Summary
Phospholipase D has long been implicated in vesicle formation and vesicular transport through the secretory pathway. The Golgi apparatus has been shown to exhibit a plethora of mechanisms of vesicle formation at different stages to accommodate a wide variety of cargo. Phospholipase D has been found on the Golgi apparatus and is regulated by ADP-ribosylation factors which are themselves regulators of vesicle trafficking. Moreover, the product of phospholipase D activity, phosphatidic acid, as well as its degradation product diacylglycerol, have been implicated in vesicle fission and fusion events. Here we summarize recent advances in the understanding of the role of phospholipase D at the Golgi apparatus.
Keywords: Phospholipase D, Phosphatidic acid, Golgi apparatus, vesicular trafficking
Introduction
Studies into the role of phospholipase D (PLD) in the regulation of vesicle transport and protein trafficking were provoked by the observation that ADP-ribosylation factor (ARF) proteins are efficacious activators of PLD [1–3]. ARF proteins have been previously implicated as factors for regulation of intracellular vesicle trafficking and are found on the Golgi apparatus and the plasma membrane [4]. The PLD discussed in this review hydrolyzes phosphatidylcholine (PC) to yield phosphatidic acid (PA) and choline. PA has been demonstrated to be a signaling molecule as well as a crucial lipid during vesicle fusion and fission [5–8]. Furthermore, PA can be hydrolyzed to generate diacylglycerol (DG) which also acts as signaling molecule as well as a functional lipid in membrane modulation [9, 10]. This review, which focuses on recent advances in the localization and function of PLD in the Golgi apparatus is dedicated to the memory of Dennis Shields, who unexpectedly passed away December 1st, 2008.
Structure and Regulation of mammalian PLD enzymes
Two mammalian PC-PLD genes have been associated with intracellular vesicle trafficking: PLD1 [11] and PLD2 [12]. Both share the same basic domain organization of a phox (PX) and a pleckstrin homology (PH) domain in tandem in their amino terminus, the conserved dual HKD motif which forms the active site, and a central phosphoinositide binding domain. This domain structure is also shared by the single PLD genes of Yeast, Nematodes and Drosophila [13], as well as by one of the plant PC-PLD families, PLDζ[6]. PLD1 exhibits low intrinsic basal activity and is strongly and synergistically activated by members of the ARF and Rho families of monomeric G proteins as well as by PKCα and PKCβ. Conversely, heterologous expression of PLD2 results in high basal activity. However, endogenous PLD2 cannot be measured without prior purification and therefore may be regulated by inhibitory proteins such as synucleins [14]. In addition, the activity of PLD1 and PLD2 is highly dependent on the presence of phosphatidylinositol 4,5-bisphosphate (PIP2) or phosphatidylinositol 3,4,5-trisphosphate (PIP3).
Subcellular localization and membrane association of mammalian PLDs
Overexpression of epitope tagged variants has been widely used to examine the subcellular localization of PLD1 and PLD2. GFP- or HA-tagged PLD1 and PLD2 localize mainly to the plasma membrane and several other organelles, including endosomes, lysosomes, secretory granules and the Golgi apparatus [12, 15, 16]. Cryo-immunoelectron microscopy using selective antibodies, and subcellular fractionation, demonstrated that 25–30% of endogenous PLD1 was localized to the Golgi apparatus. Although a fraction of endogenous PLD2 was evident on the plasma membrane, much of the enzyme localized to the region of the Golgi apparatus and cytosolic puncta [17]. Most significantly, cryoimmunoelectron microscopy demonstrated that PLD2 was present almost exclusively on Golgi cisternal rims in pituitary GH3 cells; it was enriched 80-fold in Golgi rims relative to cisternae [17]. The differential distribution of PLD1 and PLD2 suggests that these enzymes have separate functions in the Golgi apparatus.
The mechanisms by which PLD1 and PLD2 associate with cellular membranes have been studied extensively. PLD1 and PLD2 exhibit three lipid-binding domains in addition to the active site, a PX domain, a PH domain and a polybasic domain that is responsible for PIP2 stimulated enzyme activity and is sufficient for the membrane recruitment of PLD2 [18]. This multi-domain organization reflects the complex cellular distribution of these proteins. The PX domain preferentially binds to PIP3 but also mono-phosphorylated inositides, and its membrane association is enhanced by binding of acidic lipids such as PA or PS at a secondary site [19, 20]. This is thought to be responsible for internalization of PLD after recruitment to the plasma membrane [19]. PIP2 binds to the PLD2 PH domain albeit with low affinity, suggesting that this domain functions in conjunction with other membrane association domains to target the protein to different intracellular sites [21]. In addition, the PH domain of PLD1 is palmitoylated [22, 23]. Protein palmitoyl transferases are found along the secretory pathway [24] and it has not been established where PLD is acylated. In fact, the effect of palmitoylation on the subcellular localization of PLD1 is controversial [25, 26]. Both, PX and PH domains have been shown to bind to proteins in addition to lipids [27–30], and a model of combinatorial binding of such domains has been suggested [30]. Interestingly, the four-phosphate-adaptor protein PH-domain binds phosphatidylinositol 4-phosphate and ARF simultaneously to recruit these proteins to the trans-Golgi network [31]. Investigations into the role of phosphoinositides in membrane localization of PLD1 and PLD2 are complicated by the finding that certain isoforms of the enzyme responsible for PIP2 synthesis, phosphatidylinositol 4-phosphate 5-kinase (PI4P5K), are themselves stimulated by the PLD product PA [32, 33]. PIP2 is synthesized on isolated Golgi apparatus membranes incubated with cytosol in a PLD dependent manner [34]. In vivo, synthesis of PIP2 on the Golgi apparatus is required for its structural integrity and treatment of cells with primary alcohols causes rapid fragmentation of the Golgi apparatus, in part by PIP2 dependent relocalization of βIII-spectrin [35]. Stimulation of PI4P5K activity by PLD-produced PA provides an attractive explanation for these observations. This process forms a feed forward loop with PLD. PIP2 is rapidly degraded on the Golgi apparatus [35] presumably by PIP2 5-phosphatases such as Ocrl1 [36]. This suggests a tightly regulated local and temporal spike of PA and PIP2 production upon recruitment of PLD and PI4P5Ks by ARF on the Golgi apparatus, contrasting with the comparatively large and static PIP2 pool of the plasma membrane.
ARF as a PLD1 effector in membrane trafficking
PLD1 can be activated by all members of the ARF family in their GTP-bound states, and the potency of this effect is enhanced by myristoylation of ARF [37]. However, although the effects are likely to be direct because they can be readily observed using purified proteins, the mechanism whereby ARF stimulates enzyme activity and a site of direct interaction on PLD1 remain to be identified. ARF1-5 localize to the perinuclear region and the Golgi apparatus, and combinatorial RNAi mediated knock-down revealed that when ARF1 was depleted together with ARF3, ARF4 or ARF5, the distribution of the COPI coat protein β-cop was disrupted [38]. ARF1 regulated PLD activity at the Golgi apparatus has been implicated in vesicle budding [39, 40], whereas the plasma membrane localized ARF6 regulates PLD activity at the plasma membrane [41, 42]. It has been demonstrated that ARF1 is a more potent activator of PLD1 than ARF6 [43]. However, a study using effector domain mutants of ARF3 suggested that ARF activation of PLD1 and coatomer recruitment are separable processes [44]. Whereas ARF proteins are strong stimulators of PLD1 activity, their effect on PLD2 activity is low. However, paradoxically, N-terminally truncated PLD2 mutants are more sensitive to ARF stimulation [45]. Effects of ARF on PLD2 activity in cell-based assays might in fact be explained by ARF stimulated PI4P5K activity [43].
Insights from the transphosphatidylation reaction
A large body of work implicating PLD activity in an extensive variety of cellular functions, including Golgi membrane transport, is based on the long-established preference of PLD for primary alcohols over H2O to hydrolyze PC, yielding a phosphatidylalcohol instead of PA [46, 47]. This reaction, termed transphosphatidylation, has been used to infer the involvement of PLD-generated PA in cellular processes based on the following presumptions: diversion of PA to the corresponding phosphatidylalcohol would be complete, and phosphatidylalcohols, which are metabolically stable and accumulate to significant levels in primary alcohol treated cells, would be themselves inert and do not exert inhibitory effects on the cellular processes under study. In support of the suggested involvement of PLD in intracellular trafficking, primary alcohols were shown to inhibit both protein transport from the endoplasmic reticulum to the Golgi apparatus and release of secretory vesicles from the trans-Golgi network [40, 48, 49]. Moreover, the Golgi apparatus reversibly fragments in the presence of primary alcohols [34, 50].
Initiation of assembly of the COPI coat on Golgi membranes was demonstrated to occur independently of ARF in cell lines exhibiting high constitutive PLD activity [39, 48]. COPI coated vesicles mediate intra-Golgi apparatus trafficking as well as retrograde transport to the endoplasmic reticulum [51]. The formation of coated vesicles was sensitive to ethanol at concentrations that inhibit PLD catalyzed PA production. Furthermore, exogenous bacterial PLD was able to induce the binding of coatomers to Golgi membranes. Additionally, ARF1, reconstituted purified COPI coatomer proteins and chemically defined synthetic liposomes containing PA can form coated vesicles in vitro in the absence of PLD [52]. This led to the idea that PLD catalyzed production of PA is a key event in the formation of coatomer-coated vesicles, which is supported by the finding that the coatomer protein β-cop and ARF directly bind to PA in vitro [53]. Recently, PLD2 has been described as a crucial component of COPI vesicle formation [54]. The BARS protein, a structural protein acting at the fission step of COPI vesicle formation [55] requires PA in liposomes to induce tubulation. Depletion of PLD2 from Golgi membranes by either RNAi or using antibodies trapped β-cop on membranes [54]. This indicates that in absence of PA generated by PLD2 fission is not completed.
In Drosophila, absence of PLD was lethal by preventing embryonic cellularization and caused abnormal Golgi apparatus structure and vesicle trafficking [13]. The observation of abnormal Golgi apparatus structure in Pldnull Drosophila embryos suggested a role for PLD in facilitating the fission of vesicles from the trans Golgi network which are targeted to the embryonic cortex. In addition, fusion of vesicles into the plasma membrane was inhibited [13], demonstrating that Drosophila PLD is required for both events.
Interestingly, it has been demonstrated that SNARE mediated fusion of vesicles is enhanced by the presence of PA and PIP2 [56, 57], where PA has to be present on the tSNARE side whereas PIP2 is stimulatory only on the vSNARE side. Since PLD1 and PLD2 require PIP2 for their activity, it can be speculated that PLD binds to PIP2 on the vesicle while generating PA on the target membrane. The structure of PLD1 and PLD2 is unknown, however, their ability to form homo- as well as heterodimers [58] would support this scenario. However, these studies have not yet been extended to Golgi apparatus SNAREs and it is unclear if those exhibit a similar lipid requirement. Finally, the budding yeast spo14p PLD plays a conditional role in maintaining normal secretory function during the “sec14 bypass” and an obligatory role in formation of the prospore membrane during meiosis [59]. The latter process involves a redirection of the secretory pathway to sites of membrane fusion at the spindle pole bodies [60]. The identification of a PA binding function for the putative PLD effector, the SNARE protein spo20p, provides a potential mechanism for this process [56].
Role of PLD in production of diacylglycerol
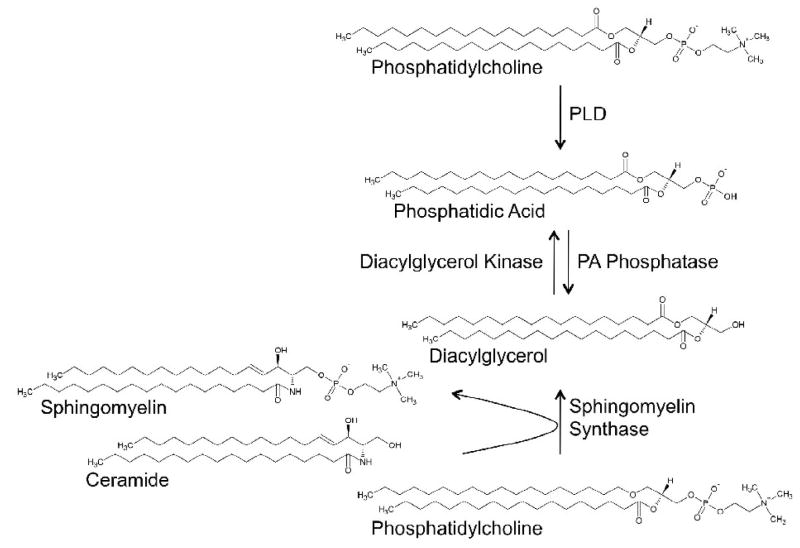
In addition to its role in production of PA, PLD may also be important in the production of other lipid messenger molecules. In many systems hormone-induced production of DG is biphasic by nature [61, 62]. A small transient increase is followed by a larger sustained increase. Polyunsaturated DG predominates during the initial phase of stimulation and is thought to be derived from PIP2 by the action of phospholipase C [63]. During the sustained phase, the concentration of mono-unsaturated and saturated DG rises. PLD has some preference for mono-unsaturated and saturated PC molecular species [64]. Therefore, it is proposed that PLD contributes to the production of DG by promoting the formation of PA, which is subsequently converted into DG by PA phosphohydrolase (Fig. 1). Since phosphatidylalcohols are not a substrate for PA phosphohydrolase, as described above, the transphosphatidylation reaction can be exploited to demonstrate that the second peak of DG accumulation results from the actions of PLD [65]. Additionally, the PA phosphohydrolase inhibitor propranolol can attenuate DG production in some systems [65]. The most prominent function of DG is activation of PKC; and PKCμ (PKD1), PKD2 and PKCν (PKD3) regulate vesicle trafficking at the Golgi apparatus [66]. Intriguingly, brief treatment with propranolol causes reversible relocalization of PKD from the Golgi apparatus into the cytoplasm [67]. Moreover, ilimaquinone, a drug that causes fragmentation of the Golgi apparatus, requires PLD-mediated PA generation and its subsequent conversion to DG to cause PKD stimulation [68]. It is important to note that DG on the Golgi apparatus can also be derived from sphingomyelin synthesis. Phosphocholine is transferred from PC to ceramide, producing sphingomyelin and DG [69]. Depletion of ceramide using fumonisin B1 therefore reduces DG production through this pathway, and it has been shown that extended treatment with fumonisin B1 causes cytosolic distribution of PKD [67]. Moreover, ten mammalian DG kinases have been described [70] which can utilize DG to create a pool of PA independent of PLD. Indeed, activation of PLD may not be an essential part of the basic ARF-mediated vesicle budding machinery in the Golgi apparatus of eukaryotic cells, because in yeast such a functionality does not exist [71], and any PA required in these processes might be produced by DG kinase [72]. The only known PLD gene in the yeast Saccharomyces cerevisiae, SPO14, is not essential for secretion, but does play a conditional role in this process during the “sec14 bypass” [73]. Spo14p is mainly involved in prospore formation which depends on vesicle transport from the Golgi apparatus to the prospore membrane [74]. The contributions of the PLD and the DG kinase pathways likely differ between cell types and for the transported cargo by way of adaptor and coat proteins.
Fig. 1.
Phosphatidic acid and diacylglycerol can be synthesized through separate routes on the Golgi apparatus. In mammalian cells all pathways are active, allowing for complexity of regulation. Note that diacylglycerol might be derived by additional pathways. PA, phosphatidic acid; PLD, phospholipase D.
Role of PLD in Golgi fragmentation during apoptosis
Recently, it has been demonstrated that fragmentation of the Golgi apparatus is an early event in apoptosis coinciding with cytochrome c release from the mitochondria [75]. Several Golgi apparatus proteins are cleaved during apoptosis by caspases, and some, such as golgin-160, or p115 are targets of upstream caspases such as caspase-2 and caspase-8 [76, 77]. Since treatment of cells with n-butanol but not t-butanol causes reversible fragmentation of the Golgi apparatus [34], the fate of PLD1 and PLD2 during apoptosis is clearly of interest. In vitro, PLD1 and PLD2 were substrates for caspases, including the upstream caspase-8 but not caspase-2 [78]. Interestingly, both isoforms retained their activity after cleavage and PLD1 showed an altered response to regulatory stimuli. Cleavage of PLD1 resulted in an enzyme not responsive to phorbol ester and increased response to GTPγS. The caspase-mediated removal of the amino terminus that is responsible for the loss of response to phorbol ester would also prevent PLD1 from being recruited by PKC to the plasma membrane. It is therefore tempting to speculate that this cleaved form of PLD1 accumulates on the Golgi apparatus and its activity is in part responsible for Golgi fragmentation during apoptosis.
Conclusion
Recent advances have provided insight as to how PLD is involved in regulating structure and function of the Golgi apparatus (Fig. 2). However, PA and DG, the immediate and secondary lipid products of PLD activity respectively, can also be derived via other pathways (Fig. 1). Mammalian cells exhibit a plethora of mechanisms of vesicle formation on the Golgi apparatus [79, 80], and alternative pathways of lipid modification add to the specific regulation of each. This complexity demands further characterization of the role of PLD in particular pathways of vesicular transport at the Golgi apparatus. Although considerable progress has been made in this area, the range of experimental tools available for manipulations of PLD activity in intact cells limits the types of experiments that can be attempted. In particular, concerns about the specificity and persistence of the effects of primary alcohols to manipulate PLD activity may limit their usefulness while more specific “epigenetic” approaches such as overexpression or RNA interference are slow in onset. The very recent identification of small molecule inhibitors of PLD1 and PLD2 activity may provide an invaluable new tool to re-examine these issues with greater precision [81, 82]. If these probes can be rapidly accumulated by, and washed out of cells, then their use in conjunction with live cell imaging techniques will provide a way to directly evaluate the role of PLD in intracellular transport and organelle dynamics.
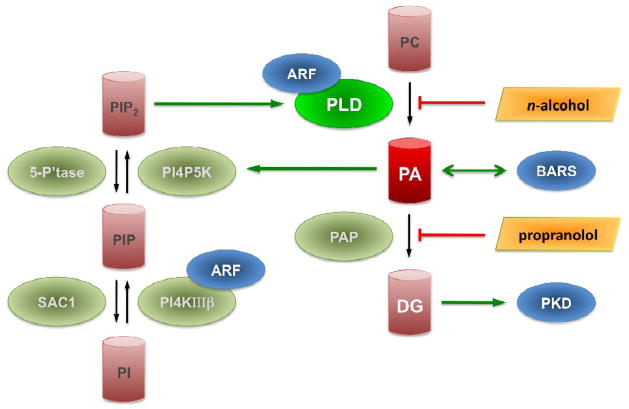
Fig. 2.
Regulation and effectors of phospholipase D and phosphatidic acid on the Golgi apparatus. See text for details. ARF, ADP-ribosylation factor; BARS, Brefeldin-A ADP-ribosylated substrate; DG, diacylglycerol; PA, phosphatidic acid; PAP, PA phosphatase; PC, phosphatidylcholine; PI, phosphatidylinositol; PIP, PI 4-phosphate; PIP2, PI 4,5-bisphosphate; PI4KIIIβ, type III PI 4-kinase β; PI4P5K, type I PIP 5-kinase; PKD, protein kinase D; PLD, phospholipase D; SAC1, PIP 4-phosphatase; 5-P’tase, PIP2 5-phosphatase.
Acknowledgments
The authors like to thank Sunandini Sridhar for very helpful suggestions with the manuscript. CR was supported by a Fellowship from the New York State Department of Health (C021327); this work was supported by a National Institutes of Health grants (DK21860) to DS and (CA096496 and GM050388) to AJM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 2.Kahn RA, Yucel JK, Malhotra V. ARF signaling: a potential role for phospholipase D in membrane traffic. Cell. 1993;75:1045–1048. doi: 10.1016/0092-8674(93)90314-g. [DOI] [PubMed] [Google Scholar]
- 3.Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 5.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 6.Bargmann BO, Munnik T. The role of phospholipase D in plant stress responses. Curr Opin Plant Biol. 2006;9:515–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Barr FA, Shorter J. Membrane traffic: do cones mark sites of fission? Curr Biol. 2000;10:R141–144. doi: 10.1016/s0960-9822(00)00326-2. [DOI] [PubMed] [Google Scholar]
- 8.Stace CL, Ktistakis NT. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta. 2006;1761:913–926. doi: 10.1016/j.bbalip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2007;32:27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Shemesh T, Luini A, Malhotra V, Burger KN, Kozlov MM. Prefission constriction of Golgi tubular carriers driven by local lipid metabolism: a theoretical model. Biophys J. 2003;85:3813–3827. doi: 10.1016/S0006-3495(03)74796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, Engebrecht J, Morris AJ, Frohman MA. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 12.Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 13.LaLonde M, Janssens H, Yun S, Crosby J, Redina O, Olive V, Altshuller YM, Choi SY, Du G, Gergen JP, Frohman MA. A role for Phospholipase D in Drosophila embryonic cellularization. BMC Dev Biol. 2006;6:60. doi: 10.1186/1471-213X-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 15.Brown FD, Thompson N, Saqib KM, Clark JM, Powner D, Thompson NT, Solari R, Wakelam MJ. Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr Biol. 1998;8:835–838. doi: 10.1016/s0960-9822(98)70326-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Kim JE, Lee SD, Lee TG, Kim JH, Park JB, Han JM, Jang SK, Suh PG, Ryu SH. Phospholipase D1 is located and activated by protein kinase C alpha in the plasma membrane in 3Y1 fibroblast cell. Biochim Biophys Acta. 1999;1436:319–330. doi: 10.1016/s0005-2760(98)00120-9. [DOI] [PubMed] [Google Scholar]
- 17.Freyberg Z, Bourgoin S, Shields D. Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells. Mol Biol Cell. 2002;13:3930–3942. doi: 10.1091/mbc.02-04-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciorra VA, Rudge SA, Prestwich GD, Frohman MA, Engebrecht J, Morris AJ. Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J. 1999;18:5911–5921. doi: 10.1093/emboj/18.21.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du G, Altshuller YM, Vitale N, Huang P, Chasserot-Golaz S, Morris AJ, Bader MF, Frohman MA. Regulation of phospholipase D1 subcellular cycling through coordination of multiple membrane association motifs. J Cell Biol. 2003;162:305–315. doi: 10.1083/jcb.200302033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahelin RV, Ananthanarayanan B, Blatner NR, Singh S, Bruzik KS, Murray D, Cho W. Mechanism of membrane binding of the phospholipase D1 PX domain. J Biol Chem. 2004;279:54918–54926. doi: 10.1074/jbc.M407798200. [DOI] [PubMed] [Google Scholar]
- 21.Sciorra VA, Rudge SA, Wang J, McLaughlin S, Engebrecht J, Morris AJ. Dual role for phosphoinositides in regulation of yeast and mammalian phospholipase D enzymes. J Cell Biol. 2002;159:1039–1049. doi: 10.1083/jcb.200205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manifava M, Sugars J, Ktistakis NT. Modification of catalytically active phospholipase D1 with fatty acid in vivo. J Biol Chem. 1999;274:1072–1077. doi: 10.1074/jbc.274.2.1072. [DOI] [PubMed] [Google Scholar]
- 23.Sugars JM, Cellek S, Manifava M, Coadwell J, Ktistakis NT. Fatty acylation of phospholipase D1 on cysteine residues 240 and 241 determines localization on intracellular membranes. J Biol Chem. 1999;274:30023–30027. doi: 10.1074/jbc.274.42.30023. [DOI] [PubMed] [Google Scholar]
- 24.Nadolski MJ, Linder ME. Protein lipidation. FEBS J. 2007;274:5202–5210. doi: 10.1111/j.1742-4658.2007.06056.x. [DOI] [PubMed] [Google Scholar]
- 25.Han JM, Kim Y, Lee JS, Lee CS, Lee BD, Ohba M, Kuroki T, Suh PG, Ryu SH. Localization of phospholipase D1 to caveolin-enriched membrane via palmitoylation: implications for epidermal growth factor signaling. Mol Biol Cell. 2002;13:3976–3988. doi: 10.1091/mbc.E02-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugars JM, Cellek S, Manifava M, Coadwell J, Ktistakis NT. Hierarchy of membrane-targeting signals of phospholipase D1 involving lipid modification of a pleckstrin homology domain. J Biol Chem. 2002;277:29152–29161. doi: 10.1074/jbc.M112169200. [DOI] [PubMed] [Google Scholar]
- 27.Park JB, Kim JH, Kim Y, Ha SH, Yoo JS, Du G, Frohman MA, Suh PG, Ryu SH. Cardiac phospholipase D2 localizes to sarcolemmal membranes and is inhibited by alpha-actinin in an ADP-ribosylation factor-reversible manner. J Biol Chem. 2000;275:21295–21301. doi: 10.1074/jbc.M002463200. [DOI] [PubMed] [Google Scholar]
- 28.Oishi K, Takahashi M, Mukai H, Banno Y, Nakashima S, Kanaho Y, Nozawa Y, Ono Y. PKN regulates phospholipase D1 through direct interaction. J Biol Chem. 2001;276:18096–18101. doi: 10.1074/jbc.M010646200. [DOI] [PubMed] [Google Scholar]
- 29.Lee CS, Kim IS, Park JB, Lee MN, Lee HY, Suh PG, Ryu SH. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat Cell Biol. 2006;8:477–484. doi: 10.1038/ncb1401. [DOI] [PubMed] [Google Scholar]
- 30.Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 31.Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- 33.Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, Davis JN. Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Ibeta for phosphatidylinositol-4-phosphate. J Cell Biochem. 2007;100:112–128. doi: 10.1002/jcb.21027. [DOI] [PubMed] [Google Scholar]
- 34.Siddhanta A, Backer JM, Shields D. Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J Biol Chem. 2000;275:12023–12031. doi: 10.1074/jbc.275.16.12023. [DOI] [PubMed] [Google Scholar]
- 35.Siddhanta A, Radulescu A, Stankewich MC, Morrow JS, Shields D. Fragmentation of the Golgi apparatus. A role for beta III spectrin and synthesis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:1957–1965. doi: 10.1074/jbc.M209137200. [DOI] [PubMed] [Google Scholar]
- 36.Dressman MA, Olivos-Glander IM, Nussbaum RL, Suchy SF. Ocrl1, a PtdIns(4,5)P(2) 5-phosphatase, is localized to the trans-Golgi network of fibroblasts and epithelial cells. J Histochem Cytochem. 2000;48:179–190. doi: 10.1177/002215540004800203. [DOI] [PubMed] [Google Scholar]
- 37.Roth MG, Bi K, Ktistakis NT, Yu S. Phospholipase D as an effector for ADP-ribosylation factor in the regulation of vesicular traffic. Chem Phys Lipids. 1999;98:141–152. doi: 10.1016/s0009-3084(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 38.Volpicelli-Daley LA, Li Y, Zhang CJ, Kahn RA. Isoform-selective effects of the depletion of ADP-ribosylation factors 1–5 on membrane traffic. Mol Biol Cell. 2005;16:4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiroyama M, Exton JH. Studies of the roles of ADP-ribosylation factors and phospholipase D in phorbol ester-induced membrane ruffling. J Cell Physiol. 2005;202:608–622. doi: 10.1002/jcp.20156. [DOI] [PubMed] [Google Scholar]
- 42.Hiroyama M, Exton JH. Localization and regulation of phospholipase D2 by ARF6. J Cell Biochem. 2005;95:149–164. doi: 10.1002/jcb.20351. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Mansilla B, Ha VL, Justin N, Wilkins AJ, Carpenter CL, Thomas GM. The differential regulation of phosphatidylinositol 4-phosphate 5-kinases and phospholipase D1 by ADP-ribosylation factors 1 and 6. Biochim Biophys Acta. 2006;1761:1429–1442. doi: 10.1016/j.bbalip.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Kuai J, Boman AL, Arnold RS, Zhu X, Kahn RA. Effects of activated ADP-ribosylation factors on Golgi morphology require neither activation of phospholipase D1 nor recruitment of coatomer. J Biol Chem. 2000;275:4022–4032. doi: 10.1074/jbc.275.6.4022. [DOI] [PubMed] [Google Scholar]
- 45.Sung TC, Altshuller YM, Morris AJ, Frohman MA. Molecular analysis of mammalian phospholipase D2. J Biol Chem. 1999;274:494–502. doi: 10.1074/jbc.274.1.494. [DOI] [PubMed] [Google Scholar]
- 46.Yang SF, Freer S, Benson AA. Transphosphatidylation by phospholipase D. J Biol Chem. 1967;242:477–484. [PubMed] [Google Scholar]
- 47.Chalifa-Caspi V, Eli Y, Liscovitch M. Kinetic analysis in mixed micelles of partially purified rat brain phospholipase D activity and its activation by phosphatidylinositol 4,5-bisphosphate. Neurochem Res. 1998;23:589–599. doi: 10.1023/a:1022422418388. [DOI] [PubMed] [Google Scholar]
- 48.Bi K, Roth MG, Ktistakis NT. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- 49.Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney DA, Siddhanta A, Shields D. Fragmentation and re-assembly of the Golgi apparatus in vitro. A requirement for phosphatidic acid and phosphatidylinositol 4,5-bisphosphate synthesis. J Biol Chem. 2002;277:3030–3039. doi: 10.1074/jbc.M104639200. [DOI] [PubMed] [Google Scholar]
- 51.Bethune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- 52.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manifava M, Thuring JW, Lim ZY, Packman L, Holmes AB, Ktistakis NT. Differential binding of traffic-related proteins to phosphatidic acid- or phosphatidylinositol (4,5)- bisphosphate-coupled affinity reagents. J Biol Chem. 2001;276:8987–8994. doi: 10.1074/jbc.M010308200. [DOI] [PubMed] [Google Scholar]
- 54.Yang JS, Gad H, Lee SY, Mironov A, Zhang L, Beznoussenko GV, Valente C, Turacchio G, Bonsra AN, Du G, Baldanzi G, Graziani A, Bourgoin S, Frohman MA, Luini A, Hsu VW. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat Cell Biol. 2008;10:1146–1153. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang JS, Lee SY, Spano S, Gad H, Zhang L, Nie Z, Bonazzi M, Corda D, Luini A, Hsu VW. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 2005;24:4133–4143. doi: 10.1038/sj.emboj.7600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coluccio A, Malzone M, Neiman AM. Genetic evidence of a role for membrane lipid composition in the regulation of soluble NEM-sensitive factor receptor function in Saccharomyces cerevisiae. Genetics. 2004;166:89–97. doi: 10.1534/genetics.166.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci USA. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kam Y, Exton JH. Dimerization of phospholipase d isozymes. Biochem Biophys Res Commun. 2002;290:375–380. doi: 10.1006/bbrc.2001.6146. [DOI] [PubMed] [Google Scholar]
- 59.Rudge SA, Zhou C, Engebrecht J. Differential regulation of Saccharomyces cerevisiae phospholipase D in sporulation and Sec14-independent secretion. Genetics. 2002;160:1353–1361. doi: 10.1093/genetics/160.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudge SA, Morris AJ, Engebrecht J. Relocalization of phospholipase D activity mediates membrane formation during meiosis. J Cell Biol. 1998;140:81–90. doi: 10.1083/jcb.140.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang CF, Cabot MC. Vasopressin-induced polyphosphoinositide and phosphatidylcholine degradation in fibroblasts. Temporal relationship for formation of phospholipase C and phospholipase D hydrolysis products. J Biol Chem. 1990;265:17468–17473. [PubMed] [Google Scholar]
- 62.Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJ. Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem Sci. 1998;23:200–204. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- 63.Pessin MS, Baldassare JJ, Raben DM. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of alpha-thrombin, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1990;265:7959–7966. [PubMed] [Google Scholar]
- 64.Heung YM, Postle AD. The molecular selectivity of phospholipase D in HL60 granulocytes. FEBS Lett. 1995;364:250–254. doi: 10.1016/0014-5793(95)00399-t. [DOI] [PubMed] [Google Scholar]
- 65.Billah MM, Eckel S, Mullmann TJ, Egan RW, Siegel MI. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989;264:17069–17077. [PubMed] [Google Scholar]
- 66.Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 68.Sonoda H, Okada T, Jahangeer S, Nakamura S. Requirement of phospholipase D for ilimaquinone-induced Golgi membrane fragmentation. J Biol Chem. 2007;282:34085–34092. doi: 10.1074/jbc.M705593200. [DOI] [PubMed] [Google Scholar]
- 69.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. J Biol Chem. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 70.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: why so many of them? Biochim Biophys Acta. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Rudge SA, Engebrecht J. Regulation and function of PLDs in yeast. Biochim Biophys Acta. 1999;1439:167–174. doi: 10.1016/s1388-1981(99)00092-x. [DOI] [PubMed] [Google Scholar]
- 72.Han GS, O’Hara L, Siniossoglou S, Carman GM. Characterization of the yeast DGK1-encoded CTP-dependent diacylglycerol kinase. J Biol Chem. 2008;283:20443–20453. doi: 10.1074/jbc.M802866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, Bankaitis VA. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudge SA, Sciorra VA, Iwamoto M, Zhou C, Strahl T, Morris AJ, Thorner J, Engebrecht J. Roles of phosphoinositides and of Spo14p (phospholipase D)-generated phosphatidic acid during yeast sporulation. Mol Biol Cell. 2004;15:207–218. doi: 10.1091/mbc.E03-04-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukherjee S, Chiu R, Leung SM, Shields D. Fragmentation of the Golgi apparatus: an early apoptotic event independent of the cytoskeleton. Traffic. 2007;8:369–378. doi: 10.1111/j.1600-0854.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 76.Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, Rosen A. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149:603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiu R, Novikov L, Mukherjee S, Shields D. A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol. 2002;159:637–648. doi: 10.1083/jcb.200208013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riebeling C, Bourgoin S, Shields D. Caspase cleavage of phospholipase D1 in vitro alters its regulation and reveals a novel property of the “loop” region. Biochim Biophys Acta. 2008;1781:376–382. doi: 10.1016/j.bbalip.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Puthenveedu MA, Linstedt AD. Subcompartmentalizing the Golgi apparatus. Curr Opin Cell Biol. 2005;17:369–375. doi: 10.1016/j.ceb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Ellis MA, Potter BA, Cresawn KO, Weisz OA. Polarized biosynthetic traffic in renal epithelial cells: sorting, sorting, everywhere. Am J Physiol Renal Physiol. 2006;291:F707–713. doi: 10.1152/ajprenal.00161.2006. [DOI] [PubMed] [Google Scholar]
- 81.Su W, Yeku O, Olepu S, Genna A, Park JS, Ren H, Du G, Gelb MH, Morris AJ, Frohman MA. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol Pharmacol. 2009;75:437–446. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]