Kinney et al. [60] reported that interfibrillar remineralization alone is insufficient in re-establishing the mechanical properties of the remineralized dentin to those present in normal mineralized dentin. Thus, intrafibrillar mineralization is critical in restoring the strength of dentin. As specimens moist-bonded with both the etch-and-rinse adhesive and the self-etching adhesive exhibited predominantly intrafibrillar remineralization, the contribution of intrafibrillar remineralization alone to the improvements in mechanical properties of remineralized dentin is currently unknown. Moreover, the effect of a less highly ordered type of intrafibrillar crystallite arrangement (i.e. crystallites that remineralize along pleaded but swollen collagen microfibrils, without banding) on the mechanical properties of remineralized dentin has never been investigated. These issues should be further examined using nanoscopic dynamic mechanical analysis.
5. Conclusion
Within the limits of this study, it may be concluded that:
Denuded collagen matrices in resin-dentin interfaces can be remineralized in the presence of the dual biomimetic analogs polyacrylic acid and polyvinylphosphonic acid;
Polymerized phosphoric acid esters in dentin adhesives cannot replace polyvinylphosphonic acid as potential phosphoprotein analogs in a biomimetic remineralization scheme;
Depending upon the extent of adhesive resin infiltration into the collagen matrices, biomimetic remineralization may be manifested as either intrafibrillar remineralization alone when the interfibrillar spaces are better filled with adhesive resin, or a combination of intrafibrillar and interfibrillar remineralization when the collagen matrix is devoid of resin.
Acknowledgments
This study was supported by Grant R21 DE019213-01 from the National Institute of Dental and Craniofacial Research (PI. Franklin R. Tay). We thank Robert Smith for TEM technical assistance, Thomas Bryan for embedding of epoxy resin and Michelle Barnes for secretarial support. This revised acknowledgments had been published on line by Dental Materials: Dent Mater (2009), doi:10.1016/j.dental.2009.05.001
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inoue S, Vargas MA, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, et al. Microtensile bond strength of eleven contemporary adhesives to dentin. J Adhes Dent. 2001;3:237–45. [PubMed] [Google Scholar]
- 2.Sadek FT, Calheiros FC, Cardoso PE, Kawano Y, Tay F, Ferrari M. Early and 24-hour bond strength and degree of conversion of etch-and-rinse and self-etch adhesives. Am J Dent. 2008;21:30–4. [PubMed] [Google Scholar]
- 3.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 4.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 5.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 6.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32:107–11. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 7.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–81. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.van Dijken JW, Sunnegardh-Gronberg K, Lindberg A. Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions. A 13 years evaluation. Dent Mater. 2007;23:1101–7. doi: 10.1016/j.dental.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang XT, Ker RF. Creep rupture of wallaby tail tendons. J Exp Biol. 1995;198:831–45. doi: 10.1242/jeb.198.3.831. [DOI] [PubMed] [Google Scholar]
- 10.Wang XT, Ker RF, Alexander RM. Fatigue rupture of wallaby tail tendons. J Exp Biol. 1995;198:847–52. doi: 10.1242/jeb.198.3.847. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Pashley DH. Biomimetic remineralization of resin-bonded acid-etched dentin. J Dent Res. 2009 doi: 10.1177/0022034509341826. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J Endod. 2007;33:1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–37. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–8. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olszta MJ, Odom DJ, Douglas EP, Gower LB. A new paradigm for biomineral formation: mineralization via an amorphous liquid-phase precursor. Connect Tissue Res. 2003;44:326–34. [PubMed] [Google Scholar]
- 16.Baht GS, Hunter GK, Goldberg HA. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008;27:600–8. doi: 10.1016/j.matbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 18.Ten Cate JM. In vitro studies on the effects of fluoride on de- and remineralization. J Dent Res. 1990;69:614–9. doi: 10.1177/00220345900690S120. [DOI] [PubMed] [Google Scholar]
- 19.Ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201–10. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 20.Engqvist H, Schultz-Walz JE, Loof J, Botton GA, Mayer D, Phaneuf MW, et al. Chemical and biological integration of a mouldable bioactive ceramic material capable of forming apatite in vivo in teeth. Biomaterials. 2004;25:2781–7. doi: 10.1016/j.biomaterials.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Yli-Urpo H, Narhi M, Narhi T. Compound changes and tooth mineralization effects of glass ionomer cements containing bioactive glass (S53P4), an in vivo study. Biomaterials. 2005;26:5934–41. doi: 10.1016/j.biomaterials.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Nakamura K, Kaga M, Yawaka Y. Crystal growth by fluoridated adhesive resins. Dent Mater. 2008;24:457–63. doi: 10.1016/j.dental.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa T, Yoshinari M, Sakae T, Nemoto T. Calcium phosphate formation on the phosphorylated dental bonding agent in electrolyte solution. J Oral Rehabil. 2004;31:67–73. doi: 10.1111/j.1365-2842.2004.01135.x. [DOI] [PubMed] [Google Scholar]
- 24.Foot L, Werner L, Gills JP, Shoemaker DW, Phillips PS, Mamalis N, et al. Surface calcification of silicone plate intraocular lenses in patients with asteroid hyalosis. Am J Ophthalmol. 2004;137:979–87. doi: 10.1016/j.ajo.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 25.Wackernagel W, Ettinger K, Weitgasser U, Bakir BG, Schmut O, Goessler W, et al. Opacification of a silicone intraocular lens caused by calcium deposits on the optic. J Cataract Refract Surg. 2004;30:517–20. doi: 10.1016/S0886-3350(03)00672-2. [DOI] [PubMed] [Google Scholar]
- 26.Werner L, Kollarits CR, Mamalis N, Olson RJ. Surface calcification of a 3-piece silicone intraocular lens in a patient with asteroid hyalosis: a clinicopathologic case report. Ophthalmology. 2005;112:447–52. doi: 10.1016/j.ophtha.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent Mater. 2001;17:296–308. doi: 10.1016/s0109-5641(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 28.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–34. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 29.Meyer JL. Phase transformation in the spontaneous precipitation of calcium phosphate. Croatica Chim Acta. 1983;56:753–67. [Google Scholar]
- 30.Xu AW, Ma Y, Cölfen H. Biomimetic mineralization. J Mater Chem. 2007;17:415–49. [Google Scholar]
- 31.Kawasaki K, Weiss KM. Evolutionary genetics of vertebrate tissue mineralization: the origin and evolution of the secretory calcium-binding phosphoprotein family. J Exp Zoolog B Mol Dev Evol. 2006;306:295–316. doi: 10.1002/jez.b.21088. [DOI] [PubMed] [Google Scholar]
- 32.Veis A. Mineral-matrix interactions in bone and dentin. J Bone Miner Res. 1993;8:S493–7. doi: 10.1002/jbmr.5650081312. [DOI] [PubMed] [Google Scholar]
- 33.Hunter GK, Hauschka PV, Poole AR, Rosenberg LC, Goldberg HA. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317:59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, et al. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–20. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 36.Imai H. Self-organized formation of hierarchical structures. Top Curr Chem. 2007;270:43–72. [Google Scholar]
- 37.Barthelat F. Biomimetics for next generation materials. Philos Transact A Math Phys Eng Sci. 2007;365:2907–19. doi: 10.1098/rsta.2007.0006. [DOI] [PubMed] [Google Scholar]
- 38.Qin C, D’Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86:1134–41. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- 39.Banks E, Nakajima S, Shapiro LC, Tilevitz O, Alonzo JR, Chianelli RR. Fibrous apatite grown on modified collagen. Science. 1977;198:1164–6. doi: 10.1126/science.929194. [DOI] [PubMed] [Google Scholar]
- 40.Tanahashi M, Matsuda T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J Biomed Mater Res. 1997;34:305–15. doi: 10.1002/(sici)1097-4636(19970305)34:3<305::aid-jbm5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–30. [PubMed] [Google Scholar]
- 42.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 2004;279:11649–56. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 43.Huq NL, Loganathan A, Cross KJ, Chen YY, Johnson NI, Willetts M, et al. Association of bovine dentin phosphophoryn with collagen fragments. Arch Oral Biol. 2005;50:807–19. doi: 10.1016/j.archoralbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 44.He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, et al. Phosphorylation of phosphophoryn is crucial for its function as a mediator of biomineralization. J Biol Chem. 2005;280:33109–14. doi: 10.1074/jbc.M500159200. [DOI] [PubMed] [Google Scholar]
- 45.Dahl T, Sabsay B, Veis A. Type I collagen-phosphophoryn interactions: specificity of the monomer-monomer binding. J Struct Biol. 1998;123:162–8. doi: 10.1006/jsbi.1998.4025. [DOI] [PubMed] [Google Scholar]
- 46.Fujisawa R, Zhou H, Kuboki Y. In vitro and in vivo association of dentin phosphophoryn with alpha1CB6 peptide of type I collagen. Connect Tissue Res. 1994;31:1–10. doi: 10.3109/03008209409005630. [DOI] [PubMed] [Google Scholar]
- 47.Niederberger M, Cölfen H. Oriented attachment and mesocrystals: non-classical crystallization mechanisms based on nanoparticle assembly. Phys Chem Chem Phys. 2006;8:3271–87. doi: 10.1039/b604589h. [DOI] [PubMed] [Google Scholar]
- 48.Onuma K. Effect of phosvitin on the nucleation and growth of calcium phosphates in physiological solutions. J Phys Chem B. 2005;109:8257–62. doi: 10.1021/jp044550l. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds EC. Calcium phosphate-based remineralization systems: scientific evidence? Aust Dent J. 2008;53:268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 50.Yu SH, Cölfen H, Antonietti M. Control of the morphogenesis of barium chromate by using double-hydrophilic block copolymers (DHBCs) as crystal growth modifiers. Chem Eur J. 2002;8:2937–45. doi: 10.1002/1521-3765(20020703)8:13<2937::AID-CHEM2937>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 51.Bozec L, van der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys J. 2007;92:70–5. doi: 10.1529/biophysj.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baeuerlein E. Handbook of biomineralization – biological aspects and structure formation. Wiley-VCH; Weinheim: 2007. [Google Scholar]
- 53.Ramachandrarao P, Bera T. A chicken’s egg as a reaction vessel to explore biomineralization. J Bionic Eng. 2007;4:133–41. [Google Scholar]
- 54.Mann S. The Chemistry of Form. Angew Chem Int Ed Engl. 2000;39:3392–406. [PubMed] [Google Scholar]
- 55.Eliades G, Palaghias G, Vougiouklakis G. Effect of acidic conditioners on dentin morphology, molecular composition and collagen conformation in situ. Dent Mater. 1997;13:24–33. doi: 10.1016/s0109-5641(97)80005-x. [DOI] [PubMed] [Google Scholar]
- 56.El Feninat F, Ellis TH, Sacher E, Stangel I. Moisture-dependent renaturation of collagen in phosphoric acid etched human dentin. J Biomed Mater Res. 1998;42:549–53. doi: 10.1002/(sici)1097-4636(19981215)42:4<549::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 57.Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008;139:705–12. doi: 10.14219/jada.archive.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suppa P, Ruggeri A, Jr, Tay FR, Prati C, Biasotto M, Falconi M, et al. Reduced antigenicity of type I collagen and proteoglycans in sclerotic dentin. J Dent Res. 2006;851:33–7. doi: 10.1177/154405910608500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakornchai S, Atsawasuwan P, Kitamura E, Surarit R, Yamauchi M. Partial biochemical characterisation of collagen in carious dentin of human primary teeth. Arch Oral Biol. 2004;49:267–73. doi: 10.1016/j.archoralbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 2003;82:957–61. doi: 10.1177/154405910308201204. [DOI] [PubMed] [Google Scholar]


