Abstract
Mitochondrial and endoplasmic reticulum (ER) networks are fundamental for the maintenance of cellular homeostasis and for determination of cell fate under stress conditions. Recent structural and functional studies revealed the interaction of these networks. These zones of close contact between ER and mitochondria called MAM (mitochondria associated membranes) support communication between the two organelles including bioenergetics and cell survival. The existence of macromolecular complexes in these contact sites has also been revealed. In this contribution, we will review: i) the ER and mitochondria structure and their dynamics, ii) the basic principles of ER mitochondrial Ca2+ transport, (iii) the physiological/pathological role of this crosstalk.
Keywords: ER, endoplasmic reticulum, mitochondria, MAM, mitochondria associated membranes, calcium, ER stress, apoptosis
ER and Mitochondria structural link
ER structure
The ER is an extensive network of cisternae and microtubules, which stretches from the nuclear envelope to the cell surface in all eukaryotic cells. It’s the largest organelle, with endomembrane accounting for more than 50% of all the cellular membranes, and occupies a substantial part (> 10%) of the cell volume.
The ER plays several vital functions. Firstly, the ER is the site of protein synthesis in the rough ER and correct post-translational “folding” of these proteins (Chevet et al., 2001). Secondly, the ER serves as a common transport route by which numerous proteins are delivered to their destination (Palade, 1975). Thirdly, the ER acts as an indispensable source for fast physiological signaling being a dynamic calcium ions (Ca2+) reservoir, which can be activated by both electrical and chemical cell stimulation (Bootman et al., 2002, Verkhratsky and Petersen, 2002).
The molecular profile of the ER reflects its signaling role and is strongly dominated by components of the Ca2+ signaling pathway. It contains the inositol-1,4,5-triphosphate (IP3) receptors (IP3Rs) and ryanodine receptors (RyRs) responsible for releasing Ca2+ in response to the input signals. Three types of RyRs and three types of IP3Rs are currently known. As well as the RyR, the IP3R complex is very large: its pore is formed by a homotetramers of ~3000 aminoacids. IP3Rs are ligand-gated channels that function in releasing Ca2+ from ER Ca2+ stores in response to inositol-1,4,5-phosphate (IP3) generation initiated by agonist binding to cell-surface receptors (Mikoshiba, 2006, Patterson et al., 2004, Patel et al., 1999). The ensuing Ca2+ signal plays a fundamental role in modulating a diverse range of cellular responses including muscle contraction, exocytosis, motility, fertilization, proliferation and gene expression. Thus the physiological release of Ca2+ through IP3Rs serves important signaling and “housekeeping” roles in maintaining normal function during agonist-mediated cell activation. However, in the presence of an apoptotic stimulus, the IP3R-mediated Ca2+ release can activate apoptotic pathways by inducing the release from mitochondria of a number of pro-apoptotic factors including cytochrome c (Joseph and Hajnoczky, 2007). In addition to these release channels, there is also a leak pathway, some of which seems to occur through an aqueous pore in the translocon that is responsible for threading newly synthesized proteins into the ER. On the other side, RyRs are the most abundant Ca2+-releasing channels in muscle (even if present in low amount in other cells), thus belonging to tissue-specific signaling pathways leading to muscle contraction (Hamilton, 2005).
Both the regulated release and the leak of Ca2+ are counteracted by the Sarco-Endoplasmic Reticulum Ca2+-ATPase (SERCA) that functions to maintain the internal store of Ca2+. SERCA transfers Ca2+ from the cytosol of the cell to the lumen of the ER at the expense of ATP hydrolysis. The SERCA pump is encoded by a family of three genes, SERCA1, 2, and 3, that are highly conserved and expressed at various levels in different cell types. These isoforms exhibit both tissue and developmental specificity, suggesting that they contribute to unique physiological properties of the tissue in which they are expressed (Clapham, 2007). The luminal Ca2+-binding proteins (CaBPs) such as calnexin and calreticulin play an important role in regulating the activity of the SERCA pump (John et al., 1998) and maintenance of a constant luminal level of Ca2+. In addition to the release channels, pumps and buffers, there are a number of ancillary proteins (FK 506-binding proteins, sorcin, triadin, phosholamban) that contribute to the ER Ca2+ signaling system (MacKrill, 1999).
Mitochondrial structure
The view of mitochondria as isolated organelle has been profoundly challenged over recent decades with the discovery that mitochondria function within a highly dynamic integrated reticular network that is continually remodeled by both fusion and fission events (see below). From a structural perspective, the mitochondrion is unusual since it contains two membranes that separate four distinct compartments, the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM) and the matrix.
The identification and characterization of the protein machinery that controls mitochondrial membranes dynamics constitutes an important step towards a better understanding of mitochondrial behavior. Mitochondria are an integrated component of the cell and their actions are undoubtedly linked to cell signaling, inter-organelle communication cell proliferation and cell death. In this sense we will focus on the structural and functional aspects of ER-mitochondria interaction that are pivotal in the control of Ca2+ signaling and of the processes that depend on them.
Ca2+ crosses the OMM then enters the mitochondrial matrix via a molecularly undefined “uniporter”, i.e. a channel located in the IMM, driven by a large electrochemical gradient (Nicholls and Crompton, 1980, Kirichok et al., 2004).
Several mechanisms of Ca2+ efflux from mitochondria have also been extensively discussed in the literature. Energized mitochondria spend a significant amount of energy to transport Ca2+ against its electrochemical gradient from the matrix to the external space. Two separate mechanisms have been found to mediate this outward transport: a Ca2+/Na+ exchanger and a Na+-independent efflux mechanism (Saris and Carafoli, 2005).
Accumulating evidence indicates another important mitochondrial actor that mediates the transport of ions, nucleotides and Ca2+ across the OMM: the voltage dependent anion channel (VDAC) (Rostovtseva and Colombini, 1996). VDAC, also referred to as the mitochondrial porin, is a 30–35 kDa protein that has been purified, cloned, sequenced and manipulated utilizing molecular biology techniques. VDAC channel forms a large voltage-gated pore and can exist in multiple conformational states with different selectivity and permeability. At zero and low transmembrane potentials, VDAC exists in a highly conductive open state (pore diameter about 2.5 nm) with a preferential selectivity against anionic compounds but it is converted into a low-conducting state (with a smaller pore diameter of about 1.8 nm) at high potentials (both positive and negative), with reduced conductivity and reversed selectivity (Shoshan-Barmatz and Gincel, 2003, De Pinto et al., 2008, Peng et al., 1992, Song et al., 1998).
Moreover, a number of reports show that numerous cytosolic components can significantly modulate VDAC gating properties, including NADH (Lee et al., 1996), metabolic enzymes (Pastorino and Hoek, 2008), chaperones (Schwarzer et al., 2002) and cytoskeletal elements (Rostovtseva et al., 2008). However, the physiological relevance of the voltage gating properties of VDAC is still matter of debates, not only because the existence of any membrane potential across the OMM has never been directly demonstrated, but also because the physiological significance of VDAC closure is still obscure. Indeed, some reports shows that VDAC closure is a sort of anti-apoptotic signal, as demonstrate, for example, by the role of hexokinases (Zaid et al., 2005) or gelsolin (Kusano et al., 2000); on the opposite side, VDAC closure has been also shown to enhance cell death through apoptosis, given that Bcl-XL, an anti-apoptotic Bcl-2 family member, triggers VDAC opening and, in parallel, some apoptotic stimuli such as serum deprivation, reduces OMM permeability (Vander Heiden et al., 1999, Vander Heiden et al., 2001). However, a recent work by Tan and Colombini demonstrates that, at least in vitro, VDAC closure enhances Ca2+ flux across the OMM. These data, together with the exhaustive demonstration of the existence of Ruthenium Red-sensitive Ca2+ binding sites (Israelson et al., 2008), strongly suggests that VDAC closure induces a higher mitochondrial Ca2+ uptake and consequently increases the probability of a mitochondrial Ca2+ overload leading to permeability transition (Tan and Colombini, 2007). Finally, very recently the 3D-structure of VDAC1 has been resolved by three groups in parallel. These data indicate that VDAC1 is β-barrel membrane protein composed of 19 β-strands with an β-helix N-terminal domain residing inside the pore: this segment most likely represents the voltage sensor since it is ideally positioned to regulate the conductance of ions and metabolites passing through the VDAC pore (Ujwal et al., 2008, Hiller et al., 2008, Bayrhuber et al., 2008). Although VDAC was originally thought to be located exclusively in the OMM, recently, immuno-electron microscopy and sub-cellular fractionation have revealed the presence of VDAC also in contact sites between mitochondria and associated ER (Shoshan-Barmatz et al., 2004). To conclude, VDAC is localized in a crucial position in the cell and, forming an important interface between mitochondria and ER, is involved in intracellular communication, including Ca2+ signal delivery between the ER and mitochondria (Lawen et al., 2005).
ER and Mitochondria dynamics
Both ER and mitochondria are now widely considered as highly dynamic organelles, capable of modifying their structure and function in response to changing environmental conditions.
ER forms a unique highly complex tubular network that is constantly remodeled through process such as “tubule sliding”, “tubule branching” and “ring closure” (Borgese et al., 2006). However, although it has been demonstrated that GTP hydrolysis is required for ER fusion, the understanding of the molecular machinery mediating this process is still lacking. The movement of ER tubules in higher eukaryotes requires the microtubule network (or actin filaments in microtubule-poor regions) and involves kinesin protein family as molecular motors (Waterman-Storer and Salmon, 1998). Moreover, ER can significantly increase in dimension in response to diverse stimuli such as ER stress (see below).
Mitochondria show a very heterogeneous shape among different cell types and, in some cases, even in the same cell in response to diverse stimuli. Indeed, they can form either short, rod-like structure or a continuous, elongated, tubular, highly dynamic and interconnected network. These differences in phenotype are the result of a complex equilibrium among organellar motility, fusion and fission events (Chan, 2006a). Since mitochondrial biogenesis occurs predominantly in the perinuclear region, eukaryotes had to evolve efficient systems to transport these organelles where energy demands are higher or where their peculiar metabolic functions are required. This is of critical relevance in cells with complex topology such as neurons, where mitochondria are abundant in the synaptic region of the axon. This unique distribution most likely reflects the high energy requirement of the synaptic transmission (ATP-driven release and recycling of vesicles, ATP-dependent pumps that control ions homeostasis, etc.) as well as the specific mitochondrial functions such as Ca2+ signaling regulation (Chan, 2006b). Similarly to ER, mitochondria exploit cytoskeletal elements as tracks for their directional movements by using a specialized molecular machinery. In mammals, mitochondrial movement is mainly a microtubules-driven process, with actin aiding in short-range mitochondrial positioning in microtubule-poor regions (Anesti and Scorrano, 2006). ER and mitochondria dynamics are not separately regulated but some cellular signals can effectively syncronize the movements of these organelles, underlying how these two subcellular compartments are closely interconnected. Indeed, ER and mitochondria movement is influenced by second messengers such as Ca2+, and it actively participates in signaling cascades: Ca2+ mobilizing agonists can effectively produce a rapid, simultaneous and reversible cessation of the movements of both organelles, which is strictly dependent on a rise in cytosolic Ca2+ concentration [Ca2+]c. This inhibition in mitochondrial motility reflects an increased mitochondrial Ca2+ uptake and thus enhances the local Ca2+ buffering capacities of mitochondria, with important consequences in signal transduction (Brough et al., 2005, Yi et al., 2004).
Apart from organelles movement along the cytoskeleton, mitochondria also continuously remodel their shape. In the first 1990s, genetic screens in yeast identified the first proteins involved in mitochondrial morphology and subsequent studies revealed that mitochondrial shape is determined by two dynamically opposed processes, fusion and fission. Indeed, genetic ablation of key regulators of the fusion machinery gives rise to cells with fragmented organelles because of unopposed ongoing fission, while the knockout (KO) of genes that mediate fission leads virtually to the formation of a single, deeply interconnected mitochondrion. Interestingly, in yeast the coinciding ablation of both fusion and fission apparatus produces a wild-type mitochondrial morphology but also shows a high frequency of mtDNA loss, suggesting an essential role of fusion and fission in maintaining the mitochondrial genome (Chan, 2006b). Considering the structural complexity of mitochondria, it should be immediately clear that the molecular machinery mediating fusion and fission has to be a quite intricate mechanism, requiring the independent but coordinated processing of both OMM and IMM. Proteins involved in mitochondrial dynamics have been originally identified in yeast but many of these genes have orthologs in mammals, mainly belonging to the large GTPase protein family. The molecular motors playing a pivotal role in OMM fusion are mitofusins (Mfn1 and Mfn2): they are characterized by the presence of a highly conserved GTPase domain, two transmembrane regions that enable the anchoring to OMM and two peculiar coiled coil structures, HR1 and HR2 (heptad repeat domain 1 and 2). During organelle fusion, mitofusins mediate the tethering of two adjacent mitochondria by forming trans homotypic (consisting of the same Mfn isotypes) or heterotypic (consisting of Mfn1 and Mfn2) complexes through the interaction of their C-terminal HR2 domains. Whether these two isoforms are functionally different or simply redundant remains to be clarified (Santel, 2006). After that, IMM fusion is achieved through the activity of another protein, OPA1 (Optic Atrophy 1). Surprisingly, this protein has been recently shown to control also IMM ultrastructure: together with the rhomboid protease PARL (presenilin-associated rhomboid like), OPA1 forms oligomers essential for the maintenance of internal cristae structure, thereby controlling their remodeling during apoptotic cell death (Cipolat et al., 2006, Frezza et al., 2006). On the other hand, the master gene regulating mitochondrial fission is DRP1 (Dynamin-related protein 1). It shares high homology with dynamin, a mechanoenzyme involved in the excision of clathrin-coated endocytic vesicles, and is normally located in the bulk cytosol. Upon induction, DRP1 redistributes into punctuated foci co-localizing with mitochondria where it mediates organelle fission. Conversely, the deciphering of the molecular players mediating remains elusive both in mammals as well as in yeast. It has been proposed that IMM processing could also be a simple mechanical consequence of OMM constriction and cleavage induced by DRP1. In any case, mitochondrial morphology regulation has profound consequences on apoptosis regulation. This fact is clearly demonstrated by the observation that in the most part of cell lines, apoptosis induction inevitably leads to the fragmentation of mitochondrial network. Indeed, DRP1 recruitment to mitochondria and its co-localization with the pro-apoptotic protein Bax has been shown during aopotosis induction (Karbowski et al., 2002, Tanaka and Youle, 2008). Thus, as a general rule, every condition causing mitochondrial fragmentation enhance cell death, while an healthy, widely interconnected mitochondrial network protects cells from apoptosis (Suen et al., 2008). However, this simple rule has been questioned by recent works that demonstrate that mitochondrial fragmentation, Bax/Bak translocation/oligomerization and release of caspases cofactors from mitochondria (which are traditionally all considered as hallmarks of the intrinsic apoptotic pathway) can be impaired and these events can occur independently one from the other (Yuan et al., 2007, Parone et al., 2006). Moreover, mitochondrial fission has been shown to protect cells from some apoptotic stimuli, such as Ca2+ induced cell death mediated by the lipid analog C2-ceramide (Szabadkai et al., 2004). These apparently conflicting data could be reconciled by considering mitochondria as organelles able to receive several different stress signals, and integrate them to commit the cell to the its appropriate fate. In this view, mitochondrial cross talk with ER play an essential role in determining cell commitment to apoptosis. Indeed, some recent works have clearly demonstrated that mitochondrial shape can be controlled by an ER dependent signaling pathway (Germain et al., 2005, Alirol et al., 2006).
ER and Mitochondria functional link
Hot spot
The transporter responsible for mitochondrial Ca2+ uptake is the Ruthenium Red sensitive Ca2+ uniporter (Gunter and Pfeiffer, 1990), which has been found to be a highly selective inward rectifying ion channel (Kirichok et al., 2004). The driving force of this transport is the mitochondrial membrane potential (Ψm), showing a value of ~180 mV (inside negative). The Ca2+ affinity of the transporter is surprisingly low in the 106–104 M range. In view of this low Ca2+-affinity the submicromolar cytosolic Ca2+ signals, observed in most cell types stimulated with a Ca2+-mobilizing agonist, seem to be insufficient to induce net mitochondrial Ca2+ uptake. The general consensus thus became that the well established capacity of mitochondria to accumulate Ca2+ would be significant only in conditions of high-amplitude, prolonged Ca2+]c increases, i.e. in the Ca2+ overload that is observed in various pathological conditions (e.g. excitotoxic glutamate stimulation of neurons), convinced the majority of specialists that these organelles had little to do with physiological Ca2+ handling.
This situation was completely reversed at the beginning of the last decade, by the observations of Miyata et al. (Miyata et al., 1991) and the development by Rizzuto and coworkers of a technique for monitoring mitochondrial Ca2+ concentration in living cells (Rizzuto et al., 1992). For this purpose, a modified Ca2+-sensitive photoprotein, aequorin, was targeted to the mitochondrial matrix in cultured epithelial cells. Surprisingly, these experiments showed that mitochondrial Ca2+ signals are relatively rapid and transient (on a second time scale) and of a larger magnitude than previously thought (Miyata et al., 1991). These studies showed that mitochondria in situ are much more efficient at taking up Ca2+ than predicted from their apparently low Ca2+ affinity, a notion that now is widely accepted. Similar conclusions was reached later also with fluorescent indicators, such as the positively charged Ca2+ indicator Rhod-2 (that accumulates within the organelle) (Boitier et al., 1999, Drummond et al., 2000) and the more recently developed green-fluorescent (GFP) protein-based fluorescent indicators (Filippin et al., 2003).
The obvious discrepancy between the low affinity of mitochondrial Ca2+ uptake mechanisms (expected based on the properties of their Ca2+ transporters established in vitro), the low concentration of global Ca2+ signals observed in cytoplasm (where Ca2+ elevations rarely exceed 2–3 μM), and the efficiency in intact cells of mitochondrial Ca2+ uptake (mitochondrial Ca2+ concentration ([Ca2+]m) rise, in a few seconds, to values above 10 μM, and in some cell types up to 500 μM) led to the formulation of the “hotspot hypothesis”. This hypothesis proposes that mitochondria preferentially accumulate Ca2+ at microdomains of high [Ca2+] that largely exceed the values reported in the bulk cytosol and meet the low affinity of the uniporter. In other word, a high- Ca2+ microdomain is transiently formed between mitochondria and the cytosolic mouth of the Ca2+ channels, localized either in the apposing ER or in the plasma membrane (Rizzuto et al., 1998). This “privileged,” local signaling is achieved through a close interaction between the mitochondria and the ER and appeared to be the key to the participation of this organelle in intracellular Ca2+ homeostasis.
MAM
Close appositions between the ER (or sarcoplasmic reticulum, SR) and mitochondria have long been known to exist and have been observed by electron microscopy (EM) in fixed samples of several cell types. These regions have long been considered to represent the sites of phospholipid exchange between the two organelles. Although such close appositions between ER and mitochondria may, at least in part, represent fixation artifacts, an EM picture taken from quickly frozen samples, which prevents most artifacts of chemical fixation, reveals that such close appositions are not only visible, but are actually more frequent than in traditionally fixed samples.
Experiments in living cells with the two organelles labeled by GFP (Rizzuto et al., 1998) and electron micrograph images of quickly frozen samples (Mannella et al., 1998) have demonstrated conclusively that such physical interactions between the two organelles indeed exist. Recently, it has also been shown by electron tomography that ER and mitochondria are adjoined by tethers (10 nm at the smooth ER and 25 nm at the rough ER) (Fig. 1) and that coupling between these two organelles can be weakened (or strengthened) by rupture (or enforcement) of this inter-organellar protein linkage (Csordas et al., 2006). Some evidence supports the hypothesis that the movement of mitochondria might occur concomitantly and in synchrony with that of specific ER regions. The mechanism of this reciprocal organelle-docking remains unresolved but it has been proposed that it depends on the expression on both membranes of complementary proteins that link the two organelles together, possibly at specific sites (Pizzo and Pozzan, 2007).
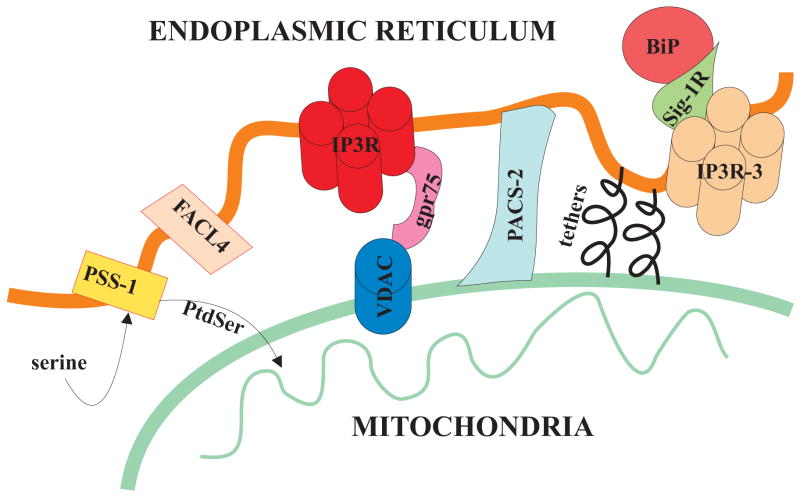
Figure 1.
A schematic representation of the organization of MAM including key players in lipid metabolism, Ca2+ signaling elements (IP3R, VDAC), chaperones (PACS-2, grp75, Sig-1R) and peptidic tethers keeping mitochondria and ER in close contact.
The close contacts through which ER communicates with mitochondria are referred as MAM (Vance, 1990). These ER-contiguous membranes contain multiple phospholipid- and glycosphingolipid-synthesizing enzymes, including long-chain fatty acid-CoA ligase type 4 (FACL4) and phosphatidylserine synthase-1 (PSS-1) (Fig. 1), and support direct transfer of lipids between the ER and mitochondria (Piccini et al., 1998, Stone and Vance, 2000). In addition to supporting lipid transfer, MAM also exchange Ca2+ ions which regulate processes ranging from ER chaperone-assisted folding of newly synthesized proteins to the regulation of mitochondria-localized dehydrogenases involved in ATP-producing Krebs cycle reactions, and the activation of Ca2+-dependent enzymes that execute cell death programs (Berridge, 2002). Recently, several mitochondria or ER bound proteins have been shown to be important for maintaining the spatial relationship between ER and mitochondria (Lebiedzinska et al., in press).
The interactions between the two organelles seem to be modulated by a family of “mitochondria-shaping proteins” and by a family of chaperone proteins. In mammals, the best characterized of the “mitochondria-shaping proteins” are DRP-1 (Smirnova et al., 2001) and Mitofusin 1 and 2, that regulate mitochondria fission and fusion (Chen et al., 2003) (see above).
MAM are also enriched in key chaperones that may play a role in regulating Ca2+ signaling between ER and Mitochondria. Specifically, Hayashi et al. identified a new, yet novel ER “ligand-operated” chaperone that specifically targets MAM: the Sig-1R (Sigma-1 receptor) chaperone (Fig. 1). Moreover they noted that in physiological conditions Sig-1R is retained in the MAM. Upon ER stress, redistribution of Sig-1Rs occurs, from MAM to the periphery of the ER.
Interestingly, they also found that Sig-1Rs and isoform 3 of IP3R co-localize and associate with each other at MAM, that Sig-1Rs form a Ca2+-sensitive chaperone machinery with GRP78/BiP (78-kDa glucose-regulated protein GRP78, also referred to as the immunoglobulin binding protein BiP) and prolong Ca2+ signaling from the ER to mitochondria by stabilizing IP3R-3 at MAM. (Hayashi and Su, 2007).
In addition, Simmen et al. demonstrated that PACS-2 (phosphofurin acidic cluster sorting protein 2) is a multifunctional sorting protein that controls the ER–mitochondria axis and the role of this axis in cellular homeostasis and apoptosis (Fig. 1). They showed that PACS-2 is required for the intimate association of mitochondria with the ER: PACS-2 depletion induces mitochondria fragmentation and uncouples this organelle from the ER raising the possibility that, in addition to mediating MAM formation, PACS-2 might also influence ER folding and Ca2+ homeostasis.
The requirement of PACS-2 for the apposition of rod-like mitochondria to the ER suggests that PACS-2 has an essential role in ER–mitochondria communication, and influences the dynamic mitochondria fusion/fission events that are coupled with mitochondria homeostasis and intermitochondria communication (Szabadkai et al., 2004).
In addition to mediating the ER–mitochondria axis, they found that PACS-2 has a profound role on ER homeostasis. Indeed, both IP3Rs and RyRs possess potential PACS-2-binding sites (Kottgen et al., 2005) and may be associated with MAM (Hajnoczky et al., 2000). Thus, disruption of PACS-2 may cause mislocalization of IP3Rs, resulting in reduced Ca2+ transfer from the ER to mitochondria.
Finally, in a recent study, our group found that the mitochondrial chaperone grp75 (glucose-regulated protein 75) regulates IP3R-mediated mitochondrial Ca2+ signaling (Szabadkai et al., 2006). In particular, we demonstrated that isoform 1 of VDAC is physically linked to the ER Ca2+-release channel IP3R through grp75, highlighting chaperone-mediated conformational coupling between the IP3R and the mitochondrial Ca2+ uptake machinery (Fig. 1).
These findings together support a new emerging picture whereby chaperone machineries at both ER and mitochondrion orchestrate the regulation of Ca2+ signaling between these two organelles.
Endoplasmic reticulum stress
Several patho-physiological stimuli as well as a number of pharmacological treatments can saturate the folding capacity of the ER, thus generating a condition called ER stress. In such a circumstance the cell triggers an evolutionarily conserved ER-to-nucleus signaling pathway known as unfolded protein response (UPR). This response decreases the whole global translation but upregulates the selective synthesis of chaperones in order to improve ER folding capacities and increases misfolded protein degradation through autophagy or the proteasome system (Schroder, 2008). ER stress can be induced by several physiological or pathological stimuli (such as cytokines inducing B cell differentiation and immunoglobulins synthesis and secretion, insulin production in glucose stimulated pancreatic β-cells, expression of aggregation prone proteins etc.) or by pharmacological treatments such as brefeldin A (disruption of ER-Golgi trafficking), thapsigargin (depletion of ER Ca2+ stores), tunicamycin (inhibition of N-linked glycosylation) or dithiothreitol (inhibition of disulfide bond formation). Moreover, given that the ER is an unique oxidizing folding-environment that favors the formation of the disulfide bonds, any change in redox state of the cell can triggers UPR and parallely antioxidants protects from ER stress (Malhotra et al., 2008).
In yeast, the signaling pathway mediating UPR relies on the transmembrane protein IRE1 (Inositol-requiring enzyme 1), which contains an ER luminal stress-sensing domain and a endoribonuclease cytosolic domain. During ER stress, the luminal chaperone BiP binds to unfolded proteins and detaches from IRE1 stress sensing domain, triggering its activation; thus, IRE1 performs its enzymatic activity by splicing an intron out of the messenger coding for Hac1 (homologous to activating transcription factor ATF/CREB1). Once translated, this trancription factor translocates to the nucleus and induces the expressions of genes involved in protein folding and post-translational modification in the ER.
In mammalian cells, ER stress signaling pathways are much more complicated and involve at least three different transduction systems operating in parallel: i) IRE1 which is similar to the yeast ortholog, leading to the synthesis of XBP1 (X-box DNA binding protein 1) transcription factor (ortholog to yeast Hac1); ii) protein kinase-like ER kinase (PERK) that mediates eIF2α (eukaryotic translation initiation factor 2α) phosphorylation thereby inhibit global mRNA translation; iii) ATF-6 (Activating transcription factor 6), a transcription factor that, upon BiP detachment, transits to the Golgi where a cytosolic fragment is released though a proteolytic cleavage (Zhang and Kaufman, 2006). The UPR is primarily a cytoprotective response, a survival mechanism that helps to fix a dysregulation in protein folding and to restore homeostatic condition. In parallel, in order to prevent ER-folding capacities poisoning, ER stress increases the cellular proteolytic machinery, through two main mechanisms: i) retrotranslocation of the unfolded polypeptide into the cytosol followed by ubiquitination and proteasomal degradation (a process named ERAD, endoplasmic reticulum associated protein degradation); ii) targeting of parts of ER to lysosomes through autophagy (Kincaid and Cooper, 2007) (Fig. 2). The requirement of autophagy during ER stress is demostrated by the fact that autophagy-incompetent yeast cells cannot grow in tunicamycin-supplemented media. However, when a normal luminal environment cannot be restored, ER stress condition can switch from survival to cell death by different mechanism (Hoyer-Hansen and Jaattela, 2007). ER stress indeed can trigger both apoptotic (type I) as well as non-apoptotic cell death (type II); the latter is often referred to as “autophagic cell death” since it occurs in the absence of chromatin condensation but is generally associated with the massive presence of autophagic vacuoles. Although the term “autophagic cell death” could suggest that cell demise is accomplished by autophagy, this notion is misleading, since there is no clear demonstration that autophagy is per se a cell death mechanism (Kroemer and Levine, 2008, Giorgi et al., 2008). In any case, ER stress has been proposed to activate the ER-localized initiator caspase-12, at least in mice. Indeed, murine caspase-12 plays a crucial role in apoptosis induced by a number of well defined ER stressors, such as tunicamicyn, tapsigargin, poly(Q) or amyloid β proteins, and has been proposed to participate in IRE1 signaling. However, caspase-12 is highly mutated in primates, and it is thus commonly inactive in human cells, where caspase-4 has been shown to be involved in ER stress to apoptosis signaling pathway. Moreover, inhibition of caspase-12 or -4 only partially protects cells from apoptosis, raising serious doubt about their requirement in the execution of cell death (Szegezdi et al., 2003). In line with this hypothesis, numerous studies indicate that the induction of apoptosis by ER stress has a mandatory mitochondrial component, further highlighting the intimate connection between these two organelles (Deniaud et al., 2008). Indeed, Bax/Bak translocation and oligomerization in OMM, cytochrome c release, loss of Ψm and caspase-9 activation are common hallmark of ER stress induced apoptosis. Parallely, mitochondrial permeability transition pore inhibitors and APAF1 or Bax/Bak genetic ablation strongly decrease cell death induced by many ER stressors.
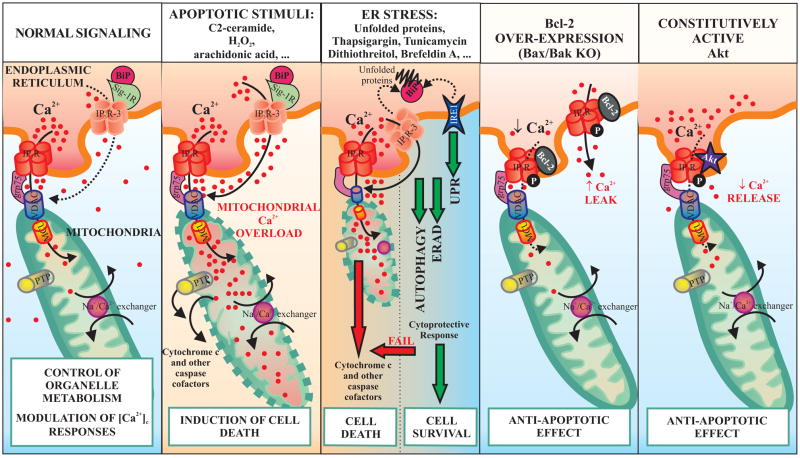
Figure 2.
ER-mitochondria cross-talk modulates Ca2+-mediated cellular responses to different patho-physiological stimuli: in normal conditions Ca2+ released from the ER to mitochondria triggers a boost in cellular metabolism and ATP production. However, mitochondrial Ca2+ overload induced by apoptotic stimuli or ER stressors sensitizes mitochondria to release caspases cofactors; conversely, anti-apoptotic elements or cell survival signals prevent Ca2+ accumulation in mitochondrial matrix through emptying intracellular stores (such as in Bcl-2 overexpression) or through inhibition of Ca2+ releasing channels (such as Akt activation).
Finally, ER stress activates another second messenger deeply involved in ER-mitochondria cross-talk, which is, again, Ca2+. Indeed, every stimulus inducing ER engulfment triggers Ca2+ release from the ER and the consequent [Ca2+] increase in the cytosol and in the mitochondrial matrix. This second messenger has been proposed to have a dual role in mediating ER stress induced cellular events. First, [Ca2+]c increase seems to be required for triggering autophagy, through a mechanism depending on Ca2+/calmodulin-dependent kinase kinase-β (Ca2+/calmodulin-dependent kinase kinase-β) and subsequent activation AMPK (AMP-activated protein kinase) leading to inhibition of mTOR (mammalian target of rapamycin) signaling complex 1 (Hoyer-Hansen et al., 2007). Moreover, prolonged ER stress conditions can cause a slow but sustained increase in mitochondrial matrix free [Ca2+], that can reach a critical threshold that is one of the strongest inducers of the pro-apoptotic mitochondrial membrane permeabilzation. However, the regulatory mechanisms, which determine the cell status leading to cell survival or cell death in response to ER stress have not been well elucidated. Not surprisingly, VDAC and IP3R has been proposed to be the main actors of this signaling event, pointing to the functional implication of intimate ER/mitochondria connection in the apoptotic cascade that leads ultimately to mitochondrial membrane permeabilization (Deniaud et al., 2008).
The ER/mitochondrial coupling in apoptosis: calcium signal and IP3R the key players
The function of the ER is intimately connected with that of the mitochondria. A major area of functional interaction between the ER and mitochondria is the control of Ca2+ signaling, that is a topic of major interest in physiology and pathology. These two organelles form a highly dynamic interconnected network within which they cooperate to generate Ca2+ signals. The mitochondria play an important role in shaping the Ca2+ signal released from the ER. The mitochondria assist in the recovery phase by rapidly sequestering Ca2+ and then later returning it to the ER. During normal signaling, therefore, there is a continuous flow of Ca2+ between these two organelles. The normal situation is for most of the Ca2+ to reside within the lumen of the ER except during Ca2+ signaling when a small bolus is periodically released to the cytoplasm and is then re-sequestered with a proportion passing through the mitochondria (Fig. 2). At equilibrium, therefore, the bulk of internal Ca2+ is in the ER where it not only functions as a reservoir of signal Ca2+ but it also plays an essential role in maintaining the activity of the chaperones responsible for protein processing (Rizzuto and Pozzan, 2006). Alterations in the operation of the ER/mitochondrial couple provide a pathway for activating apoptosis (Pinton et al., 2008). The key process connecting apoptosis to ER-mitochondria interactions is an alteration in Ca2+ homeostatic mechanisms that results in massive and/or a prolonged mitochondrial Ca2+ overload. It is firmly established that Ca2+ is an important regulator of both cell proliferation and apoptosis. It has been argued that the switch from a life to a death signal occur when its normal distribution between the ER and the mitochondria is distorted leading to a breakdown of mitochondrial function. Impairment of ER functioning, including depletion of ER Ca2+ stores, induced by i) protein expression alteration or by chemical agents (e.g. H2O2, arachidonic acid), ii) block of the proteasome that is required to degrade unfolded proteins (e.g. tunicamycin and brefeldin A), or iii) genetic mutations resulting in proteins that cannot be properly folded, can induce apoptosis (Ferri and Kroemer, 2001, Kaufman, 2002) (Fig. 2). Previous studies have established that the reduction in the Ca2+ amount that can be released from ER to mitochondria decreases the probability of Ca2+-dependent apoptosis. On the other hand conditions that increase ER Ca2+ storage have the opposite effect on Ca2+-dependent apoptosis. Indeed, in this context Pinton at al. demonstrated that over-expression of the anti-apoptotic proteins Bcl-2 can influence the distribution of Ca2+ within the ER/mitochondrial couple. In particular, compared to controls, Bcl-2 over-expressing cells showed a ~30% reduction in the Ca2+ levels within both the ER and the Golgi apparatus due to an increased Ca2+ leak from the lumen of the organelles (Foyouzi-Youssefi et al., 2000, Pinton et al., 2000).
In good agreement with these observations, the green tea compound epigallocatechin gallate, known to bind and inactivate Bcl-2, reduced Ca2+ leakage from the ER and restored [Ca2+]ER of Bcl-2 overexpressing cells to values similar to those of control cells (Palmer et al., 2004). As a consequence, the [Ca2+] increases elicited in these cells by stimuli coupled to IP3 generation were reduced both in the cytoplasm and in the mitochondria (Pinton et al., 2000). Manipulating the level of ER Ca2+ was able to reproduce the effect of Bcl-2. C2-ceramide-induced apoptosis in HeLa cells seems to depend on a release of Ca2+ from the ER and its accumulation in the mitochondria (Pinton et al., 2001). The ER can thus play an important role in regulating apoptosis by adjusting the load of Ca2+ imposed upon the mitochondrion. This concept was confirmed by the elegant work of the Korsmeyer’s group that showed that the KO of the pro-apoptotic protein Bax and Bak, localized to the ER, reduced the resting concentration of ER Ca2+ ([Ca2+]ER) thus decreasing the uptake of Ca2+ by mitochondria after Ca2+ release from the ER (Fig. 2). They demonstrated that expression of SERCA corrects [Ca2+]ER and mitochondrial Ca2+ uptake in Bax/Bak double KO cells (DKO), restoring apoptotic death in response to agents that release Ca2+ from intracellular store (such as arachidonic acid and oxidative stress) (Scorrano et al., 2003). Finally, this year the effect of the anti-apoptotic protein PKB or Akt on subcellular Ca2+ homeostasis has been revealed. Indeed, cells with the active form of Akt have a reduced ER Ca2+ release, that in turn affected cytosolic and mitochondria Ca2+ response. Reduction of mitochondrial Ca2+ accumulation by activated Akt results in diminished cellular sensitivity to Ca2+-mediated apoptotic stimuli (such as arachidonic acid and H2O2) (Marchi et al., 2008, Szado et al., 2008) (Fig. 2).
As mentioned above, the most important molecular component of Ca2+ handling machinery of the ER is represented by IP3Rs. Immunocytochemical studies show that regions of the ER apposed to mitochondria are enriched in IP3Rs, identifying these zones as ‘hotspots’ of Ca2+ transfer from the ER to the mitochondria (Rizzuto et al., 1998).
The release of Ca2+ from ER stores by IP3Rs has been implicated in multiple models of apoptosis as being directly responsible for mitochondrial Ca2+ overload.
The requirement of IP3Rs for Ca2+-dependent cell death is exemplified by the resistance to apoptosis of cells with antisense KO or genetic deletion of IP3R gene (Blackshaw et al., 2000) (Jayaraman and Marks, 1997, Khan et al., 1996, Sugawara et al., 1997). In this picture the three isoforms of the IP3R, appear to play distinct roles (Assefa et al., 2004, Hirota et al., 1999). Initial evidence suggested that Ca2+-dependent apoptotic death was mediated by the type 3 IP3R (Khan et al., 1996), but subsequent studies have shown that the type 1 isoform can also mediate apoptosis (Boehning et al., 2003). Interestingly, Korsmeyer’s group found that Bcl-2 and IP3R-1 physically interact at the ER surface and proposed a model in which Bcl-2 family members regulate IP3R-1 phosphorylation to control the rate of ER Ca2+ leak from intracellular stores and as consequence, apoptosis. In support of this view, the [Ca2+]ER reduction of Bax/Bak DKO was reversed by siRNA silencing of IP3R-1 (Oakes et al., 2005).
Additional mechanisms, however, have been proposed. Bcl-XL was shown to directly bind to the IP3R and sensitize it to low agonist doses. Bax prevents the effect of Bcl-XL, both in terms of its binding to the IP3R and of capacity of modifying the sensitivity to IP3 (White et al., 2005).
Expression of Bcl-XL reduced [Ca2+]ER in type 3 but not type 1 or 2 IP3R-expressing cells. In contrast, Bcl-XL enhanced spontaneous [Ca2+]c signaling in all three IP3R isoform-expressing cell lines. These results suggest that modulation of [Ca2+]ER is not a specific requirement for ER-dependent antiapoptotic effects of Bcl-XL. Rather, apoptosis protection is conferred by enhanced spontaneous [Ca2+]c signaling by Bcl-XL interaction with all isoforms of the IP3R (Li et al., 2007).
In this complex scenario, recent data showed that IP3R-3, localized in the MAM, plays a selective role in the induction of apoptosis by preferentially transmitting apoptotic Ca2+signals into mitochondria, whereas IP3R-1 predominantly mediates cytosolic Ca2+ mobilization (Mendes et al., 2005). Accordingly siRNA silencing of IP3R-3 blocked apoptosis, whereas transfection of IP3R-1 antisense constructs was ineffective (Blackshaw et al., 2000). Mitochondria appear to be the downstream effectors of this pathway, as KO of IP3R-3 significantly decreased agonist-induced mitochondrial Ca2+ uptake (Hayashi and Su, 2007).
A final crucial aspect is that, in response to survival signals, Akt interacts with and phosphorylates IP3Rs, significantly reducing their Ca2+ release activity (Khan et al., 2006, Szado et al., 2008). Moreover, phosphorylation of IP3Rs by Akt reduced cellular sensitivity to apoptotic stimuli through a mechanism that involved diminished Ca2+ flux from the ER to the mitochondria. In particular Joseph et al. demonstrated that all three isoforms present a consensus sequence for phosphorylation by Akt kinase and that IP3R-1 and IP3R-3 are substrate for activated Akt in vivo, but IP3R-1 phosphorylation did not affect Ca2+ homeostasis. IP3R-3 appears thus as a likely effector of the antiapoptic activity of Akt.
The elucidation of the role of IP3R-3 in Ca2+ transfer from the ER to mitochondria, of its molecular mechanism and of the regulatory effect of phosphorylation, may reveal a novel unexplored pharmacological target in apoptosis.
Table 1.
Summary of cited proteins involved in ER-Mitochondria structural link
| SYMBOL | FULL NAME | DETAILS |
|---|---|---|
| ER and Mitochondria structure | ||
| CaBPs | Ca2+-binding proteins | Large family of eukaryotic proteins containing a specific helix-loop-helix structure, referred to as the EF-hand motif; many of the Ca2+-mediated processes are carried out through the cooperation of CaBPs |
| IP3Rs | Inositol-1,4,5-trisphosphate receptors | Family of ligand-gated channels that function to release Ca2+ from intracellular Ca2+ stores (ER and Golgi apparatus) in response to agonist stimulation |
| RyRs | Ryanodine receptors | Ca2+ release channels located in the Sarcoplasmic Reticulum membrane |
| SERCA | Sarco-Endoplasmic Reticulum Ca2+- ATPase | Highly conserved family of Ca2+ pumps which actively transfer Ca2+ from the cytosol to the lumen ER/SR at the expense of ATP hydrolysis |
| VDAC | Voltage dependent anion channel | The VDAC family of proteins are the most abundant proteins of the outer mitochondrial membrane and mediate the flow of ions and metabolites between the cytosol and the mitochondrial intermembrane space |
| ER and Mitochondria dynamics | ||
| DRP1 | Dynamin-related protein 1 | Main protein controlling mitochondrial fission in mammalian cells. DRP-1 is located in the cytoplasm but during fission translocates to mitochondria surface, where constriction of the membranes takes place by direct or indirect interaction with hFis1, its adaptor in the OMM |
| Mfn1 and Mfn2 | Mitofusins 1 and 2 | GTPases localized to the outer mitochondrial membrane, playing a pivotal role in mitochondrial fusion |
| OPA1 | Optic Atrophy 1 | Profusion dynamin-related protein of the inner mitochondrial membrane mutated in dominant optic atrophy; this protein has been recently shown to participate in the biogenesis of the mitochondrial cristae and regulate the cristae remodelling pathway |
| PARL | Presenilin-associated rhomboid like | Rhomboid protease of the inner mitochondrial membrane (originally identified in a yeast two-hybrid screen as an interactor of presenilin) required for the correct assembly of the OPA1-containing structures that regulate the integrity of the mitochondrial cristae junctions |
Table 2.
Summary of cited proteins involved in ER-Mitochondria functional link
| SYMBOL | FULL NAME | DETAILS |
|---|---|---|
| ER and Mitochondria functional link: MAM | ||
| FACL4 | Long-chain fatty acid-CoA ligase type 4 | Key enzyme involved in the metabolism of arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid, and thereby plays a key role in lipid biosynthesis and fatty acid degradation. FACL4 preferentially utilizes arachidonate as substrate and selectively esterifies it with coenzyme A, forming acyl-CoA, which can then be incorporated into membrane phospholipid |
| grp75 | glucose-regulated protein 75 | Molecular chaperone which regulate the association between ER and mitochondria: cytosolic grp75 links IP3Rs to VDAC at the OMM, the resulting association presumably enhances the Ca2+ accumulation in mitochondria by stabilizing conformations or the coupling of the two receptors |
| IP3Rs | Inositol-1,4,5-trisphosphate receptors | See Table 1 |
| GRP78/BiP | 78-kDa glucose-regulated protein GRP78, also referred to as the immunoglobulin binding protein BiP | Central regulator of ER functions due to its roles in protein folding and assembly, targeting misfolded protein for degradation, ER Ca2+-binding and controlling the activation of trans-membrane ER stress sensors |
| PACS-2 | Phosphofurin acidic cluster sorting protein 2 | Multifunctional sorting protein that integrates ER-mitochondria communication, ER homeostasis, homeostasis of ER Ca2+ and apoptosis. PACS-2 controls the apposition of mitochondria with the ER and formation of ER lipid-synthesizing centers found on MAM, and regulates the distribution and activity of calnexin |
| PSS-1 | Phosphatidylserine synthase-1 | Enzyme involved in phosphatidylserine biosynthesis in mammalian cells: exchanges serine for choline of phosphatidylcholine; PSS-1 is highly enriched in MAM and is largely excluded from the bulk of the ER |
| Sig-1R | Sigma-1 receptor | Ca2+-sensitive and ligand-operated receptor chaperone that specifically targets MAM. Sig-1Rs form a Ca2+-sensitive chaperone machinery with BiP and prolong Ca2+ signaling from ER into mitochondria by stabilizing IP3R-3 at MAM; Sig-1Rs can translocate under chronic ER stress |
| VDAC | Voltage dependent anion channel | See Table 1 |
Table 3.
Summary of cited proteins involved in ER stress
| SYMBOL | FULL NAME | DETAILS |
|---|---|---|
| ER stress | ||
| AMPK | AMP-activated protein kinase | Metabolite sensing serine/threonine kinase that has been termed the master regulator of cellular energy metabolism due to its numerous roles in the regulation of glucose, lipid, and protein metabolism |
| APAF1 | apoptotic peptidase activating factor 1 | Cytoplasmic protein that initiates apoptosis: if cytochrome c is released into the cytosol, in the presence of ATP or dATP it mediates the allosteric activation and hepta-oligomerization of APAF1, generating the complex known as apoptosome. The apoptosome recruits and processes caspase-9 to form a holoenzyme complex, which in turn recruits and activates the effector caspases, thus beginning the proteolytic cascade that results in the morphological features of apoptosis |
| ATF-6 | Activating transcription factor 6 | Endoplasmic Reticulum stress-regulated transmembrane transcription factor required for activating many UPR target genes |
| CaMKKβ | Ca2+/calmodulin-dependent kinase kinase-β | CaMKKβ belongs to the Serine/Threonine protein kinase family, and to the Ca2+/calmodulin-dependent protein kinase subfamily. Ca2+-mediated autophagy depends on CaMKKβ-dependent activation of AMPK that ultimately leads to the inhibition of mTORC signaling complex 1 |
| eIF2α | eukaryotic translation initiation factor 2α | Phosphorylated eIF2α interferes with the formation of the 43S translation initiation complex, leading to overall translational repression in UPR-induced cells presumably to alleviate ER stress by reducing the influx of the newly synthesized proteins into the ER |
| GRP78/BiP | 78-kDa glucose-regulated protein GRP78, also referred to as the immunoglobulin binding protein BiP | see Table 2 |
| IRE1 | Inositol-requiring enzyme 1 | ER-resident transmembrane protein kinase and endoribonuclease that induces the non-conventional splicing of XBP1 mRNA and so transmits an ER stress signal to the cytoplasm and mediates the UPR |
| mTOR | mammalian target of rapamycin | Serine/threonine kinase that controls many aspects of cellular physiology, including transcription, translation, cell size, cytoskeletal organization and autophagy. Recent advances in the mTOR signaling field have found that mTOR exists in two heteromeric complexes, mTORC1 and mTORC2. The activity of mTORC1 is regulated by the integration of many signals, including growth factors, insulin, nutrients, energy availability and cellular stressors such as hypoxia, osmotic stress, reactive oxygen species, viral infection and ER stress |
| PERK | Protein kinase-like ER kinase | ER-transmembrane kinase that phosphorylates the a subunit of eIF2α thereby reducing cellular protein synthesis and with it the load of proteins entering into the ER |
| Sig-1R | Sigma-1 receptor | see Table 2 |
| XBP1 | X-box DNA binding protein 1 | XBP1 pre-mRNA is converted to spliced mRNA (by activated IRE1) in response to UPR, leading to the production of an activated/spliced XBP-1 (XBP-1s), a potent transcription factor responsible for UPR |
Table 4.
Summary of cited proteins involved in the ER/mitochondria coupling in apoptosis
| SYMBOL | FULL NAME | DETAILS |
|---|---|---|
| apoptosis | ||
| Akt (PKB) | v-akt murine thymoma viral oncogene homolog, also known as protein kinase B (PKB) | Serine/threonine kinase, involved in the control of cellular processes as diverse as glucose metabolism, cell proliferation, apoptosis, transcription and cell migration. AKT has a key role in promoting cell survival |
| Bak | Bcl-2 antagonist/killer | Pro-apoptotic Bcl-2 family members; Bax and Bak are required for the initiation of mitochondrial dysfunction during apoptosis and for maintaining the ER Ca2+ stores necessary for Ca2+-dependent cell death |
| Bax | Bcl-2 associated X protein | |
| Bcl-2 | B-cell lymphoma/leukemia 2 | Anti-apoptotic Bcl-2 family member; Bcl-2 has been shown to decrease Ca2+ concentration in the ER |
| Bcl-XL | B-cell lymphoma/leukemia XL | Anti-apoptotic Bcl-2 family members; Bcl-XL have been reported to function at the ER by lowering its Ca2+ content only in the type 3 IP3R- expressing cells |
| IP3Rs | Inositol-1,4,5-trisphosphate receptors | see Table 1 |
| VDAC | Voltage dependent anion channel | see Table 1 |
Acknowledgments
The authors are deeply indebted to past and present collaborators. This work was supported by the Italian Association for Cancer Research (AIRC), Telethon, local funds from the University of Ferrara, the Italian University Ministry, the PRRIITT program of the Emilia Romagna Region, the Italian Space Agency (ASI), NIH (Grant #1P01AG025532-01A1), the Italian Multiple Sclerosis Foundation (FISM) and the United Mitochondrial Disease Foundation (UMDF).
Abbreviations
- Ca2+
calcium ions
- [Ca2+]
Ca2+ concentration
- [Ca2+]c
cytosolic Ca2+ concentration
- [Ca2+]ER
resting concentration of ER Ca2+
- [Ca2+]m
mitochondrial Ca2+ concentration
- DKO
double knockout cells
- EM
electron microscopy
- ER
endoplasmic reticulum
- ERAD
Endoplasmic reticulum associated protein degradation
- GFP
Green-fluorescent protein
- IMM
inner mitochondrial membrane
- IMS
intramembrane space
- IP3
Inositol-1,4,5-triphosphate
- IP3Rs
Inositol-1,4,5-trisphosphate receptors
- KO
knockout
- MAMs
mitochondria associated membranes
- OMM
outer mitochondrial membrane
- SR
sarcoplasmic reticulum
- UPR
Unfolded protein response
- VDAC
Voltage dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alirol E, James D, Huber D, Marchetto A, Vergani L, Martinou JC, Scorrano L. The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol Biol Cell. 2006;17:4593–4605. doi: 10.1091/mbc.E06-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta. 2006;1757:692–699. doi: 10.1016/j.bbabio.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Assefa Z, Bultynck G, Szlufcik K, Nadif KN, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB, De Smedt H. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. Journal of Biological Chemistry. 2004;279:43227–43236. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Sawa A, Sharp AH, Ross CA, Snyder SH, Khan AA. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. FASEB J. 2000;14:1375–1379. doi: 10.1096/fj.14.10.1375. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. The Journal of Cell Biology. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Petersen OH, Verkhratsky A. The endoplasmic reticulum is a focal point for co-ordination of cellular activity. Cell Calcium. 2002;32:231–234. doi: 10.1016/s0143416002002002. [DOI] [PubMed] [Google Scholar]
- Borgese N, Francolini M, Snapp E. Endoplasmic reticulum architecture: structures in flux. Curr Opin Cell Biol. 2006;18:358–364. doi: 10.1016/j.ceb.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough D, Schell MJ, Irvine RF. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J. 2005;392:291–297. doi: 10.1042/BJ20050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006a;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006b;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevet E, Cameron PH, Pelletier MF, Thomas DY, Bergeron JJ. The endoplasmic reticulum: integration of protein folding, quality control, signaling and degradation. Curr Opin Struct Biol. 2001;11:120–124. doi: 10.1016/s0959-440x(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pinto V, Reina S, Guarino F, Messina A. Structure of the voltage dependent anion channel: state of the art. J Bioenerg Biomembr. 2008;40:139–147. doi: 10.1007/s10863-008-9140-3. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Drummond RM, Mix TC, Tuft RA, Walsh JV, Jr, Fay FS. Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus. J Physiol. 2000;522(Pt 3):375–390. doi: 10.1111/j.1469-7793.2000.t01-2-00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. Journal of Biological Chemistry. 2003;278:39224–39234. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, Demaurex N, Krause KH. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci USA. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Krishnamurthy R, Szalai G. Mitochondrial calcium signaling driven by the IP3 receptor. J Bioenerg Biomembr. 2000;32:15–25. doi: 10.1023/a:1005504210587. [DOI] [PubMed] [Google Scholar]
- Hamilton SL. Ryanodine receptors. Cell Calcium. 2005;38:253–260. doi: 10.1016/j.ceca.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hirota J, Furuichi T, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J Biol Chem. 1999;274:34433–34437. doi: 10.1074/jbc.274.48.34433. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Israelson A, Zaid H, Abu-Hamad S, Nahon E, Shoshan-Barmatz V. Mapping the ruthenium red-binding site of the voltage-dependent anion channel-1. Cell Calcium. 2008;43:196–204. doi: 10.1016/j.ceca.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Marks AR. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol Cell Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LM, Lechleiter JD, Camacho P. Differential modulation of SERCA2 isoforms by calreticulin. The Journal of Cell Biology. 1998;142:963–973. doi: 10.1083/jcb.142.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Hajnoczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 2007;12:951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. Journal of Biological Chemistry. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- Kincaid MM, Cooper AA. ERADicate ER stress or die trying. Antioxid Redox Signal. 2007;9:2373–2387. doi: 10.1089/ars.2007.1817. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. Embo J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H, Shimizu S, Koya RC, Fujita H, Kamada S, Kuzumaki N, Tsujimoto Y. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 2000;19:4807–4814. doi: 10.1038/sj.onc.1203868. [DOI] [PubMed] [Google Scholar]
- Lawen A, Ly JD, Lane DJ, Zarschler K, Messina A, De Pinto V. Voltage-dependent anion-selective channel 1 (VDAC1)--a mitochondrial protein, rediscovered as a novel enzyme in the plasma membrane. Int J Biochem Cell Biol. 2005;37:277–282. doi: 10.1016/j.biocel.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Lebiedzinska M, Szabadkai G, Jones AWE, Duszynski J, Wieckowski MR. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other Subcellular organelles . Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.02.017. In press. [DOI] [PubMed] [Google Scholar]
- Lee AC, Xu X, Colombini M. The role of pyridine dinucleotides in regulating the permeability of the mitochondrial outer membrane. J Biol Chem. 1996;271:26724–26731. doi: 10.1074/jbc.271.43.26724. [DOI] [PubMed] [Google Scholar]
- Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKrill JJ. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem J. 1999;337(Pt 3):345–361. [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA, Buttle K, Rath BK, Marko M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- Marchi S, Rimessi A, Giorgi C, Baldini C, Ferroni L, Rizzuto R, Pinton P. Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem Biophys Res Commun. 2008;375:501–505. doi: 10.1016/j.bbrc.2008.07.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. Journal of Biological Chemistry. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. Inositol 1,4,5-trisphosphate IP(3) receptors and their role in neuronal cell function. J Neurochem. 2006;97:1627–1633. doi: 10.1111/j.1471-4159.2006.03985.x. [DOI] [PubMed] [Google Scholar]
- Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980;111:261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Peng S, Blachly-Dyson E, Forte M, Colombini M. Large scale rearrangement of protein domains is associated with voltage gating of the VDAC channel. Biophys J. 1992;62:123–131. doi: 10.1016/S0006-3495(92)81799-X. discussion 131–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini M, Vitelli F, Bruttini M, Pober BR, Jonsson JJ, Villanova M, Zollo M, Borsani G, Ballabio A, Renieri A. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics. 1998;47:350–358. doi: 10.1006/geno.1997.5104. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. The Journal of Cell Biology. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. Embo J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Simpson AW, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J Biol Chem. 1996;271:28006–28008. doi: 10.1074/jbc.271.45.28006. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A. Get the balance right: mitofusins roles in health and disease. Biochim Biophys Acta. 2006;1763:490–499. doi: 10.1016/j.bbamcr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Barnikol-Watanabe S, Thinnes FP, Hilschmann N. Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex1 and the heat-shock protein PBP74. Int J Biochem Cell Biol. 2002;34:1059–1070. doi: 10.1016/s1357-2725(02)00026-2. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Gincel D. The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem Biophys. 2003;39:279–292. doi: 10.1385/CBB:39:3:279. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M. The sensor regions of VDAC are translocated from within the membrane to the surface during the gating processes. Biophys J. 1998;74:2926–2944. doi: 10.1016/S0006-3495(98)78000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. Embo J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. The Journal of Cell Biology. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg O, Bootman MD, Roderick HL. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci U S A. 2008;105:2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell. 2008;29:409–410. doi: 10.1016/j.molcel.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. Journal of Biological Chemistry. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Petersen OH. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol. 2002;447:141–154. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Gerencser AA, Liot G, Lipton SA, Ellisman M, Perkins GA, Bossy-Wetzel E. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ. 2007;14:462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- Zaid H, Abu-Hamad S, Israelson A, Nathan I, Shoshan-Barmatz V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]