Summary
There is a growing acceptance that tumor-infiltrating myeloid cells play an active role in tumor growth and mast cells are one of the earliest cell types to infiltrate developing tumors. Mast cells accumulate at the boundary between healthy tissues and malignancies and are often found in close association with blood vessels within the tumor microenvironment. They express many pro-angiogenic compounds, and may play an early role in angiogenesis within developing tumors. Mast cells also remodel extracellular matrix during wound healing, and this function is subverted in tumor growth, promoting tumor spread and metastasis. In addition, mast cells modulate immune responses by dampening immune rejection or directing immune cell recruitment, depending on local stimuli. In this review, we focus on key roles for mast cells in angiogenesis, tissue remodeling and immune modulation and highlight recent findings on the integral role that mast cells play in tumor growth. New findings suggest that mast cells may serve as a novel therapeutic target for cancer treatment and that inhibiting mast cell function may lead to tumor regression.
Keywords: Mast cells, angiogenesis, remodeling, immune-modulation, cancer, immunity
1. Introduction
The rate of development and growth of tumors is regulated by a delicate balance between pro- and anti-tumorigenic effects, stimulated by the tumor cells themselves, as well as the surrounding microenvironment. Local inflammation at the site of tumor growth results in the accumulation of a variety of cell types, and it is now generally accepted that these cell populations are intimately linked to the kinetics of tumor growth. Infiltrating cell populations include myeloid cells such as macrophages, neutrophils, eosinophils, mast cells and dendritic cells [1], as well as T cells, NK cells and endothelial precursors, many of which are highlighted elsewhere in this journal. As recently reviewed, there is a growing acceptance in the field that bone marrow-derived myeloid cells can play an active role in tumor growth and angiogenesis [1].
Mast cells were recognized to infiltrate the interface between developing tumors and healthy tissues as early as 1891 by Westphal, a student of Paul Ehrlich, using early metachromatic staining techniques on primary tumors [2]. Since that time, they have been found to accumulate around and within many types of solid cancers and recently, mast cell function in developing tumors has been extensively reviewed, with varying suggestions that they may shift the balance either in favour of or against tumor growth.
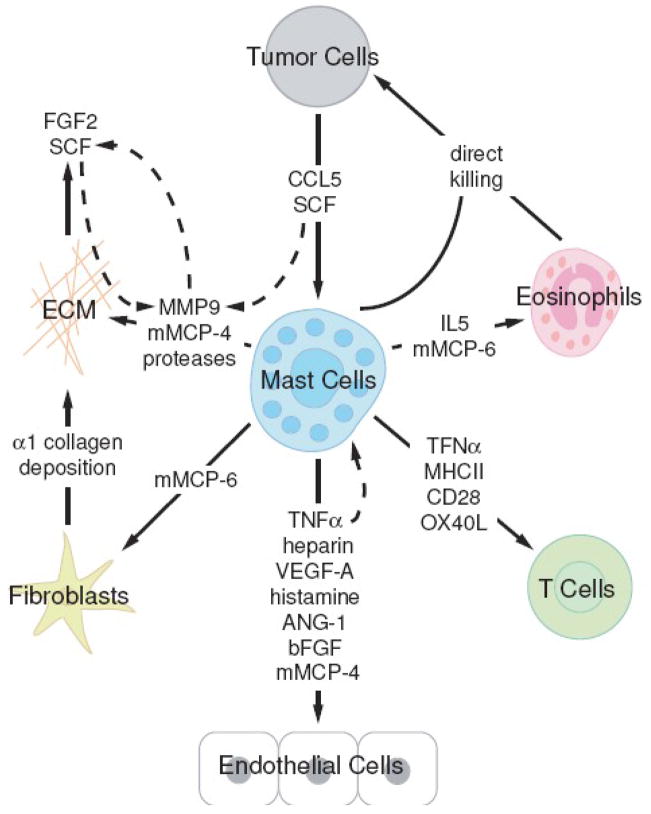
Potential mast cell effects on tumor growth can be categorized as either direct effects on tumor cells, such as mast cell-mediated cytotoxicity, or indirect effects such as mast cell-directed angiogenesis, tissue remodelling of the neighboring environment and immune cell recruitment. These functions are often interlinked and difficult to separate as will be highlighted in this review. While classically, direct mast cell-mediated killing of tumor cells has been the focus of research and plays an obvious role in tumor growth, here we have chosen to focus on three novel areas of mast cell interactions with tumor cells; 1) mast cell effects on tumor angiogenesis, 2) mast cell-mediated tissue remodelling and 3) mast cell-dependent immune regulation via recruitment of immune cells and immunosuppression. The goal of this review is to provide a critical evaluation of the literature, suggesting a role for mast cells in tumor progression and highlight recent advances that suggest they may be viable therapeutic targets for cancer treatment. We will highlight the interplay between mast cells and infiltrating cell types in the tumor environment and the interactions that occur between the released molecules within the local stroma (Figure 1).
Figure 1. Schematic representation of interactions between mast cells and cells within the tumor microenvironment.
Developing tumor cells secrete a number of molecules promoting mast cell recruitment. Within the tumor microenvironment, mast cells release a range of mediators affecting tumor cell survival, as well as remodeling of local tissues and recruitment of immune effector cells. Local cells and infiltrating populations also produce and secrete their own subsets of molecules, creating a complex set of interactions. This figure highlights the cell types and molecules mentioned in this review.
2. Mast cells in the tumor microenvironment
Mast cells were first described in 1878 [3] and, like all hematopoietic lineage cells, originate from hematopoietic stem cells (HSCs) in the bone marrow. They are atypical however, in that they branch off very early from HSCs and exit the bone marrow as committed, but undifferentiated, precursors before trafficking through the circulation to their target tissues, where they ultimately undergo terminal differentiation [4;5]. Mature mast cells populate most tissues, but are found in highest numbers in the skin, airways and digestive tract [6], where they are thought to act as a first line of defense against infiltrating pathogens and parasites. Over the past century, studies of mast cell functions have revealed diverse roles in tissue remodelling during wound healing and chronic asthma [7], as well as inflammatory roles in conditions such as arthritis [8], allergy and asthma [9].
Data on mast cell function in developing tumors has largely resulted from mouse models of cancer, with complementary, correlative studies in human patients. As such, it is important to note that important differences exist between human and mouse mast cell subsets. In mice, mast cells are broadly distinguished as the short-lived mucosal or long-lived connective tissue subtypes based on: 1) their location 2) their complement of proteases, and 3) their growth factor requirements [10]. Human mast cells are generally distinguished as either those containing both chymase and tryptase, or those containing tryptase alone. We mention the subtype designation differences between mouse and human to draw attention to the variation across species. However, it should be noted that extensive work has demonstrated plasticity between mast cell subsets in tissues [11–13], demonstrating that subtype classifications are not rigid and may be shifting within the tumor microenvironment. Thus, while characterization of mast cell subtypes within the tumor microenvironment may be useful in describing the cellular infiltrate, these classifications should not be considered to remain static throughout the development of a tumor.
Similar to the subtype distinctions between human and mouse mast cells, there are differences between the growth factors required for precursor expansion, with IL-3 driving development in mouse, and IL-6 in addition to IL-3 driving development in humans [14;15]. Differences also exist in the release of certain mediators such as TNFαmurine mast cells are suggested to be a more prominent source than human mast cells) [16;17], and IL-5 (human mast cells produce larger amounts at sites of allergic inflammation) [18]. Differences in growth factor requirements and effector molecule expression may have major effects in the tumor environment and should be considered when applying data from mouse models to the human population.
Mast cells are now recognized as an early and persistent infiltrating cell type in many tumors, often entering before significant tumor growth and angiogenesis have occurred. They have been shown to accumulate in and around adenomatous polyps (precursors to invasive colon cancer) [19] and skin dysplasias [20] prior to tumor development and around many developing tumors, particularly malignant melanoma [21], breast carcinoma [22] and colorectal carcinoma [23]. Much of the speculation on mast cell function in tumors results from findings in wound healing, allergic asthma and parasite infection, where their functions are generally better understood. However, recent work has focussed on the role of the mast cell within the tumor microenvironment, and their roles in cancer are becoming clearer.
Mast cells migrate toward supernatants from a number of tumorigenic cell lines, but not from primary cells or non-tumorigenic cell lines, suggesting tumor-intrinsic factors in mast cell recruitment across a range of tumor types [24]. More recently, stem cell factor (SCF) produced by tumor cells in vivo has been implicated in mast cell accumulation at the periphery of developing tumors [25;26]. SCF over-expression in developing mammary tumors increases mast cell accumulation at local sites of tumor growth, whereas inhibition of SCF expression results in decreased mast cell accumulation and decreased angiogenesis [25]. In addition to tumor-specific homing, mast cell progenitors home constitutively to mucosal tissues including the gut [27;28] and mast cell accumulation also occurs at sites of inflammation in allergic asthma, wound healing and parasite infection [29;30]. Thus, the tissue site of tumor development and localized inflammation also likely contribute to mast cell recruitment via tumor-independent pathways.
Intriguingly, the majority of reports on mast cell accumulation in tumors show the presence of mast cells predominantly at the tumor periphery, at the interface with healthy tissues, rather than within the tumor [21;31–37]. Often these cells are associated with vasculature in the healthy regions surrounding malignant tissues, leading to the suggestions that mast cells play a pro-angiogenic role. The scarcity of mast cells within the tumor core has been suggested to be a histology artefact, resulting from mast cell degranulation leading to “ghosts” following staining [38;39]. However, as peripheral mast cell distribution has been reported in studies using a wide variety of histological stains ranging from toluidine blue and chloracetate esterase to immuno-staining for tryptase, VEGF and other markers, this seems unlikely to be true in all cases.
Peripheral mast cell localization suggests that recruitment occurs either from a) resident mast cells migrating from neighbouring healthy tissue or, b) de novo recruitment of mast cell progenitors via healthy vasculature close to the tumor site (but not through tumor vasculature) or both. While we recently showed that efficient mast cell migration is a critical feature for their function in the tumor environment in the gastrointestinal tract [19], the original location of these migrating mast cell populations during active tumorigenesis has not been addressed. Few studies, if any, have specifically investigated the origins of tumor mast cells (i.e. local resident migration vs. blood infiltrate), and these findings could have important implications on future mast cell-based tumor therapies.
Difficulties interpreting the significance of mast cells within tumors have been compounded by the fact that many reports have failed to characterize them as potential infiltrating cells. As we have shown, many of the tumor-infiltrating stromal cell populations that have been studied express mast cell markers [19]. For example, reports of immature myeloid cells (iMCs) recruited to the front of developing tumors described these cells as expressing CD34, CCR1, MMP2 and MMP9 [40], all of which are expressed by mast cells at different stages in development [41–45]. This is just one example of the emerging cell types thought to play a role in tumor growth, and we suggest that in many reports mast cells may, in fact, be the key support cells within the tumor. In addition to the afore mentioned molecules, mast cells also express CD45, c-kit, Sca-1 and low levels of CD11b [42;46;47], all of which are shared with other groups of tumor infiltrating cells, making it difficult to compile findings on mast cell association with tumor progression (Table 1). The variability across reports makes it understandably hard to predict the direct effects of tailored drug treatments aimed at modifying specific infiltrating cell functions.
Table 1.
Mast cell-expressed markers shared with alternate tumor infiltrating cell populations
Human Patient Samples
Difficulties aside, while it is clear that mast cells accumulate around many types of tumors, there are conflicting reports on the prognostic value of mast cell numbers within tumors (as assessed from biopsies) on patient survival [23]. In the majority of human tumors, higher mast cell numbers are associated with greater vascularity, and sometimes (but not always) decreased patient survival [48;49]. A large study of 331 rectal cancer patients found that the presence of low mast cells numbers (0–3 in 30 fields) in tumor samples was associated with better patient survival [50]. In addition, several studies of mast cell infiltration in Hodgkin lymphoma also demonstrated a poor prognosis associated with infiltration of CD30L-expressing mast cells [51]. Intriguingly, this effect appears to occur independent of mast cell-mediated effects on angiogenesis [52], potentially via direct interaction between mast cells and Hodgkin and Reed-Sternberg cells expressing CD30.
However, the association of mast cell infiltration with poor prognosis depends on the type of cancer studied and several large human studies have demonstrated the opposite effect, showing a correlation between high mast cell counts and improved prognosis. In two recent studies, one of 187 breast carcinoma [53] and the other of 348 invasive breast carcinoma samples [54], high mast cells numbers in tumor stroma was associated with an improved prognosis. A study of 175 patients with non-small-cell lung cancer also demonstrated a correlation between improved prognosis and the presence of mast cells in islets, but not surrounding stroma [55]. These studies suggest an anti-tumor role for mast cells and likely reflect the ability of infiltrating mast cells to mediate direct tumor killing. It suggests that in certain tumor environments, anti-tumor functions may supersede the tumor promoting functions that will be discussed further in this review. It is important to acknowledge these findings when discussing mast cell-targeted therapeutics, as mast cells may play very different roles in different tumor types and stages. Thus, therapies interfering with mast cell function may have very different outcomes across a range of cancer types.
Mouse Tumor Models
In mouse models, mast-cell deficient W/Wv mice exhibit decreased tumor growth at early stages in a B16 melanoma model, which could be recovered by reconstituting mast cell populations [56]. However, to date very few studies have evaluated the growth of different tumor types in W/Wv or other mast cell deficient mouse models, and it remains unclear whether this observation is universal across a range of tumors or applies directly to human tumor growth. Interestingly, similar to the conflicting roles for mast cells in human cancer patients, a recent article on the effects of imatinib mesylate (Gleevec) in a mammary adenocarcinoma tumor model in mice demonstrated increased tumor growth when mast cell-derived heparin was inhibited [33].
These studies highlight that mast cells play a complex role in the tumor environment, and a better understanding of mast cell function will undoubtedly improve both the treatment of cancer and patient health. However, currently specific knowledge in many types of human cancers and factors involved in balancing mast cell pro- and anti-tumor effects remains lacking.
3. Mast cells and angiogenesis
Angiogenesis is integral to tumor development, providing nutrients necessary for cell growth [57;58] and is required for tumor growth > 1 mm3 [59]. In addition, angiogenesis is a necessary step for metastasis of solid tumors to distal sites in the body [60]. For many years, mast cells have been known to accumulate around blood vessels during homeostasis [61–63] and produce pro-angiogenic compounds [31;64], leading to suggestions that they play an important role in tumor angiogenesis as reviewed in [1;39;48;49;59]. Recent studies have also suggested that mast cells play an important role in the “angiogenic switch” during tumor growth [34].
Mast cells synthesize many pro-angiogenic molecules, including VEGF [65], bFGF [64], heparin [66], histamine [67], TNFα [68] and various proteases, as well as ANG-1 [69] (Table 2). In addition, in vitro studies have shown that addition of whole mast cells can drive neovascularization in the CAM assay [70;71], a rat mesentery assay [72] and a limb ischemic reperfusion assay [73]. These studies have demonstrated that vascularization requires mast cell granular components, while the presence of mast cell membranes alone is insufficient [71]. Several studies have also suggested that SCF, which attracts mast cells into the tumor environment, also increases mast cell release and production of both VEGF and bFGF [25].
Table 2.
Mast cell-expressed factors involved in angiogenesis, tissue remodelling and immune modulation within the developing tumor microenvironment
Coussens et al. [34] suggest that mast cell infiltration into a developing tumor triggers an “angiogenic switch”; at early stages in tumor development, mast cells direct angiogenesis in the developing tumor, while at later stages tumor cells take control of growth and angiogenesis and growth becomes mast cell-independent. Using a transgenic mouse model expressing HPV16 early region genes in basal keratinocytes, which develop tumors over the course of the first year of life, they showed accumulation of mast cells increased at the hyperplastic stage of tumor growth, with localization proximal to small blood vessels [34]. Mast cell density increased near angiogenic lesions, during stages of intense angiogenesis, but remained absent within the tumor mass itself [34]. The mast cells residing near developing tumors expressed mMCP-4 and mMCP-6, and addition of mMCP-4 alone was sufficient to stimulate an angiogenic phenotype when coincubated with a hyperplastic skin sample in vitro, while mMCP-6 exhibited a role in tissue remodelling, as discussed in the next section [34]. This study highlights the key role that mast cell-derived mMCP-4 and mMCP-6 play in tumor growth and spread.
Recently, we showed the presence of both connective tissue and mucosal (intraepithelial) mast cell subtypes in developing colorectal polyps [19]. The screening of human polyp tissue samples demonstrated an unusually high frequency of mast cells in developing polyps compared to neighboring healthy tissue. To confirm a functional role for mast cells, we used APCΔ468 mice and a transgenic mouse overexpressing an inducible, stabilized β-catenin in intestinal enterocytes; two independent hereditary models of polyposis [19]. In both strains of mice, developing polyps were rapidly infiltrated by CD34+ mast cells, in a T and B cell-independent manner (demonstrated by crossing to Rag2−/− mice). Staining confirmed the presence of both connective tissue and intraepithelial, mucosal type mast cells in the developing preneoplastic lesions [19]. Interestingly, the latter appeared to dominate at the site of newly developing polyps, while the former were more prevalent in the stroma at later stages of invasive tumor development, suggesting a switch in mast cell subtype during tumor development. Pharmacological intervention with anti-TNFα antibodies resulted in fewer polyps, reduced polyp growth and decreased mast cell infiltration, even when administered after polyp formation had commenced [19]. Reconstitution of Wsh/Wsh mast cell-deficient mice with Cd34−/−Cd43−/− bone marrow also resulted in a decrease in polyp growth, compared to mice reconstituted with wildtype bone marrow [19]. Our previous work demonstrated highly impaired mast cell migration in the absence of CD34 and CD43 expression [41] and these findings suggest a requirement for mast cell precursor migration from the bone marrow to the tumor site to promote and sustain early tumor growth. This study also demonstrates the important role of TNFα in mast cell function within early neoplastic growths, and suggests that inhibition of mast cell function or migration in developed tumors may be sufficient to slow growth and induce regression. In addition, a more recent study has shown that macrophage-derived TNFα has direct effects on promoting tumor growth by activating Wnt signalling in K19-Wnt1 transgenic mice [74]. Thus, inhibition of TNFα may have beneficial effects by suppressing both mast cell activation and tumor growth directly.
A similar study by Soucek et al. [35] suggests that CCL5 production in developing tumors triggers mast cell recruitment to the periphery of pancreatic islet tumors. Through transcriptional profiling of Myc-dependent inducible and reversible pancreatic β-cell carcinomas, this group demonstrated the induction of several chemokines in developing tumors, including CCL2 and CCL5 [35]. Since CCL5 acts as a mast cell chemoattractant [75], the group evaluated mast cell accumulation in developing pancreatic tumors. Myc activation resulted in a rapid recruitment of mast cells to the tissue surrounding the pancreas, although not into the islets themselves [35]. Tumor expansion was shown to be mast cell-dependent, with decreased tumor growth and increased tumor cell apoptosis in both Wsh/Wsh mast cell-deficient animals and in wild type mice following intervention using cromolyn, a common inhibitor of mast cell degranulation [35]. The tumor environment in animals lacking mast cells was hypoxic, whereas the presence of mast cells was correlated with extensive angiogenesis and growth. Importantly, addition of cromolyn after Myc-induction resulted in regression of tumors, demonstrating a continued requirement for mast cell degranulation in tumor maintenance [35]. These results combined with our findings, suggest targeting mast cells, even after tumor induction, as a strategy to trigger tumor regression and block tumor growth.
Finally, a study performed by Nakayama et al. [69] demonstrates a clear role for mast cell derived angiopoeitin 1 (Ang-1) in a mouse model of multiple myeloma. Ang-1 and VEGF-A expression were found in murine mast cells, along with high levels of VEGF-A expression by two plasmacytoma cell lines [69]. In implanted Matrigel plugs, vascularization was induced in the presence of bone marrow-derived mast cells and plasmacytoma cells, with little angiogenesis in the presence of each cell type individually and this effect was inhibited by addition of antibodies against Ang-1, VEGF-A or both [69]. Following injection into Balb/c nude mice, mast cells greatly increased both plasmacytoma tumor size and vascularization [69], suggesting mast cell-derived Ang-1 plays a potent role in promoting angiogenesis in multiple myeloma.
These recent studies demonstrate the effectiveness of mast cell-targeted interventions in several tumor models, and confirm that mast cells are not merely innocent bystanders in the tumor environment. Mast cells express a range of potent pro-angiogenic factors (Table 2), are associated with vessels in developing tumors in humans and have been shown to be integral in tumor angiogenesis in mouse models. Mast cell-targeted approaches could limit angiogenesis in developing tumors, resulting in increased tumor cell apoptosis and decreased tumor growth and metastasis throughout the body.
4. Mast cells and tissue remodelling
In addition to producing pro-angiogenic compounds, mast cells are major sources of proteases, which act on the surrounding extracellular matrix (ECM), and have been demonstrated to play a role in tissue remodelling during wound healing [7] and in autoimmune inflammatory arthritis [76] (Table 2). It has been suggested that in the context of developing tumors, the ability of mast cells to remodel tissues is subverted, disrupting surrounding ECM and increasing tumor spread [77]. In addition to providing room for tumor spread, the disruption of local ECM leads to the release of matrix-bound factors including SCF and FGF-2, and thereby increases endothelial cell migration and proliferation and likely promotes angiogenesis, tumor spread and growth [78–81].
In the study by Coussens et al. mentioned above [34], release of mMCP-4 (chymase) and mMCP-6 (tryptase) by mast cells were both demonstrated to play roles in tissue remodelling. mMCP-6 was found to stimulate dermal fibroblasts, inducing DNA synthesis in quiescent cells and also increased α-1 collagen production in MC-rich areas in vivo [34]. In addition, mMCP-4 was shown to activate progelatinase B (MMP9) early in tumor development, further inducing ECM remodelling. In turn, mMCP-4 and MMP9 activity led to release of bound pro-angiogenic compounds from the ECM, highlighting the tight link between tissue remodelling and angiogenesis [34]. For an extensive review of mast cell proteases (MCPs) and their functions see Stevens & Adachi [82].
Huang et al. demonstrated that tumor-derived SCF both recruits mast cells to the tumor environment and also activates them [26]. SCF-knockdown in an H22 hepatocarcinoma model led to decreased tumor growth, correlated with a reduction in mast cell accumulation in the developing tumors, suggesting a role for SCF in mast cell recruitment [26]. Interestingly, injection of bone marrow-derived, cultured mast cells directly into the tumor site was not sufficient to recover tumor growth. Thus, there is an additional requirement for SCF to activate recruited cells following their migration to the tumor site [26]. SCF-stimulated mast cells released active MMP9 into the local environment, disrupting ECM and released further matrix-bound SCF, acting as a positive feedback loop on mast cell activation within the tumor [26]. Activation by high levels of SCF in vitro led to upregulation of the proinflammatory genes such as IL-6, TNFα and VEGF, which could be inhibited by antibodies against the SCF receptor, c-kit [26]. In addition, mast cell presence resulted in a shift towards increased IL-10 expression, increased Treg cell numbers within the tumor and suppression of T lymphocytes and NK cells, suggesting roles in immune suppression, which will be discussed in the next section [26]. This study illustrates a clear feedback loop whereby mast cell recruitment by SCF can stimulate localized tissue remodelling, which in turn results in further SCF release and ultimately in modulation of local immune responses.
Mast cell roles in tissue remodelling have obvious beneficial effects in wound healing but, in the tumor environment, promote tumor growth and spread. In addition, as shown, their role in remodelling demonstrates obvious links to angiogenesis and immune regulation highlighting the diverse range of functions that mast cells can have in a developing tumor environment. Further, these findings suggest that local stimuli within the microenvironment can alter these functions to shift the immune response.
5. Mast cells and immune activation/suppression
Within the developing tumor environment, mast cells do not act alone, and while little data exists on their interactions with resident immune cells in cancer specifically, much is known about their ability to recruit and activate immune cells in other disease models [83;84]. Mast cells have been recognized for their ability to recruit eosinophils and neutrophils and to activate adaptive T and B cell responses. In addition, mast cells have been reported to express antigen presentation molecules, suggesting a potential role in initiating adaptive immunity, and thereby potentially modulating tumor rejection (Table 2)
Upon activation, mast cells express antigen presentation molecules, such as MHC class II and costimulatory molecule CD28, and activate T cells in vitro [85;86]. However, their relative importance as key antigen presenting cells in vivo remains controversial. Their presence at tissue boundaries ideally positions mast cells to be among the first immune cells encountering invading pathogens and parasites, and they have been suggested to play a role in initiating immune responses. Similarly, their presence at the boundaries of tumors could serve to initiate rejection by activating anti-tumoral T and NK cells, although it remains unclear whether this occurs.
Several findings suggest that the presence of mast cells may promote initiation of tumorigenesis. In the skin, mast cells degranulate in response to UV-B exposure, suggesting a potential link between UV exposure and skin cancer onset. Local mast cell degranulation leads to neutrophil recruitment [20], as well as UV-B induced elastosis [87]. In addition, mast cell degranulation is linked to localized UV-B-induced immunosuppression [21]. Following UV-B exposure, cis-urocanic acid (cis-UCA) and nerve growth factor (NGF) activate neuropeptide secretion from sensory nerves, which in turn induces mast cell degranulation [88]. Mast cells release TNFα and histamine [89], activating local keratinocytes to release PGE2. Finally, PGE2 triggers release of IL-10 by dendritic cells, which plays an immunosuppressive role [90]. These findings demonstrate that UV exposure results in a number of mast cell-mediated effects, including immune cell recruitment, suppression and facilitating tumor initiation. Interventions blocking mast cell degranulation during UV exposure may shift responses from immune suppression during early tumorigenic events and promote active immune responses against malignancies.
In addition to roles as antigen presenting cells, mast cells are able to recruit and activate various immune cells, particularly eosinophils. In an asthma model, we demonstrated that defective mast cell and eosinophil migration (through loss of CD34 expression), resulted in reduced infiltration of both cell types as well as macrophages and lymphocytes into the lung, suggesting an integral role in immune cell recruitment [91]. Intriguingly, eosinophils have also recently been suggested to play active roles in tumor rejection.
In a mouse model of melanoma metastasis, eosinophils infiltrate developing tumors in the lung, degranulate and cause tumor regression when present in a TH2-shifted environment [92]. Eosinophils migrate into the core of solid melanoma tumors and occupy the necrotic region of the tumor, suggesting a possible role in tumor cell killing [93]. In addition, MC-derived IL-5 promotes eosinophil survival and results in high eosinophil numbers in the fibrotic capsule surrounding many tumor types [94]. Intriguingly, mast cell-derived mMCP-6, which was mentioned in relation to tissue remodeling, was also recently shown to be critical for eosinophil recruitment during parasite infection, and may play a similar role in the tumor environment [95]. In vitro, eosinophils can initiate killing of tumor cells and decreased tumor growth has been observed in mouse models of eosinophilia [94]. The ability of eosinophils to play major roles in modulating immune cell recruitment and antigen presentation was also recently reviewed, revealing many similarities between mast cells and eosinophils [96]. We suggest that mast cells recruit eosinophils into developing tumors and modulate their ability to kill tumor cells. Thus, when considering the role that mast cells play in the developing tumor, it is also important to consider not only direct mast cell function, but also downstream effects of mast cell-mediated immune modulation.
In addition to interactions with eosinophils, mast cells also activate and modulate T cell function. Mast cell-derived lymphotoxin supports lymph node expansion during immune responses, while mast cell-derived TNFα promotes hypertrophy of draining lymph nodes [97] and modulates dendritic cell migration [98]. In addition, mast cells promote lymph node hypertrophy independent of TNFα release [99]. Intriguingly, mast cells also appear to interact with Treg cells, with reports that Treg cells recruit and activate mast cells during immune suppression [100], or alternatively may suppress mast cell degranulation depending on local stimuli [101].
A recent study suggests that mast cells activate T cells via TNFα release and through cell-cell interactions via OX40L [86]. Nakae et al. demonstrated expression of the B7 and CD28 costimulatory molecules and OX40L on bone marrow cultured mast cells in vitro. OX40L expression was also slightly upregulated on C57Bl/6 mast cells following activation by IgE and Ag [86]. In culture, T cell activation was promoted more by secreted TNFα, derived from mast cells, than membrane bound TNFα, suggesting mast cells may be able to modulate T cell activation at a distance in the tissue. Exposure to secreted TNFα also resulted in increased OX40 levels on CD3+ T cells [86]. In turn, the interaction between OX40 on T cells and OX40L on mast cells via direct cell-cell contact initiated T cell proliferation, which was inhibited with antibodies against OX40L [86]. These studies demonstrate several mechanisms whereby activated mast cells activate T cell responses through both direct and indirect interactions, which may carry over to T cell activity in the tumor microenvironment.
Most of the research supporting mast cell roles in immune modulation result from mouse studies in non-tumor situations. These studies have found a diverse range of interactions between mast cells and other hematopoietic cell types (many of which are being reviewed separately in this issue) and suggest a prominent role for mast cells not just as immune effectors, but also in modulating the immune response in the tumor microenvironment. Many of these complex interactions likely contribute to the differences seen across studies on whether mast cells promote or inhibit tumor growth.
6. Discussion
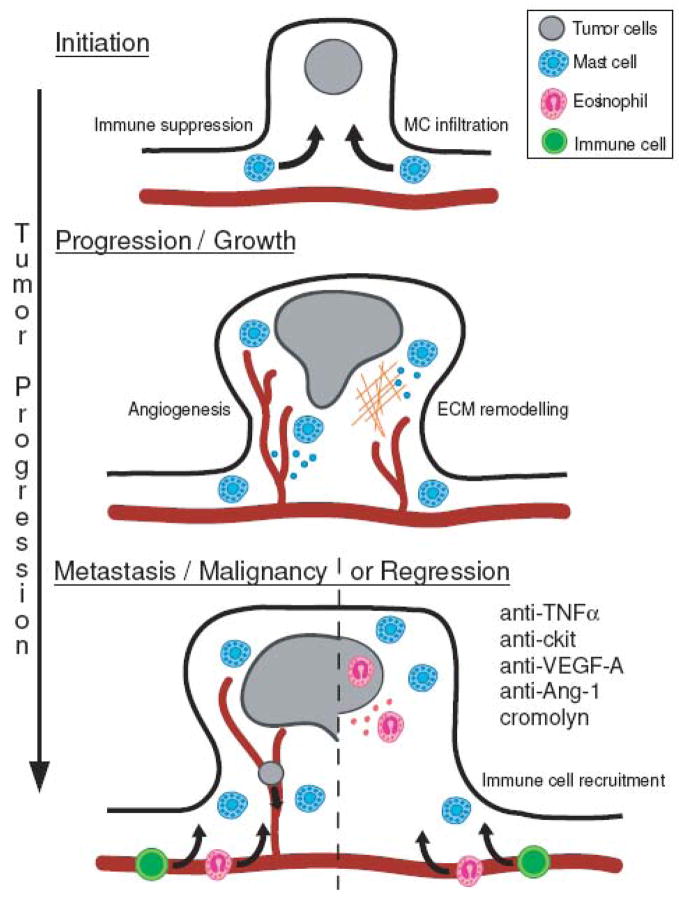
The significance of mast cell functions within the tumor environment are only beginning to be revealed and it is important to recognize that these roles likely vary based on tumor type, stage and interactions with other cells in the microenvironment. While mast cells can have roles in angiogenesis, remodeling and immune regulation in many situations, the ultimate result of all of these effects on tumor growth will vary depending on specific circumstances. Mast cells are recruited early in tumor development and play a key role in both angiogenesis and tissue remodeling, facilitating tumor initiation and growth. As tumor growth progresses, mast cells recruit immune cells or alternatively suppress anti-tumoral responses (Figure 2).
Figure 2. Schematic representation of mast cell functions at different stages in tumor progression, using colon polyp formation as an example.
Mast cell infiltration occurs rapidly following tumor initiation. Mast cells accumulate at the boundary between healthy tissue and the tumor and early in tumor growth mast cells play an active role in promoting angiogenesis and tissue remodeling. At later stages, mast cells induce immune suppression, promoting tumor growth or alternatively, recruitment of mature immune effector cells, leading to rejection. Interventions with antibodies recognizing TNFα c-kit, VEGF-A or Ang-1 or the chemical cromolyn induce regression, by blocking mast cell maintenance of the tumor.
Our ability to apply new knowledge of mast cell function in tumor growth is still limited by three factors; 1) differences between mouse and human mast cell function, 2) variability in staining and marker usage across studies and 3) the paucity of data demonstrating causal relationship between mast cells and many types of primary human tumors.
While many of the differences between human and mouse mast cells appear quite subtle, they can have major effects on the development of mast cell-directed therapies. It remains unclear whether the key molecules driving mast cell infiltration and activation in the mouse system will translate to the human system.
Differences in staining techniques and marker usage between reports make it difficult to determine which cell populations actually enter the tumor. As “novel” subsets are characterized within tumors based on varying cell marker combinations, distinctions between these populations become increasingly difficult. These differences, in turn, make it difficult to assign functions to specific cell types and hard to predict the effects of tailored drug regimens.
Finally, as with all mouse model research, findings must be translated into human patients. Data on mast cell presence and function in a range of human tumors will provide insight into both mast cell function and ultimately the treatment of human disease.
7. Concluding Remarks
Recognition of mast cells as integral to tumor growth opens an avenue for novel approaches to cancer therapies or combination-therapies to decrease tumor growth and metastasis in patients. As we have highlighted in this article, preliminary studies using antibodies against c-kit [26], TNFα [19], VEGF-A [69] or Ang-1 [69] or administration of cromolyn [35] in mouse models demonstrated promising results, even if administered after the initiation of tumor development. Using compounds known to modify mast cell function and already in use for the treatment of human diseases (e.g. STI571 (imatinib mesylate, Gleevec) and cromolyn, it may be possible to get mast cell-targeted therapies into the clinic quickly for cancer treatment. STI571 is already in use for certain gastrointestinal stromal tumors and some varieties of mastocytosis, while cromolyn has been administered with very limited side effects for various allergic inflammatory conditions [102–104]. A number of new cancer therapeutics, which target c-kit and other mast cell-associated molecules, was also recently reviewed by Galinsky & Nechushtan [105]. These include sorafenib, suntinib, pazopanib, axitinib and dasatinib, which are all targeted against c-kit and are currently in clinical trials. Clinical trial findings using these new drugs have reported promising effects demonstrating that targeting mast cells in a viable approach to treating cancer.
In addition, compiling data on tumor growth in individuals already taking drugs affecting mast cell function for pre-existing conditions would also provide insight into mast cell function in human cancers. This analysis could provide pilot data on the applicability of mast cell-based interventions and provide warning of potential side effects in future drug trials.
By characterizing cells within tumors using a range of markers, and data derived from mouse models of cancer, we gain an improved understanding of the diverse interactions that occur with a growing tumor. Through this understanding, the development of novel therapies to alter mast cell function in the tumor environment could inhibit angiogenesis and tissue remodelling, reducing tumor growth and activate the immune system to clear developing malignancies, while inhibiting mast cell-mediated immune suppression and promoting direct killing of tumor cells by mast cells.
Acknowledgments
The authors would like to thank Vicki MacDonald for assistance in preparing this manuscript and producing the figures. SM holds a Canadian Institutes of Health Research (CIHR) and the Heart and Stroke Foundation of Canada Transfusion Science Fellowship from the Centre for Blood Research (CBR) at the University of British Columbia. KK is the recipient of an American Society for Cancer Research Award. KMM is a Michael Smith Foundation for Health Research Scholar (Senior) and CBR Member. This work was funded by grants from NIH (R01-CA104547), the Canadian Institutes of Health Research (MOP-84545) and the Allergen Network Centre of Excellence (Project 3.13).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 2.Westphal E. In: Uber mastzellen. Ehrlich P, editor. Ferbenanlytische Untersuchungen Zur Histologie Und Klinik Des Blutes; Hirschwald, Berlin: 1891. p. 17. [Google Scholar]
- 3.Ehrlich P. Thesis. Leipzig University; 1878. Beitrage Zur Theorie Und Praxis Der Histologischen Farbung. [Google Scholar]
- 4.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- 6.Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18:751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Hebda PA, Collins MA, Tharp MD. Mast cell and myofibroblast in wound healing. Dermatol Clin. 1993;11:685–696. [PubMed] [Google Scholar]
- 8.Nigrovic PA, Lee DM. Mast cells in inflammatory arthritis. Arthritis Res Ther. 2005;7:1–11. doi: 10.1186/ar1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce JA. The role of mast cells in asthma. Prostaglandins Leukot Essent Fatty Acids. 2003;69:195–205. doi: 10.1016/s0952-3278(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu H, Nagakui Y, Tsuchiya K, Horii Y. Demonstration of chymotryptic and tryptic activities in mast cells of rodents: comparison of 17 species of the family Muridae. J Comp Pathol. 2001;125:76–79. doi: 10.1053/jcpa.2001.0469. [DOI] [PubMed] [Google Scholar]
- 11.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levi-Schaffer F, Austen KF, Gravallese PM, Stevens RL. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci USA. 1986;83:6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurish MF, Pear WS, Stevens RL, Scott ML, Sokol K, Ghildyal N, Webster MJ, Hu X, Austen KF, Baltimore D. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity. 1995;3:175–186. doi: 10.1016/1074-7613(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 14.Nakahata T, Toru H. Cytokines regulate development of human mast cells from hematopoietic progenitors. Int J Hematol. 2002;75:350–356. doi: 10.1007/BF02982123. [DOI] [PubMed] [Google Scholar]
- 15.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–112. doi: 10.1385/1-59259-967-2:105. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff SC, Lorentz A, Schwengberg S, Weier G, Raab R, Manns MP. Mast cells are an important cellular source of tumour necrosis factor alpha in human intestinal tissue. Gut. 1999;44:643–652. doi: 10.1136/gut.44.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. 1999;29:1496–1503. doi: 10.1002/(SICI)1521-4141(199905)29:05<1496::AID-IMMU1496>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimbaldeston MA, Finlay-Jones JJ, Hart PH. Mast cells in photodamaged skin: what is their role in skin cancer? Photochem Photobiol Sci. 2006;5:177–183. doi: 10.1039/b504344a. [DOI] [PubMed] [Google Scholar]
- 21.Ch’ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19:149–159. doi: 10.1038/modpathol.3800474. [DOI] [PubMed] [Google Scholar]
- 22.Amini RM, Aaltonen K, Nevanlinna H, Carvalho R, Salonen L, Heikkila P, Blomqvist C. Mast cells and eosinophils in invasive breast carcinoma. BMC Cancer. 2007;7:165. doi: 10.1186/1471-2407-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2007 doi: 10.1111/j.1440-1746.2007.05009.x. [DOI] [PubMed] [Google Scholar]
- 24.Poole TJ, Zetter BR. Stimulation of rat peritoneal mast cell migration by tumor-derived peptides. Cancer Res. 1983;43:5857–5861. [PubMed] [Google Scholar]
- 25.Zhang W, Stoica G, Tasca SI, Kelly KA, Meininger CJ. Modulation of tumor angiogenesis by stem cell factor. Cancer Res. 2000;60:6757–6762. [PubMed] [Google Scholar]
- 26.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, Feng ZH. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, Koni PA, Gurish MF. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood. 2005;105:4308–4313. doi: 10.1182/blood-2004-09-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, Austen KF. Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J Exp Med. 2001;194:1243–1252. doi: 10.1084/jem.194.9.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abonia JP, Hallgren J, Jones T, Shi T, Xu Y, Koni P, Flavell RA, Boyce JA, Austen KF, Gurish MF. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, Gurish MF. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA. 2007;104:20478–20483. doi: 10.1073/pnas.0709651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawatsubashi M, Yamada T, Fukushima N, Mizokami H, Tokunaga O, Shin T. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch. 2000;436:243–248. doi: 10.1007/s004280050037. [DOI] [PubMed] [Google Scholar]
- 32.Toth T, Toth-Jakatics R, Jimi S, Takebayashi S. Increased density of interstitial mast cells in amyloid A renal amyloidosis. Mod Pathol. 2000;13:1020–1028. doi: 10.1038/modpathol.3880184. [DOI] [PubMed] [Google Scholar]
- 33.Samoszuk M, Corwin MA. Acceleration of tumor growth and peri-tumoral blood clotting by imatinib mesylate (Gleevec) Int J Cancer. 2003;106:647–652. doi: 10.1002/ijc.11282. [DOI] [PubMed] [Google Scholar]
- 34.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 36.Humphreys TR, Monteiro MR, Murphy GF. Mast cells and dendritic cells in basal cell carcinoma stroma. Dermatol Surg. 2000;26:200–3. doi: 10.1046/j.1524-4725.2000.09207.x. discussion 203–4. [DOI] [PubMed] [Google Scholar]
- 37.Aoki M, Pawankar R, Niimi Y, Kawana S. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int Arch Allergy Immunol. 2003;130:216–223. doi: 10.1159/000069515. [DOI] [PubMed] [Google Scholar]
- 38.Claman HN, Choi KL, Sujansky W, Vatter AE. Mast cell “disappearance” in chronic murine graft-vs-host disease (GVHD)-ultrastructural demonstration of “phantom mast cells”. J Immunol. 1986;137:2009–2013. [PubMed] [Google Scholar]
- 39.Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355–371. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, Minato N, Taketo MM. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 41.Drew E, Merzaban JS, Seo W, Ziltener HJ, McNagny KM. CD34 and CD43 inhibit mast cell adhesion and are required for optimal mast cell reconstitution. Immunity. 2005;22:43–57. doi: 10.1016/j.immuni.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Drew E, Merkens H, Chelliah S, Doyonnas R, McNagny KM. CD34 is a specific marker of mature murine mast cells. Exp Hematol. 2002;30:1211. doi: 10.1016/s0301-472x(02)00890-1. [DOI] [PubMed] [Google Scholar]
- 43.Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999;162:5528–5535. [PubMed] [Google Scholar]
- 44.Tanaka A, Arai K, Kitamura Y, Matsuda H. Matrix metalloproteinase-9 production, a newly identified function of mast cell progenitors, is downregulated by c-kit receptor activation. Blood. 1999;94:2390–2395. [PubMed] [Google Scholar]
- 45.Juremalm M, Nilsson G. Chemokine receptor expression by mast cells. Chem Immunol Allergy. 2005;87:130–144. doi: 10.1159/000087640. [DOI] [PubMed] [Google Scholar]
- 46.Berger SA, Mak TW, Paige CJ. Leukocyte common antigen (CD45) is required for immunoglobulin E-mediated degranulation of mast cells. J Exp Med. 1994;180:471–476. doi: 10.1084/jem.180.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 48.Ribatti D, Vacca A, Nico B, Crivellato E, Roncali L, Dammacco F. The role of mast cells in tumour angiogenesis. Br J Haematol. 2001;115:514–521. doi: 10.1046/j.1365-2141.2001.03202.x. [DOI] [PubMed] [Google Scholar]
- 49.Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett. 2008;269:1–6. doi: 10.1016/j.canlet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 50.Fisher ER, Paik SM, Rockette H, Jones J, Caplan R, Fisher B. Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01) Hum Pathol. 1989;20:159–163. doi: 10.1016/0046-8177(89)90180-9. [DOI] [PubMed] [Google Scholar]
- 51.Molin D, Edstrom A, Glimelius I, Glimelius B, Nilsson G, Sundstrom C, Enblad G. Mast cell infiltration correlates with poor prognosis in Hodgkin’s lymphoma. Br J Haematol. 2002;119:122–124. doi: 10.1046/j.1365-2141.2002.03768.x. [DOI] [PubMed] [Google Scholar]
- 52.Glimelius I, Edstrom A, Fischer M, Nilsson G, Sundstrom C, Molin D, Amini RM, Enblad G. Angiogenesis and mast cells in Hodgkin lymphoma. Leukemia. 2005;19:2360–2362. doi: 10.1038/sj.leu.2403992. [DOI] [PubMed] [Google Scholar]
- 53.Aaltomaa S, Lipponen P, Papinaho S, Kosma VM. Mast cells in breast cancer. Anticancer Res. 1993;13:785–788. [PubMed] [Google Scholar]
- 54.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, Gelmon K, Chia S, Hayes M. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod Pathol. 2004;17:690–695. doi: 10.1038/modpathol.3800094. [DOI] [PubMed] [Google Scholar]
- 55.Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 56.Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42:48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- 57.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 58.Hamada J, Cavanaugh PG, Lotan O, Nicolson GL. Separable growth and migration factors for large-cell lymphoma cells secreted by microvascular endothelial cells derived from target organs for metastasis. Br J Cancer. 1992;66:349–354. doi: 10.1038/bjc.1992.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ribatti D, Crivellato E, Roccaro AM, Ria R, Vacca A. Mast cell contribution to angiogenesis related to tumour progression. Clin Exp Allergy. 2004;34:1660–1664. doi: 10.1111/j.1365-2222.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- 60.Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 61.Eady RA, Cowen T, Marshall TF, Plummer V, Greaves MW. Mast cell population density, blood vessel density and histamine content in normal human skin. Br J Dermatol. 1979;100:623–633. doi: 10.1111/j.1365-2133.1979.tb08065.x. [DOI] [PubMed] [Google Scholar]
- 62.Rhodin JA, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989;21:1–34. [PubMed] [Google Scholar]
- 63.Rakusan K, Sarkar K, Turek Z, Wicker P. Mast cells in the rat heart during normal growth and in cardiac hypertrophy. Circ Res. 1990;66:511–516. doi: 10.1161/01.res.66.2.511. [DOI] [PubMed] [Google Scholar]
- 64.Qu Z, Huang X, Ahmadi P, Stenberg P, Liebler JM, Le AC, Planck SR, Rosenbaum JT. Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int Arch Allergy Immunol. 1998;115:47–54. doi: 10.1159/000023829. [DOI] [PubMed] [Google Scholar]
- 65.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.JORPES JE. Heparin: a mucopolysaccharide and an active antithrombotic drug. Circulation. 1959;19:87–91. doi: 10.1161/01.cir.19.1.87. [DOI] [PubMed] [Google Scholar]
- 67.Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 68.Okayama Y, Ono Y, Nakazawa T, Church MK, Mori M. Human skin mast cells produce TNF-alpha by substance P. Int Arch Allergy Immunol. 1998;117(Suppl 1):48–51. doi: 10.1159/000053571. [DOI] [PubMed] [Google Scholar]
- 69.Nakayama T, Yao L, Tosato G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J Clin Invest. 2004;114:1317–1325. doi: 10.1172/JCI22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clinton M, Long WF, Williamson FB, Duncan JI, Thompson WD. Effect of the mast cell activator compound 48/80 and heparin on angiogenesis in the chick chorioallantoic membrane. Int J Microcirc Clin Exp. 1988;7:315–326. [PubMed] [Google Scholar]
- 71.Ribatti D, Crivellato E, Candussio L, Nico B, Vacca A, Roncali L, Dammacco F. Mast cells and their secretory granules are angiogenic in the chick embryo chorioallantoic membrane. Clin Exp Allergy. 2001;31:602–608. doi: 10.1046/j.1365-2222.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 72.Norrby K, Jakobsson A, Sorbo J. Mast-cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52:195–206. doi: 10.1007/BF02889963. [DOI] [PubMed] [Google Scholar]
- 73.Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer M, Juremalm M, Olsson N, Backlin C, Sundstrom C, Nilsson K, Enblad G, Nilsson G. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int J Cancer. 2003;107:197–201. doi: 10.1002/ijc.11370. [DOI] [PubMed] [Google Scholar]
- 76.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 78.Meininger CJ. Mast cells and tumor-associated angiogenesis. Chem Immunol. 1995;62:239–257. [PubMed] [Google Scholar]
- 79.Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 80.Friedl A, Chang Z, Tierney A, Rapraeger AC. Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparan sulfate: comparison of normal and abnormal human tissues. Am J Pathol. 1997;150:1443–1455. [PMC free article] [PubMed] [Google Scholar]
- 81.Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- 82.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 83.Kinet JP. The essential role of mast cells in orchestrating inflammation. Immunol Rev. 2007;217:5–7. doi: 10.1111/j.1600-065X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 84.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 85.Vincent-Schneider H, Thery C, Mazzeo D, Tenza D, Raposo G, Bonnerot C. Secretory granules of mast cells accumulate mature and immature MHC class II molecules. J Cell Sci. 2001;114:323–334. doi: 10.1242/jcs.114.2.323. [DOI] [PubMed] [Google Scholar]
- 86.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez S, Moran M, Kochevar IE. Chronic photodamage in skin of mast cell-deficient mice. Photochem Photobiol. 1999;70:248–253. [PubMed] [Google Scholar]
- 88.Grimbaldeston MA, Skov L, Finlay-Jones JJ, Hart PH. Increased dermal mast cell prevalence and susceptibility to development of basal cell carcinoma in humans. Methods. 2002;28:90–96. doi: 10.1016/s1046-2023(02)00213-x. [DOI] [PubMed] [Google Scholar]
- 89.Hart PH, Townley SL, Grimbaldeston MA, Khalil Z, Finlay-Jones JJ. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods. 2002;28:79–89. doi: 10.1016/s1046-2023(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 90.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 91.Blanchet MR, Maltby S, Haddon DJ, Merkens H, Zbytnuik L, McNagny KM. CD34 facilitates the development of allergic asthma. Blood. 2007;110:2005–2012. doi: 10.1182/blood-2006-12-062448. [DOI] [PubMed] [Google Scholar]
- 92.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 95.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 98.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 99.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006;177:1755–1762. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- 100.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 101.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP, Pucillo CE. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pardanani A, Elliott M, Reeder T, Li CY, Baxter EJ, Cross NC, Tefferi A. Imatinib for systemic mast-cell disease. Lancet. 2003;362:535–536. doi: 10.1016/s0140-6736(03)14115-3. [DOI] [PubMed] [Google Scholar]
- 103.Edwards AM. Oral sodium cromoglycate: its use in the management of food allergy. Clin Exp Allergy. 1995;25(Suppl 1):31–33. doi: 10.1111/j.1365-2222.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 104.Norris AA. Pharmacology of sodium cromoglycate. Clin Exp Allergy. 1996;26(Suppl 4):5–7. doi: 10.1111/j.1365-2222.1996.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 105.Galinsky DS, Nechushtan H. Mast cells and cancer--no longer just basic science. Crit Rev Oncol Hematol. 2008;68:115–130. doi: 10.1016/j.critrevonc.2008.06.001. [DOI] [PubMed] [Google Scholar]