Summary
There are ten mammalian diacylglycerol kinases (DGKs) whose primary role is to terminate diacylglycerol (DAG) signaling. However, it is becoming increasingly apparent that DGKs also influence signaling events through their product, phosphatidic acid (PA). They do so in some cases by associating with proteins and then modifying their activity by generating PA. In other cases, DGKs broadly regulate signaling events by virtue of their ability to provide PA for the synthesis of phosphatidylinositols (PtdIns).
Keywords: diacylglycerol kinase, diacylglycerol, phosphatidic acid, phosphatidylinositol, lipid signaling
1. Introduction
DAG is generated by the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) by PtdIns-specific phospholipase C (PLC) enzymes [1]. Remaining in the membrane, it binds proteins with cysteine-rich, C1 domains, and activates several of these proteins including protein kinase C (PKC) isoforms [2] and Ras guanyl nucleotide-releasing proteins (RasGRPs) [3]. In other cases, DAG recruits but does not activate proteins such as protein kinase D, the Munc13 proteins, and the chimaerins [3]. Additionally, DAG appears to activate some transient receptor potential channels that do not harbor C1 domains [4]. Its effects on numerous and diverse targets underscores the importance of DAG signaling and indicates that DAG modulates a broad array of biological events. It is critical then that intracellular DAG levels be tightly regulated and it is now widely agreed that under most circumstances, conversion of DAG to PA by the DGKs is the major route to terminate DAG signaling. But PA, itself, has a broad array of signaling properties that are very distinct from those of DAG. Their ability to generate PA suggests that DGKs might also influence biological events by generating this lipid. In fact, there are now several examples indicating that DGKs modulate signaling events not only by metabolizing DAG to terminate its effects, but also by producing PA.
DGKs have been identified in unicellular organisms such as bacteria, but these DGKs are structurally different from those identified in higher eukaryotes such as Drosophila melanogaster [5-7], Arabidopsis thaliana [8, 9], Caenorhabditis elegans [10, 11], and mammals [12]. For example, bacterial DGK, unlike higher eukaryote DGKs, is a small, integral membrane protein that phosphorylates other lipids in addition to DAG [13]. And a recently identified yeast DGK is similar to cytidyltransferases and uses CTP as a phosphate donor rather than the ATP used by DGKs in higher eukaryotes [14, 15]. Finally, a multisubstrate lipid kinase (MuLK) that also phosphorylates DAG was recently identified [16, 17]. Unlike mammalian DGKs, MuLK phosphorylates non-DAG lipids and its structure is not very homologous to that of DGKs. This review will focus on DGKs in higher eukaryotes, with a specific focus on their role in generating PA that influences biological events.
2. Common structural features of the mammalian DGK enzymes
DGK activity was first identified about forty years ago [18], and an 80kDa DGK enzyme was purified to homogeneity from pig brain cytosol in 1983 [19]. Based on the partial sequence from the 80kDa enzyme, Sakane and colleagues isolated a full-length, porcine DGK cDNA in 1990 [20]. But based on antibody studies, additional DGK enzymes appeared to exist [21], prompting an exhaustive search for other DGK isoforms. Nine additional isoforms have since been identified (Fig. 1), making this a rather large family of enzymes. Their diversity is even broader because DGKs β, γ, δ, ζ, ι, and η are alternatively spliced [22]. All mammalian DGKs have two common structural elements: a catalytic domain and at least two C1 domains. The functions and structural properties of these domains have been described in detail in recent reviews [22, 23], so only the important features of these motifs are highlighted below.
Fig. 1.
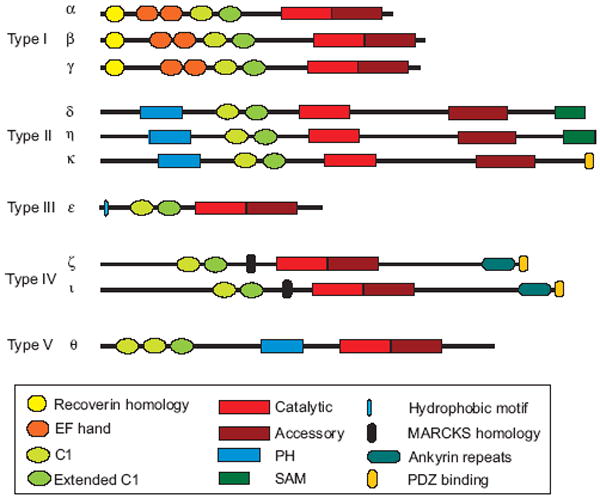
Stucture of mammalian DGK isoforms. The ten members of the mammalian DGK family are grouped by sequence homology into five subtypes. Shown are protein motifs common to several DGKs. The significance of several of these motifs is discussed in the text. Additional motifs of uncertain significance are not shown.
2.1 The catalytic domain
DGK catalytic domains are composed of accessory and catalytic subunits. In most cases, these subunits are joined to create an uninterrupted catalytic domain. However, in DGKs δ, η and κ [24-26], these domains are separated by a long peptide sequence that does not have any apparent functional motif. Each catalytic subunit has an ATP binding site where mutation of a glycine in this motif to an aspartate or alanine renders the DGK kinase dead [27-29]. The DGK catalytic domains may also require other motifs for maximal activity because catalytic domains of DGKs ε, ζ, and θ have very little DGK activity when expressed as isolated subunits (M.K.T. unpublished observations and [30]). Moreover, the isolated catalytic domain of DGKα retained only about 1/3 the activity of this fully active mutant [25]. Thus, it appears that mammalian DGK catalytic domains, unlike bacterial DGK, require other motifs for maximal activity. These other motifs likely function in coordination with the catalytic domain or confer structural stability that allows full catalytic activity.
2.2 The C1 domains
All DGKs have at least two cysteine-rich regions homologous to the DAG-binding C1A and C1B motifs of PKCs [31]. The C1 domain closest to the catalytic domain has an extended region of fifteen amino acids not present in C1 domains from other proteins or in the other C1 domains of DGKs. This extended motif appears to contribute to DGK activity because mutations within this domain significantly reduced the kinase activity of the enzyme [30]. In theory, C1 domains bind DAG, perhaps localizing DGKs to where DAG accumulates. However, no DGK C1 domain has so far been conclusively shown to bind DAG, but some bind phorbol esters [32-34], which are DAG analogues. Based on sequence alignments, Hurley and colleagues [31] proposed that most DGK C1 domains were sufficiently different from those in PKCs that they might not bind DAG. Instead of binding DAG, the C1 domains of some DGKs appear to act as protein-protein interaction sites. For example, the C1 domains of DGKζ directly bind to Rac1 [35] and they also associate with β-arrestins [36]. Collectively, these data have led to the suggestion that the C1 domains in some DGKs might not bind DAG. However, it seems unlikely that a DAG kinase would have C1 domains without using them to bind DAG. The question of whether or not DGK C1 domains bind DAG will not be answered until the crystal structure of a DGK C1 domain has been solved.
2.3 The five DGK subfamilies
Based on other structural motifs, mammalian DGK isoforms are classified into five subtypes (Fig. 1). Type I DGKs [20, 37, 38] have calcium-binding EF hand motifs that make them more active in the presence of calcium [39]. Type II DGKs have pleckstrin homology (PH) domains at their amino termini [24-26]. This domain in DGKδ has been shown to bind weakly and non-selectively to PtdIns [40]. Some Type II DGKs also have sterile alpha motifs (SAM domains) at their carboxy termini that appear to act as localization cues [28] and/or cause homo- and hetero-oligomerization of DGKs δ and η [41, 42]. DGKε, the only type III enzyme, has an unusual specificity toward acyl chains of DAG, strongly preferring a specific fatty acid—arachidonate—in the sn-2 (middle) position of the glycerol backbone [43]. Its preference for arachidonoyl-DAG suggests that DGKε may be a component of the biochemical pathway that accounts for the enrichment of PtdIns(4,5)P2 with arachidonate [44]. This possibility is discussed in more detail below. Type IV DGKs [45, 46] have domains similar to the phosphorylation site domain of the MARCKS protein. This motif in type IV DGKs appears to function as a localization cue. Type IV DGKs also have four ankyrin repeats and a carboxy terminal PDZ binding domain [47]. The type V enzyme, DGKθ, has three C1 domains and a putative PH domain with a Ras association (RA) domain embedded within it [48]. There is no evidence that the RA domain is functional [49] and the affinity of the PH domain for PtdIns has not been tested. Overall, the DGK family is structurally diverse, which indicates that these enzymes likely modulate numerous important biological events.
3. DGKs influence specific biological events depending on their binding partners
Each DGK isotype is expressed in numerous tissues, and usually multiple DGK isotypes are expressed in the same tissue and even within the same cell [50]. For example we detected all known DGK isoforms in mouse brain extracts [51] and have found expression of at least six DGK isoforms in mouse embryo fibroblasts (J.C. and M.K.T. unpublished observations). When multiple DGK isoforms are expressed in a cell type, they are usually from different subfamilies, suggesting that the subfamilies have distinct functional roles. Thus, specific pools of DAG could be uniquely regulated by directing DGK isoforms to appropriate cellular compartments in order to metabolize the DAG. Conversely, DGK isotypes could uniquely generate PA depending on their intracellular localization and modes of regulation. Consistent with this model, evidence indicates that DGKs achieve functional specificity by accessing specific pools of DAG and binding to a unique subset of DAG- or PA-activated proteins in order to regulate their activity (Fig. 2). This concept agrees with an emerging body of evidence indicating that specificity in signal transduction is often achieved by gathering together signaling proteins in common pathways along with their regulators [52].
Fig. 2.
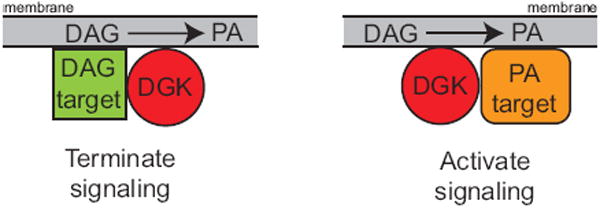
Model of compartmentalized DGK function. Evidence indicates that DGKs bind and influence the activity of proteins regulated by either DAG or PA. In the left panel, a DGK binds and inhibits a DAG-activated protein. In the right panel, a DGK binds a protein and, by generating PA, increases its function.
The outcome of DGK function will thus depend on the binding partners of each DGK isoform, and the effects that they exert can be quite different. A clear example of this concept has been demonstrated for the type IV DGKs, ζ and ι, which are structurally very similar but have opposing effects on Ras signaling. DGKζ was found to attenuate Ras signaling, both in vitro [53, 54] and in vivo [55]. Its effects on Ras are due to the ability of DGKζ to bind and inhibit RasGRP1 [53], a Ras activator that requires DAG for its function. By metabolizing DAG, DGKζ inhibits the activity of RasGRP1. Its ability to regulate RasGRP1 was unique among the five other DGK isotypes that were tested; even an alternatively spliced form DGKζ did not inhibit RasGRP1.
Given the structural similarity between DGKζ and DGKι, one would predict that they would have similar signaling outcomes. It was surprising then when we subsequently discovered that DGKι had the opposite effects on Ras signaling: while DGKζ deficiency enhanced Ras activity, DGKι deficiency reduced it [56]. The effects of DGKι on Ras signaling were caused by its inhibition of RasGRP3. In conditions of DGKι deficiency, RasGRP3 activity was augmented, which led to activation Rap1 that then interfered with Ras signaling [56]. Collectively these observations indicate that DGKs achieve functional specificity based upon the company that they keep.
Additional examples of DGKs specifically binding to DAG target proteins to regulate their activity have been published [57, 58], indicating that this is a common way to regulate DAG levels and the proteins that this lipid influences. Based on the structural diversity of the DGK family, it is likely that each DGK regulates a distinct set of DAG signaling proteins, a concept that is supported by mouse knockout studies showing that mice with targeted deletion of individual DGK isoforms have distinct phenotypes [51, 55, 56, 59, 60]. But, in addition to inhibiting the activity of proteins influenced by DAG, DGKs also appear to modulate proteins that are influenced by PA (Fig. 2, right panel). Several examples of DGKs modulating signaling events by producing PA are discussed in detail below.
4. PA signaling events mediated by DGKs
Most of the mechanisms described below involve DGKs ζ and α, suggesting that these might be the only DGK isoforms that modulate signaling events by producing PA. However, other DGK isoforms have not been similarly tested, so one cannot rule out the possibility that they might also function in this manner.
4.1 DGKζ activates phosphatidylinositol-4-phosphate 5-kinase type Iα
Almost seventeen years ago, phosphatidylinositol-4-phosphate (PtdIns4P) 5-kinase enzymes were shown to be potently activated by PA [61]. A subsequent study demonstrated that DGK activity co-immunoprecipitated with a complex that included a PtdIns4P 5-kinase [62], suggesting that DGKs might bind to PtdIns4P 5-kinases and then generate PA to modulate their activity. We investigated this possibility in more detail and found that DGKζ co-localized and co-immunoprecipitated with PtdIns4P 5-kinase type Iα, and we showed that expression of DGKζ dramatically increased generation of PtdIns(4,5)P2 in cells [63]. A kinase dead DGKζ also co-immunoprecipitated with the PtdIns4P 5-kinase, but failed to enhance its activity. Together, these data strongly argue that localized PA generation, rather than a conformational change mediated by association of the PtdIns4P 5-kinase with DGKζ, augmented PtdIns4P 5-kinase activity.
In a separate study, DGKζ was shown to mediate DAG signaling downstream of the M1 muscarinic receptor (M1R), a seven-transmembrane receptor (7TMR) [36, 64]. Its translocation to M1R required binding to β-arrestins—which are scaffolding proteins that bind 7TMRs. The binding site on DGKζ for β-arrestins mapped to the C1 domains [36], and mutating either of the C1 domains abolished translocation of DGKζ [64]. Blocking the interaction of β-arrestins with DGKζ attenuated DAG metabolism, which led to the conclusion that the function of DGKζ in this complex was to terminate DAG signaling that was initiated by M1R. Since β-arrestins bind other 7TMRs, this mechanism is likely broadly applied to limit DAG signaling initiated by many different 7TMR agonists. Indeed, over-expressing DGKζ enhanced decay of ERK phosphorylation following activation of the gonadotropin-releasing hormone receptor, another 7TMR [65].
It was subsequently shown that PtdIns4P 5-kinase type Iα also translocated to 7TMRs by binding to β-arrestins. Its function at the 7TMR was to promote internalization of the receptor [66]. Since DGKζ also binds β-arrestins, this collection of observations raises the possibility that DGKζ might function in this complex not only to metabolize DAG, but also to promote PtdIns4P 5-kinase activity by generating PA. This would provide a two step mechanism to shut down the receptor (Fig. 3). First, DGKζ could metabolize DAG to reduce the impact of this signaling lipid, and then the PA that is produced could activate the PtdIns4P 5-kinase enzyme in order to promote receptor internalization. This hypothetical model has not been specifically tested, but it agrees with data showing that transgenic over-expression of DGKζ in mouse myocardium protects the mice against cardiac hypertrophy initiated by excessive activation of a 7TMR [67].
Fig. 3.
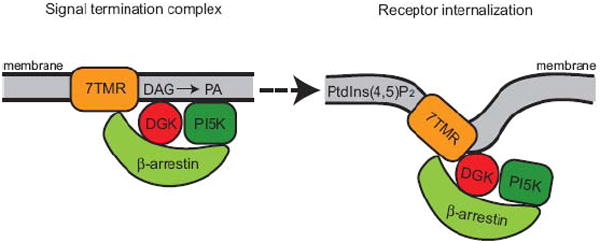
Hypothetical model in which DGKζ could negatively regulate 7TMR signaling. Upon activation of the 7TMR, β-arrestin is recruited to the receptor along with its bound DGKζ. The DGK terminates DAG signaling by converting this lipid to PA. The PA, in turn, might serve to activate a PtdIns4P 5-kinase (PI5K), which generates PtdIns(4,5)P2. This lipid promotes internalization of the 7TMR to limit access to its ligand.
4.2 DGKζ modulates Rac1 activation to influence neurite outgrowth
As noted above, DGK activity was found to co-immunoprecipitate with a PtdIns4P 5-kinase [62]. In addition to these two proteins, the precipitate also contained Rac1, a member of the Rho family of GTPases that helps regulate changes in actin organization. Rho GTPases are molecular switches that oscillate between an inactive GDP-bound state and an active GTP-bound state. We followed up these studies and found that DGKζ directly interacted with Rac1 and colocalized with it at sites of actin remodeling [68]. DGKζ appeared to promote the activity of Rac1 because its over-expression in a neuronal cell line induced neurite outgrowth that was inhibited by a dominant-negative Rac1 [35]. As an upstream activator of Rac1, it is appealing to speculate that DGKζ provides a component that is necessary for Rac1 activation. PA is a likely candidate because it is known to promote dissociation of Rac1 from its inhibitor Rho guanine dissociation inhibitor (RhoGDI) [69], a protein that was also identified in the DGK precipitates [62]. PA is also known to activate p21-activated kinase 1 (PAK1) [70], which phosphorylates RhoGDI, causing it to release Rac1 to enable its activation [71]. Thus, in this complex of proteins, there are at least two mechanisms by which DGK-derived PA might promote actin reorganization and cell motility. First, PA could activate PtdIns4P 5-kinase activity that would provide localized PtdIns(4,5)P2 to promote actin polymerization [72]. Second, the PA could also activate Rac1 by causing its dissociation from RhoGDI. Supporting the second mechanism, Abramovici et al. recently found reduced PAK1 phosphorylation and attenuated dissociation of RhoGDI from Rac1 in DGKζ-deficient fibroblasts [93]. These defects were rescued by exogenous PA and by expression of wild-type DGKζ, but not by kinase dead DGKζ. Additionally, they found that DGKζ stably associated with both PAK1 and RhoGDI. Together, these data indicate that DGKζ has a major role in regulating Rac1-mediated signaling. It should be noted that DGKζ might not be the only isoform that can regulate Rac1, because there is also evidence that DGKα positively influences Rac1 following activation of tyrosine kinase receptors [73].
4.3 DGKζ regulates mTor activation
The serine/threonine kinase mammalian target of rapamycin (mTOR) is an important intermediate in several pathways that manage cellular responses to environmental stress. Its activity is regulated, in part, by PA, which appears to bind the same region of mTOR to which rapamycin binds. This observation has led to a hypothetical model in which rapamycin inhibits mTOR by competing with PA or displacing it from its binding site so that mTOR can’t be activated by PA. There is strong evidence indicating the phospholipase D (PLD) isoforms are largely responsible for providing the pool of PA that activates mTOR [74]. But a recent report indicates that DGKζ might also activate mTOR under some circumstances. Avila-Flores and colleagues demonstrated that over-expression of DGKζ led to enhanced, serum-induced phosphorylation of p70 S6 kinase (p70S6K)—a major downstream target of mTOR—and rendered the cells resistant to the effects of rapamycin. Conversely, RNAi-mediated knockdown of DGKζ reduced phosphorylation of p70S6K. It appears that PA is important in this mechanism to activate mTOR, because DGKζ could not promote activation of a mutant mTOR that had reduced ability to bind PA. And demonstrating that among the DGKs, this might be a specific property of DGKζ, over-expression of DGKα did not promote p70S6K phosphorylation. Collectively, these data indicate that DGKζ can activate mTOR, presumably through its ability to generate PA. The target of this PA, however, is not clear because another report showed in the same cell line that inhibiting PLD almost completely abolished serum-induced S6 kinase activity, indicating that PLD is largely responsible for activating mTOR [75]. It is possible then that instead of directly activating mTOR, DGKζ activates PtdIns4P 5-kinases, which could provide PtdIns(4,5)P2, an important activator of PLD enzymes [76]. Regardless of the mechanism, these data suggest that DGKζ can potentially activate mTOR and that it does so by producing PA.
4.4 DGKs ζ and α modulate immune cell signaling
Gene knockout studies in mice have demonstrated that DGKs ζ and α have central roles in modulating immune cell function. Most data indicate that their primary function in immune cells is to metabolize DAG, and that in their absence DAG accumulates and causes aberrant immune cell function. For example, deficiency of either DGKζ or DGKα in T cells causes hyper-reactive responses largely due to excess DAG that promotes Ras activation [55, 60, 77]. But there is also evidence that DGKs ζ and α might also provide PA that is critical for proper immune cell function. For example, compound mutant mice lacking both DGKζ and DGKα have defects in T cell development that can be partially rescued by exogenous PA [78]. Additionally, generation of PA by DGKζ also appears to be important for proper signaling from Toll-like receptors (TLRs) in macrophages [79]. TLRs are key mediators that promote the production of pro-inflammatory cytokines which are important for both innate and adaptive immune responses. In DGKζ-deficient macrophages, production of two cytokines, TNFα and IL-12, was blunted. The reduced levels of cytokines suggested that DGKζ might provide a component, such as PA, that is necessary for their production. Supporting this possibility, exogenous PA restored the levels of IL-12 in DGKζ-deficient macrophages. The role of PA is not clear, but it might be necessary to inhibit PtdIns 3-kinases, which were excessively active in the DGKζ deficient cells. Finally, a recent report suggested that PA derived from DGKα influenced neutrophil responses to anti-neutrophil cytoplasmic antibodies [80]. Collectively, these observations indicate that DGKs α and ζ regulate immune cell function not only by influencing DAG levels, but also by producing PA.
4.5 Plant DGKs affect stress responses by generating PA
DGK isoforms have also been identified in several plant species. For example, seven DGK genes (AtDGK1-7) has been identified in Arabidopsis thaliana [81] and in rice there are eight putative DGK isoforms [82]. There is very little data to indicate their specific roles, but based on current information indicating that the number of PA targets in plants vastly outnumbers DAG targets, it has been hypothesized that the primary role of DGKs in plants is to generate PA rather than to consume DAG [83]. In plants, PA is usually produced in response to stress, suggesting that DGKs might influence the stress response. Supporting this possibility, expression of plant DGKs is induced in response to stresses such as wounding, chemicals, and fungal infection [81, 82], and over-expression of a rice DGK in tobacco plants enhanced the resistance of those plants to disease [82]. Although it is not clear exactly how these DGKs are protective in conditions of stress, numerous PA targets have been identified in plants [84] and these proteins are probably critical effectors in the stress response.
5. Role of DGKs in regenerating PtdIns
PtdIns(4,5)P2 is enriched in unsaturated fatty acids [44], so there must be a mechanism that promotes this enrichment. PtdIns(4,5)P2 is re-synthesized from DAG in a series of reactions known as the PtdIns cycle (Fig. 4) and the DGK reaction is the first step in this sequence. Evidence indicates that DGKε, by virtue of its specificity for DAG that has unsaturated fatty acids, helps enrich PtdIns(4,5)P2 with unsaturated fatty acids. But there is also evidence that other DGK isoforms might also contribute.
Fig. 4.
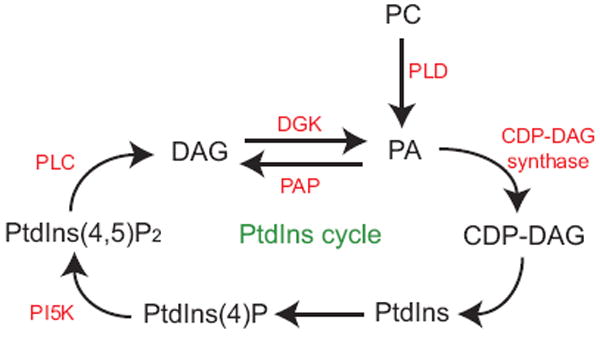
A simplified diagram of the PtdIns cycle. Shown are lipids that are components of the PtdIns cycle and enzymes in this cycle that are relevant to the discussion in the text.
5.1 DGKε in neural tissues
As noted above, DGKε prefers to phosphorylate DAG with an arachidonate group in the sn-2 position [43]. PtdIns, including PtdIns(4,5)P2, are enriched at the sn-2 position with unsaturated fatty acids—usually arachidonate [44]. Consequently, the DAG generated by PtdIns-specific PLC isoforms such as PLCβ and PLCγ is enriched with this fatty acid. While it may seem that the fatty acid components of DAG would not significantly affect its signaling ability, some DAG targets, including PKCs, appear to be specifically activated by unsaturated DAG [85]. How the fatty acid components of DAG affect target proteins is unclear, but it is possible that the fatty acids in PtdIns(4,5)P2 and/ or DAG might help enrich these lipids in membrane microdomains that may, in turn, recruit other necessary signaling components. In vitro, most DGKs don’t distinguish between DAG species with fatty acid components. But DGKε is an exception [43]. Its selectivity suggests that DGKε may have a prominent role in the re-synthesis of PtdIns from DAG, because incorporating DGKε into the cycle would maintain the enrichment of PtdIns with arachidonate.
To examine the biological function of DGKε, we disrupted the gene encoding DGKε in mice. Since proper PtdIns signaling is important for normal neuronal transmission, in a collaborative effort we studied seizure threshold in the mice. We found that DGKε null mice had significantly shorter seizures following electroconvulsive shock and they recovered faster than wild type mice[59]. Examination of brain lipids showed reduced levels of arachidonate in both PtdIns(4,5)P2 and DAG in the DGKε-deficient mice. This lipid profile demonstrated a critical role for DGKε in maintaining a proper balance of arachidonate-enriched PtdIns. Subsequent analysis of lipids in embryo fibroblasts from the DGKε knockout mice also revealed reduced levels of arachidonate in PtdIns [86]. There were minimal changes in the lipid species of DAG in the embryo fibroblasts, probably because those experiments were conducted under basal conditions. Together these observations indicate that DGKε is a component of the PtdIns cycle and that through its selectivity for arachidonoyl-DAG it helps maintain the fatty acid composition of PtdIns and consequently modulates DAG signaling events and seizure susceptibility.
But DGKε does not appear to be expressed in some tissues [59], suggesting that there are other mechanisms to enrich PtdIns with unsaturated fatty acids. Since other DGKs do not seem to exhibit specificity for DAG that has unsaturated fatty acids, one way to maintain this enrichment of PtdIns with unsaturated fatty acids would be to couple PtdIns-specific phospholipase C enzymes with DGKs. Indeed, we found that DGKζ co-immunoprecipitated with the PtdIns-specific PLCs β and γ (M.K.T., unpublished observations and [87]). Collectively, these observations suggest that by producing PA, DGKε and possibly other DGKs, help maintain the proper fatty acid composition of PtdIns, and consequently DAG. They may do this either by having specificity for the fatty acid components of DAG or by associating with elements of the PtdIns cycle that have specificity.
5.2 A Drosophila DGK in the retina
A Drosophila eye-enriched DGK encoded by the rdgA locus that is similar to mammalian type IV DGKs also appears to be important for PtdIns re-synthesis. This DGK functions downstream from the 7TMR Drosophila photoreceptors. When a photon of light is absorbed by these receptors, PLC enzymes produce DAG, which is critical, because flies that harbor mutant PLC enzymes do not respond to light [88]. This DAG probably activates PKCs and other targets that promote activation of TRP channels, which leads to increased intracellular calcium[89]. The importance of the Drosophila DGK is indicated by rdgA mutant flies, which have constitutively active TRP channels and eventually develop retinal degeneration [90]. Presumably, the channels are constitutively active because of excess DAG, but no one has yet demonstrated high levels of DAG in rdgA mutant flies.
In addition to providing an avenue to metabolize DAG, the DGK reaction also seems to be important for regenerating PtdIns(4,5)P2 from DAG. This re-synthesis cycle appears to be disrupted in rdgA mutant flies because there are dramatic reductions in the levels of PA [91] and PtdIns [92] in their retinae. Once PA is produced by the DGK, a series of reactions occurs to regenerate PtdIns(4,5)P2, and the first step in this sequence is the production of CDP-DAG by CDP-DAG synthase (Fig. 4). Supporting the possibility that impaired re-synthesis of PtdIns(4,5)P2 contributes to retinal degeneration in rdgA mutant flies, mutation of the CDP-DAG synthase gene enhanced the retinal degeneration in rdgA mutant flies [92]. Presumably their phenotype is made worse because mutation of CDP-DAG synthase shuts off other avenues—such as the PLD reaction—to synthesize PtdIns(4,5)P2. Moreover, mutating a PtdIns4P 5-kinase that catalyzes the final step of PtdIns(4,5)P2 synthesis had the same effect as mutating CDP-DAG synthase in the rdgA mutant background [92]. Conversely, mutating lazaro, a lipid phosphate phosphohydrolase (LPP) that might function as a phosphatidic acid phosphatase (PAP) to reverse the DGK reaction, reduced the rate of retinal degeneration in rdgA mutant flies [92]. Presumably the lazaro mutation is protective because, by blocking the reverse reaction, it leads to enhanced synthesis of PtdIns(4,5)P2. However, LPP and PAP enzymes are structurally distinct and it has not been conclusively demonstrated that the lazaro gene product can, indeed, dephosphorylate PA [92]. Nonetheless, this collection of data strongly suggests that DGK activity in the Drosophila retina is not only important to metabolize DAG, but is also critical as an initial step in regenerating PtdIns(4,5)P2. While these data indicate that mammalian type IV DGKs—the orthologs of the rdgA gene product—might have important roles in visual signal transduction, there is no evidence that deleting type IV DGK genes in mice affects their vision (M.K.T. unpublished data).
6. Summary and conclusions
While DGKs are better known as enzymes that metabolize diacylglycerol, there are also several reports indicating that they can additionally function by providing PA. In some cases, this PA binds and activates proteins such as PtdIns4P 5-kinases to influence cell function, while in other cases the PA is a critical component for the re-synthesis of PtdIns. In either case, disrupting DGK function causes aberrant signaling. This concept in which DGKs provide PA to influence signaling events is relatively new and it is very likely that additional examples will be identified in the future.
Acknowledgments
This work was supported by the Huntsman Cancer Foundation (to M.K.T.), the R. Harold Burton Foundation (to M.K.T.), the National Institutes of Health Grants R01-CA95463 (to M.K.T.) and The Cancer Research Society, Inc. (to S.H.G.).
Abbreviations used
- DGKs
diacylglycerol kinases
- DAG
diacylglycerol
- PA
phosphatidic acid
- PtdIns
phosphatidylinositols
- PtdIns(4,5)P2
phosphatidylinositol-4,5-bisphosphate
- PLC
phospholipase C
- PKC
protein kinase C
- RasGRPs
Ras guanyl nucleotide-releasing proteins
- MuLK
multisubstrate lipid kinase
- PH
pleckstrin homology
- SAM
sterile alpha motif
- RA
Ras association domain
- 7TMR
seven-transmembrane receptor
- M1R
M1 muscarinic receptor
- RhoGDI
Rho guanine dissociation inhibitor
- PAK1
p21-activated kinase 1
- mTOR
mammalian target of rapamycin
- PLD
phospholipase D
- p70S6K
p70 S6 kinase
- TLR
Toll-like receptor
- LPP
lipid phosphate phosphohydrolase
- PAP
PA phosphatase
- PtdIns4P
phosphatidylinositol-4-phosphate
- PI5K
PtdIns4P 5-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 2.Newton AC. Regulation of protein kinase C. Cell Biology. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 3.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–94. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 4.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A Diacylglycerol-Gated Cation Channel in Vomeronasal Neuron Dendrites Is Impared in TRPC2 Mutant Mice. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- 5.Masai I, Hosoya T, Kojima S-I, Hotta Y. Molecular cloning of a Drosophila diacylglycerol kinase gene that is expressed in the nervous system and muscle. Proc Natl Acad Sci USA. 1992;89:6030–6034. doi: 10.1073/pnas.89.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masai I, Okazaki A, Hosoya T, Hotta Y. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc Natl Acad Sci USA. 1993;90:11157–11161. doi: 10.1073/pnas.90.23.11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harden N, Yap SF, Chiam M-A, Lim L. A Drosophila gene encoding a protein with similarity to diacylglycerol kinase is expressed in specific neurons. Biochem J. 1993;289:439–444. doi: 10.1042/bj2890439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katagiri T, Mizoguchi T, Shinozaki K. Molecular cloning of a cDNA encoding diacylglycerol kinase (DGK) in Arabidopsis thaliana. Plant Mol Biol. 1996;30:647–653. doi: 10.1007/BF00049339. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Merino FC, Brearley CA, Ornatowska M, Abdel-Haliem ME, Zanor MI, Mueller-Roeber B. AtDGK2, a Novel Diacylglycerol Kinase from Arabidopsis thaliana. J Biol Chem. 2004;279:8230–8241. doi: 10.1074/jbc.M312187200. [DOI] [PubMed] [Google Scholar]
- 10.Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Gαo decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 11.Miller KG, Emerson MD, Rand JB. Goα and diacylglycerol kinase negatively regulate the Gqα pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topham MK. Signaling roles of diacylglycerol kinases. J Cell Biochem. 2006;97:474–484. doi: 10.1002/jcb.20704. [DOI] [PubMed] [Google Scholar]
- 13.Badola P, Sanders CR., II Escherichia coli diacylglycerol kinase is an evolutionarily optimized membrane enzyme and catalyzes direct phosphoryl transfer. J Biol Chem. 1997;272:24176–24182. doi: 10.1074/jbc.272.39.24176. [DOI] [PubMed] [Google Scholar]
- 14.Han GS, O’Hara L, Siniossoglou S, Carman GM. Characterization of the yeast DGK1-encoded CTP-dependent diacylglycerol kinase. J Biol Chem. 2008;283:20443–53. doi: 10.1074/jbc.M802866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han GS, O’Hara L, Carman GM, Siniossoglou S. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J Biol Chem. 2008;283:20433–42. doi: 10.1074/jbc.M802903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waggoner DW, Johnson LB, Mann PC, Morris V, Guastella J, Bajjalieh SM. MuLK, a Eukaryotic Multi-substrate Lipid Kinase. J Biol Chem. 2004;279:38228–38235. doi: 10.1074/jbc.M405932200. [DOI] [PubMed] [Google Scholar]
- 17.Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol. 2005;169:801–11. doi: 10.1083/jcb.200407123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hokin LE, Hokin MR. Diglyceride phosphokinase: an enzyme which catalyzes the synthesis of phosphatidic acid. Biochim Biophys Acta. 1959;31:285–7. doi: 10.1016/0006-3002(59)90481-0. [DOI] [PubMed] [Google Scholar]
- 19.Kanoh H, Kondoh H, Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983;258:1767–1774. [PubMed] [Google Scholar]
- 20.Sakane F, Yamada K, Kanoh H, Yokoyama C, Tanabe T. Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature. 1990;344:345–348. doi: 10.1038/344345a0. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, Kanoh H. Occurrence of immunoreactive 80 kDa and non-immunoreactive diacylglycerol kinases in different pig tissues. Biochem J. 1988;255:601–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: why so many of them? Biochim Biophys Acta. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 24.Sakane F, Imai SI, Kai M, Wada I, Kanoh H. Molecular cloning of a novel diacylglycerol kinase isozyme with a pleckstrin homology domain and a C-terminal tail similar to those of the EPH family of protein tyrosine kinases. J Biol Chem. 1996;271:8394–8401. doi: 10.1074/jbc.271.14.8394. [DOI] [PubMed] [Google Scholar]
- 25.Klauck TM, Xu X, Mousseau B, Jaken S. Cloning and characterization of a glucocorticoid-induced diacylglycerol kinase. J Biol Chem. 1996;271:19781–19788. doi: 10.1074/jbc.271.33.19781. [DOI] [PubMed] [Google Scholar]
- 26.Imai H, Kai M, Yasuda S, Kanoh H, Sakane F. Identification and characterization of a novel type II diacylglycerol kinase, DGK kappa. J Biol Chem. 2005;280:39870–39881. doi: 10.1074/jbc.M500669200. [DOI] [PubMed] [Google Scholar]
- 27.Sanjuan MA, Jones DA, Izquierdo M, Merida I. Role of diacylglycerol kinase α in the attenuation of receptor signaling. J Cell Biol. 2001;153:207–219. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaya H, Wada I, Jia Y-J, Kanoh H. Diacylglycerol Kinase d Suppresses ER-to-Golgi Traffic via Its SAM and PH Domains. Mol Biol Cell. 2002;13:302–316. doi: 10.1091/mbc.01-05-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topham MK, Bunting M, Zimmerman GA, McIntyre TM, Blackshear PJ, Prescott SM. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature. 1998;394:697–700. doi: 10.1038/29337. see comments. [DOI] [PubMed] [Google Scholar]
- 30.Los AP, van Baal J, de Widt J, Divecha N, van Blitterswijk WJ. Structure-activity relationship of diacylglycerol kinase theta. Biochim Biophys Acta. 2004;1636:169–174. doi: 10.1016/j.bbalip.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Hurley JH, Newton AC, Parker PJ, Blumberg PM, Nishizuka Y. Taxonomy and function of C1 protein kinase C homology domains. Protein Science. 1997;6:477–480. doi: 10.1002/pro.5560060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shindo M, Irie K, Ohigashi H, Kuriyama M, Saito N. Diacylglycerol kinase gamma is one of the specific receptors of tumor-promoting phorbol esters. Biochem Biophys Res Commun. 2001;289:451–456. doi: 10.1006/bbrc.2001.5935. [DOI] [PubMed] [Google Scholar]
- 33.Shindo M, Irie K, Masuda A, Ohigashi H, Shirai Y, Miyasaka K, Saito N. Synthesis and Phorbol Ester Binding of the Cysteine-rich Domains of Diacylglycerol Kinase (DGK) Isozymes. J Biol Chem. 2003;278:18448–18454. doi: 10.1074/jbc.M300400200. [DOI] [PubMed] [Google Scholar]
- 34.Sakane F, Kai M, Wada I, Imai S-i, Kanoh H. The C-terminal part of diacylglycerol kinase α lacking zinc fingers serves as a catalytic domain. Biochem J. 1996;318 doi: 10.1042/bj3180583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yakubchyk Y, Abramovici H, Maillet J, Daher E, Obagi C, Parks RJ, Topham MK, Gee SH. Regulation of neurite outgrowth in N1E-115 cells through PDZ-mediated recruitment of diacylglycerol kinase zeta. Mol Cell Biol. 2005;25:7289–7302. doi: 10.1128/MCB.25.16.7289-7302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by -arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- 37.Goto K, Kondo H. Molecular cloning and expression of a 90-kDa diacylglycerol kinase that predominantly localizes in neurons. Proc Natl Acad Sci USA. 1993;90:7598–7602. doi: 10.1073/pnas.90.16.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kai M, Sakane F, Imai S-i, Wada I, Kanoh H. Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in human retina with a truncated and inactive enzyme expression in most other human cells. J Biol Chem. 1994;269:18492–18498. [PubMed] [Google Scholar]
- 39.Yamada K, Sakane F, Matsushima N, Kanoh H. EF-hand motifs of α, β and γ isoforms of diacylglycerol kinase bind calcium with different affinities and conformational changes. Biochem J. 1997;321:59–64. doi: 10.1042/bj3210059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeuchi H, Kanematsu T, Misumi Y, Sakane F, Konishi H, Kikkawa U, Watanabe Y, Katan M, Hirata M. Distinct specificity in the binding of inositol phosphates by pleckstrin homology domains of pleckstrin, RAC-protein kinase, diacylglycerol kinase and a new 130 kDa protein. Biochimica et Biophysica Acta. 1997;1359:275–285. doi: 10.1016/s0167-4889(97)00109-2. [DOI] [PubMed] [Google Scholar]
- 41.Murakami T, Sakani F, Imai S, Houkin K, Kanoh H. Identification and Characterization of Two Splice Variants of Diacylglycerol Kinase η. J Biol Chem. 2003;278:34364–34372. doi: 10.1074/jbc.M301542200. [DOI] [PubMed] [Google Scholar]
- 42.Sakane F, Imai S, Yamada K, Murakami T, Tsushima S, Kanoh H. Alternative Splicing of the Human Diacylglycerol Kinase d Gene Generates Two Isoforms Differing in Their Expression Patterns and in Regulatory Functions. J Biol Chem. 2002;277:43519–43526. doi: 10.1074/jbc.M206895200. [DOI] [PubMed] [Google Scholar]
- 43.Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Biol Chem. 1996;271:10237–10241. [PubMed] [Google Scholar]
- 44.Prescott SM, Majerus PW. The fatty acid composition of phosphatidylinositol from thrombin-stimulated human platelets. J Biol Chem. 1981;256:579–582. [PubMed] [Google Scholar]
- 45.Bunting M, Tang W, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning and characterization of a novel human diacylglycerol kinase ζ. J Biol Chem. 1996;271:10230–10236. [PubMed] [Google Scholar]
- 46.Ding L, Traer E, McIntyre TM, Zimmerman GA, Prescott SM. The cloning and characterization of a novel human diacylglycerol kinase, DGKι. J Biol Chem. 1998;273:32746–32752. doi: 10.1074/jbc.273.49.32746. [DOI] [PubMed] [Google Scholar]
- 47.Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH. Interaction of γ1-syntrophin with Diacylglycerol Kinase-ζ. J Biol Chem. 2001;276:26526–26533. doi: 10.1074/jbc.M104156200. [DOI] [PubMed] [Google Scholar]
- 48.Houssa B, Schaap D, van der Wal J, Goto K, Yamakawa A, Shibata M, Takenawa T, van Blitterswijk WJ. Cloning of a novel human diacylglycerolkinase (DGKθ) containing three cysteine-rich domains, a proline-rich region and a pleckstrin homology domain with overlappping ras-associating domain. J Biol Chem. 1997;272:10422–10428. doi: 10.1074/jbc.272.16.10422. [DOI] [PubMed] [Google Scholar]
- 49.Houssa B, de Widt J, Kranenburg O, Moolenaar WH, van Blitterswijk WJ. Diacylglycerol Kinase θ binds to and is negatively regulated by active RhoA. J Biol Chem. 1999;274:6820–6822. doi: 10.1074/jbc.274.11.6820. [DOI] [PubMed] [Google Scholar]
- 50.Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem. 1999;274:11447–50. doi: 10.1074/jbc.274.17.11447. [DOI] [PubMed] [Google Scholar]
- 51.Crotty T, Cai J, Sakane F, Taketomi A, Prescott SM, Topham MK. Diacylglycerol kinase δ regulates protein kinase C and epidermal growth factor receptor signaling. Proc Natl Acad Sci (USA) 2006;103:15485–15490. doi: 10.1073/pnas.0604104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pawson T, Scott JD. Signaling through scaffolding, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 53.Topham MK, Prescott SM. Diacylglycerol kinase ζ regulates Ras activation by a novel mechanism. J Cell Biol. 2001;152:1135–1143. doi: 10.1083/jcb.152.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase ζ. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 55.Zhong X, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase ζ deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 56.Regier DS, Higbee J, Lund KM, Sakane F, Prescott SM, Topham MK. Diacylglycerol kinase iota regulates ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc Natl Acad Sci (USA) 2005;102:7595–7600. doi: 10.1073/pnas.0500663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones DR, Sanjuan MA, Stone JC, Merida I. Expression of a catalytically inactive form of diacylglycerol kinase α induces sustained signaling through RasGRP. FASEB J. 2002;16:595–597. doi: 10.1096/fj.01-0762fje. [DOI] [PubMed] [Google Scholar]
- 58.Luo B, Prescott SM, Topham MK. Association of diacylglycerol kinase ζ with protein kinase C α: spatial regulation of diacylglycerol signaling. J Cell Biol. 2003;160:929–937. doi: 10.1083/jcb.200208120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriquez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott SM, Bazan NG. Diacylglycerol kinase e regulates siezure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proc Natl Acad Sci USA. 2001;98:4740–4745. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 61.Moritz A, De Graan PN, Gispen WH, Wirtz KW. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992;267:7207–10. [PubMed] [Google Scholar]
- 62.Tolias KF, Couvillon AD, Cantley LC, Carpenter CL. Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Molecular and Cellular Biology. 1998;18:762–770. doi: 10.1128/mcb.18.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo B, Prescott SM, Topham MK. Diacylglycerol kinase z regulates phosphatidylinositol 4-phosphate 5-kinase by a novel mechanism. Cell Signalling. 2004;16:891–897. doi: 10.1016/j.cellsig.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Santos T, Carrasco S, Jones DR, Merida I, Eguinoa A. Dynamics of diacylglycerol kinase zeta translocation in living T-cells. Study of the structural domain requirements for translocation and activity. J Biol Chem. 2002;277:30300–9. doi: 10.1074/jbc.M200999200. [DOI] [PubMed] [Google Scholar]
- 65.Davidson L, Pawson AJ, Lopez de Maturana R, Freestone SH, Barran P, Millar RP, Maudsley S. Gonadotropin-releasing hormone-induced activation of diacylglycerol kinase-zeta and its association with active c-src. J Biol Chem. 2004;279:11906–16. doi: 10.1074/jbc.M310784200. [DOI] [PubMed] [Google Scholar]
- 66.van Baal J, de Widt J, Divecha N, van Blitterswijk WJ. Translocation of diacylglycerol kinase theta from cytosol to plasma membrane in response to activation of G protein-coupled receptors and protein kinase C. J Biol Chem. 2005;280:9870–9878. doi: 10.1074/jbc.M409301200. [DOI] [PubMed] [Google Scholar]
- 67.Arimoto T, Takeishi Y, Takeishi H, Shishido T, Niizeki T, Koyama Y, Shiga R, Nozaki N, Nakajima O, Nishimaru K, Abe J, Endoh M, Walsh RA, Goto K, Kubota I. Cardiac-specific overexpression of diacylglycerol kinase ζ prevents Gq protein-coupled receptor agonist-induced cardiac hypertrophy in transgenic mice. Circulation. 2006;113:60–66. doi: 10.1161/CIRCULATIONAHA.105.560771. [DOI] [PubMed] [Google Scholar]
- 68.Abramovici H, Hogan AB, Obagi C, Topham MK, Gee SH. Diacylglycerol Kinase-z Localization in Skeletal Muscle Is Regulated by Phosphorylation and Interaction with Syntrophins. Mol Biol Cell. 2003;14:4499–4511. doi: 10.1091/mbc.E03-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chuang TH, Bohl BP, Bokoch GM. Biologically active lipids are regulators of Rac.GDI complexation. J Biol Chem. 1993;268:26206–11. [PubMed] [Google Scholar]
- 70.Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, Knaus UG. A GTPase-independent mechanism of p21-activated kinase activation. Jrnl Biol Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- 71.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117–27. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y. Massive Actin Polymerization Induced by Phosphatidylinositol-4-phosphate 5-Kinase in Vivo. J Biol Chem. 1997;272:7578–7581. doi: 10.1074/jbc.272.12.7578. [DOI] [PubMed] [Google Scholar]
- 73.Chianale F, Cutrupi S, Rainero E, Baldanzi G, Porporato PE, Traini S, Filigheddu N, Gnocchi VF, Santoro MM, Parolini O, van Blitterswijk WJ, Sinigaglia F, Graziani A. Diacylglycerol Kinase-{alpha} Mediates Hepatocyte Growth Factor-induced Epithelial Cell Scatter by Regulating Rac Activation and Membrane Ruffling. Mol Biol Cell. 2007;18:4859–4871. doi: 10.1091/mbc.E07-02-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 75.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–5. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 76.Powner DJ, Wakelam MJ. The regulation of phospholipase D by inositol phospholipids and small GTPases. FEBS Lett. 2002;531:62–4. doi: 10.1016/s0014-5793(02)03410-5. [DOI] [PubMed] [Google Scholar]
- 77.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7:1166–73. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 78.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A. 2008;105:11909–14. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu CH, Machado FS, Guo R, Nichols KE, Burks AW, Aliberti JC, Zhong XP. Diacylglycerol kinase zeta regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007;204:781–92. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams JM, Pettitt TR, Powell W, Grove J, Savage CO, Wakelam MJ. Antineutrophil cytoplasm antibody-stimulated neutrophil adhesion depends on diacylglycerol kinase-catalyzed phosphatidic acid formation. J Am Soc Nephrol. 2007;18:1112–20. doi: 10.1681/ASN.2006090973. [DOI] [PubMed] [Google Scholar]
- 81.Gomez-Merino FC, Arana-Ceballos FA, Trejo-Tellez LI, Skirycz A, Brearley CA, Dormann P, Mueller-Roeber B. Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration: the DGK inhibitor R59022 differentially affects AtDGK2 and AtDGK7 activity in vitro and alters plant growth and development. J Biol Chem. 2005;280:34888–99. doi: 10.1074/jbc.M506859200. [DOI] [PubMed] [Google Scholar]
- 82.Zhang W, Chen J, Zhang H, Song F. Overexpression of a rice diacylglycerol kinase gene OsBIDK1 enhances disease resistance in transgenic tobacco. Mol Cells. 2008;26:258–64. [PubMed] [Google Scholar]
- 83.Meijer HJ, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 84.Testerink C, Dekker HL, Lim ZY, Johns MK, Holmes AB, Koster CG, Ktistakis NT, Munnik T. Isolation and identification of phosphatidic acid targets from plants. Plant J. 2004;39:527–36. doi: 10.1111/j.1365-313X.2004.02152.x. [DOI] [PubMed] [Google Scholar]
- 85.Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJO. Diacylglycerols and phosphatidates: which molecular species are intracellular messenger? Trends Biochem Sci. 1998;23:200. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- 86.Milne SB, Ivanova PT, Armstrong MD, Myers DS, Lubarda J, Shulga YV, Topham MK, Brown HA, Epand RM. Dramatic differences in the roles in lipid metabolism of two isoforms of diacylglycerol kinase. Biochemistry. 2008;47:9372–9. doi: 10.1021/bi800492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luo B, Prescott SM, Topham MK. Protein Kinase Cα Phosphorylates and Negatively Regulates Diacylglycerol Kinase ζ. J Biol Chem. 2003;278:39542–39547. doi: 10.1074/jbc.M307153200. [DOI] [PubMed] [Google Scholar]
- 88.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–33. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 89.Raghu P. Regulation of Drosophila TRPC channels by protein and lipid interactions. Semin Cell Dev Biol. 2006;17:646–53. doi: 10.1016/j.semcdb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Harris WA, Stark WS. Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J Gen Physiol. 1977;69:261–91. doi: 10.1085/jgp.69.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inoue H, Yoshioka T, Hotta Y. Diacylglycerol kinase defect in a drosophila retinal degeneration mutant rdgA. J Biol Chem. 1989;264:5996–6000. [PubMed] [Google Scholar]
- 92.Garcia-Murillas I, Pettitt T, Macdonald E, Okkenhaug H, Georgiev P, Trivedi D, Hassan B, Wakelam M, Raghu P. lazaro encodes a lipid phosphate phosphohydrolase that regulates phosphatidylinositol turnover during Drosophila phototransduction. Neuron. 2006;49:533–46. doi: 10.1016/j.neuron.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Abramovici H, Mojtabaie P, Parks RJ, Zhone X-P, Koretzky GA, Topham MK, Gee SH. Diacylglycerol kinase ζ regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell. 2009 doi: 10.1091/mbc.E07-12-1248. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


