Abstract
Endocytosis and endosomal trafficking have emerged as important cell biological steps in the Notch developmental signaling pathway. Ligand endocytosis helps generate the physical forces needed to dissociate and activate the receptor, and activated receptors enter endosomes to signal productively. Endosomal trafficking is also responsible for down-regulating Notch receptors that have not been activated by ligand. Recent studies have provided new insights into these Notch trafficking steps, and have uncovered additional endosomal mechanisms that contribute to asymmetric Notch activation and ligand-independent Notch signaling.
Keywords: Notch, receptor, ligand, endocytosis, endosome, lysosome, developmental signaling, γ-secretase
Introduction
The Notch signaling pathway, operating in concert with other signal transduction mechanisms, controls a wide range of developmental processes in many animal tissues (reviewed in [1]). One of the most striking features of Notch signaling is that the receptor undergoes intramembrane proteolysis to release a soluble fragment that participates directly in nuclear gene regulation. Receptor proteolysis is generally triggered by ligand binding and it involves a series of proteolytic cleavages in the vicinity of the Notch transmembrane domain (reviewed in [2,3]). In spite of the apparent simplicity of this mechanism, it has become increasingly clear that a variety of post-translational events, including glycosylation, ubiquitination, and endocytic trafficking, regulate the activities of both the Notch receptor and its ligands. The core features of Notch signaling and regulatory modifications have been covered in several excellent recent reviews [4–7]. In this brief article, we instead concentrate on recent developments and emerging ideas in one area of the pathway – endocytosis and endosomal trafficking of Notch and its ligands –focusing particularly on insights from research in Drosophila.
Notch receptor and ligand endocytosis: new insights from structural studies
The importance of endocytosis for Notch signaling was first appreciated through genetic studies which revealed that blocking dynamin-dependent internalization interfered with Notch signaling in Drosophila [8,9]. These studies pointed to roles for endocytosis in both the signal-sending and signal-receiving cells, suggesting that internalization of both ligand and receptor regulates pathway activation. Structural studies have now provided a glimpse into the biochemical details of how endocytic forces could promote Notch activation. Most Notch receptors at the cell surface are heterodimeric, single-pass transmembrane molecules in which the large extracellular domain (or ectodomain) is non-covalently attached to a membrane-embedded C-terminal transmembrane/intracellular fragment (CTF); the heterodimer is generated in the secretory pathway by a cleavage called S1. The first ligand-induced cleavage, which is termed S2 and is performed by members of the ADAM/TACE family of metalloproteases, occurs at a site in the short extracellular stub of the CTF [9–11]. High-resolution crystal structures have now revealed that this site is deeply embedded within the Notch heterodimer prior to ligand stimulation, and hence protected from metalloprotease cleavage [12]. Ligand binding triggers conformational changes that lift the Notch ectodomain away from the membrane-anchored CTF and provide access to the ADAM/TACE cleavage site. Where do the physical forces for these critical conformational changes originate? In an unusual application of atomic force microscopy to Notch signaling in cell culture, Ahimou and colleagues [13] demonstrated that the binding of Notch to its ligand Delta is remarkably strong, consistent with the idea that internalization of either ligand or receptor could pull the ligand-bound Notch ectodomain away from the CTF. In this model, the dynamic membrane invaginations that take place during endocytosis are utilized to generate the forces that dissociate the Notch heterodimer and induce its proteolytic activation [9].
Initial studies of endocytosis in Notch signaling focused on the role of ligand internalization in the signal-sending cell. Blocking dynamin-dependent endocytosis interferes with the ability of ligand-presenting cells to signal [8], and a specialized endocytic pathway involving the epsin Liquid facets and the E3 ubiquitin ligases Neuralized and Mind bomb is required for ligand signaling [14–16]. Endocytosis of ligand within the signal-sending cell could potentiate signaling by contributing to the physical forces needed to separate the Notch heterodimer. Indeed, a recent study in mammalian cell culture indicates that endocytosis of Notch-bound ligand leads to the non-enzymatic dissociation of Notch heterodimers, rendering them susceptible to S2 cleavage and activation [17]. In addition, endocytosis followed by subsequent recycling to the plasma membrane might serve to spatially concentrate ligand molecules in a specialized membrane environment that promotes receptor activation. These events might also be associated with post-translational modifications of ligands in endosomal compartments that increase ligand signaling potency. For example, monoubiquitination of the intracellular domain of the ligand Delta is associated with increased signaling [16], although the biochemical basis for this effect remains to be elucidated.
Notch signaling and its attenuation in endocytic compartments
Recent work has highlighted an important role for endocytosis of not only Notch ligands but also the Notch receptor itself in productive signaling. Notch is continuously internalized into early endosomes and subsequently sorted to other endocytic compartments, including recycling endosomes, multivesicular bodies/late endosomes, and lysosomes. These trafficking steps have complex effects on Notch signaling, both promoting signaling from ligand-induced Notch while preventing inappropriate signaling from the pool of Notch that has not been activated by ligand. A systematic analysis of mutations inactivating endocytic regulators has allowed a general paradigm to emerge. Factors that promote cargo internalization from the cell surface and entry into early endosomes (for instance, Dynamin and Rab5) are needed for productive ligand-dependent Notch signaling [18,19]. In contrast, mutations in ESCRT components, which sort ubiquitinated proteins from early endosomes into multivesicular bodies, prevent Notch degradation and exhibit elevated, ligand-independent signaling [19–23]. Inactivation of the endosomal C2 domain-containing protein Lethal giant discs (Lgd) also results in Notch endosomal accumulation and ectopic ligand-independent signaling activity [24–26]. Although the early endosome cargo recruitment factor Hrs is not generally required for Notch signaling [27,28], it is needed for the ectopic Notch signaling seen in lgd mutants [24–26], again underscoring the link between active Notch signaling and entry into early endosomes. The observation that endosomal access is required for effective Notch activation while endosomal trapping is sufficient to activate even unliganded Notch indicates that receptor kinetics and flux through endosomal compartments can have important consequences for Notch signaling. Viewing Notch trafficking as a continuous flow of activated and unactivated receptors through the various endosomal compartments at a relatively steady rate in a given cell raises the possibility that flow-modulating effects might be physiologically relevant in vivo.
While much remains to be learned about how different forms of internalized Notch are correctly sorted into trafficking routes needed for signaling, recycling, or degradation, it is evident that Notch ubiquitination plays a central role. Several E3 ubiquitin ligases, including members of the conserved Cbl family as well as Drosophila Suppressor of Deltex, mammalian Itch, and C. elegans SEL-10, have been implicated in the sorting and lysosomal degradation of unactivated Notch [29–32]. Although the genetic activities of these ubiquitin ligases are consistent with the idea that they directly modify the Notch intracellular domain, causing it to be recognized by the ESCRT trafficking machinery or associated sorting factors, direct evidence for this model still needs to be obtained. The precise biochemical effects of ubiquitination on Notch, the locations and functional consequences of specific ubiquitination events, and the identity of key accessory factors are all areas that deserve further attention.
New studies have also clarified an auxiliary mode of Notch signaling that involves activity of the E3 ubiquitin ligase Deltex. Deltex orthologues are found in Drosophila and mammals, although not in C. elegans, and complete elimination of Deltex function affects only a narrow subset of Notch-dependent patterning processes in Drosophila, indicating that Deltex augments signaling in some contexts but is not required for most Notch signaling [33]. Deltex is needed for normal endosomal trafficking of Notch [34] and it physically associates with both Notch and a protein that promotes Notch degradation – the β-arrestin Kurtz [35]. Taken together, these observations suggest that Kurtz acts as an E3 adapter for Deltex, allowing it to modulate the differential sorting of Notch into the degradative late endosomal/lysosomal compartment, as opposed to other endosomal compartments that facilitate ligand-induced signaling. Further support for this model has come from a new analysis of the fly HOPS and AP-3 complexes, which regulate aspects of late endosomal/lysosomal biogenesis and protein trafficking. Genetic impairment of these complexes in Drosophila causes a loss of Deltex-dependent Notch signaling, strongly suggesting that Deltex targets a pool of intact Notch to the limiting membrane of the lysosome, where Notch heterodimer dissociation and/or degradation generates signaling activity from the resulting Notch CTF [36]. What is the biological significance of this lysosomal Notch signaling? An important clue is provided by the fact that Deltex-mediated Notch signaling does not require ligand, and hence reflects a cell-intrinsic signal that evidently is not initiated by neighboring cell contact. This mechanism might be utilized by some cells to maintain a basal level of Notch activation that dampens signaling noise or potentiates signaling induced by ligand.
From the cell surface to the lysosome – where does γ-secretase fit in?
Following ligand binding and Notch heterodimer dissociation, the resulting membrane-tethered Notch CTF undergoes the S3 cleavage mediated by γ-secretase, an intramembrane aspartyl protease, to release a soluble intracellular Notch signaling fragment (reviewed in [2,3]). The growing appreciation of the extent to which Notch activation and down-regulation are influenced by endocytic trafficking raises questions about how γ-secretase proteolysis of Notch is related to Notch trafficking. Genetic results demonstrate that Notch signaling emanating from certain endosomal compartments, including the Hrs-positive endosomal compartment in lgd and ESCRT gene mutants as well as the lysosomal compartment associated with Deltex-dependent signaling, requires functional γ-secretase [19,26,36]. Thus γ-secretase might be capable of cleaving Notch at several different points in the endocytic trafficking pathway. One simple idea is that γ-secretase is able to cleave and activate Notch receptors wherever they are encountered in the trafficking system as long as the Notch ectodomain has been shed, which can be accomplished by ligand binding, lysosomal degradation, or other mechanisms that cause heterodimer dissociation. Arguing against this idea is the fact that Notch cleavage by γ-secretase in an ex vivo assay that forces heterodimer dissociation still depends on endosomal access, indicating that entry of Notch into endosomes has another, essential regulatory function in Notch activation [19].
An alternative possibility is that the membrane composition or microenvironment of each trafficking compartment might exert direct effects on the intramembrane proteolysis of Notch by γ-secretase. In the case of Amyloid Precursor Protein (APP), a well-characterized γ-secretase substrate, cleavage products are produced in distinct membrane compartments including the cell surface and the endosomal/lysosomal system [37–39]. The γ-secretase cleavage site near the middle of the APP transmembrane domain displays considerable positional heterogeneity, producing peptides from APP that differ by only a few amino acids, yet show markedly different biological toxicities (reviewed in [40]). A new analysis of intracellular Notch fragments generated from the γ-secretase transmembrane cleavage site near the inner leaflet of the membrane suggests a similar potential for cleavage site heterogeneity. Amino acid substitutions in the Notch transmembrane domain or juxtamembrane intracellular region can shift the precise location of γ-secretase cleavage by up to a few residues, producing Notch signaling fragments with different stabilities and, hence, different signal-transducing potencies [41]. It will be fascinating to determine whether these alternative cleavage sites are ulitized in vivo to accomplish specific signaling outcomes.
Hints that specific biophysical features of the different endocytic compartments might impinge upon Notch signaling have emerged from genetic studies. Drosophila mutants lacking phosphocholine cytidyltransferase activity show aberrant Notch signaling and trafficking, raising the possibility that altering lipid biosynthesis and membrane composition can have subtle effects on Notch signaling [42]. The Big brain (Bib) protein is a Drosophila channel of the aquaporin family that localizes to the plasma membrane and intracellular vesicles [43] and is needed for optimal Notch signaling in some cell lineages [43,44]. Bib was reported recently to promote endosome maturation, Notch trafficking, and acidification of the endosome pathway [45], but subsequent studies indicate that the overt Notch trafficking effects are due to an unlinked locus (R. Kanwar, M. E. Fortini, S. Bray, and T. Klein, unpubl. results). Analysis of new bib mutant stocks confirms that Bib is needed for acidification of the endocytic pathway, which might potentially impact alternative γ-secretase cleavage site usage in Notch. While it remains to be determined whether and how endocytic acidification might affect Notch activation, progressive acidification is a highly conserved feature of the endocytic trafficking system [46], and γ-secretase is reported to be enzymatically more active in the low pH environment of the lysosome [39].
Asymmetric segregation of endosomes during cell division: biasing Notch signaling through new trafficking patterns
Recent studies have led to a growing appreciation that the asymmetric segregation of endosomes during cell division plays a prominent role in the regulation of Notch signaling. Remarkably, several different asymmetrical trafficking mechanisms appear to operate simultaneously in the sensory organ precursor (SOP) lineages of the Drosophila peripheral nervous system. In one mechanism, a membrane-associated inhibitor of Notch signaling, termed Numb, is localized along a crescent-shaped zone along one side of the dividing cell, such that it segregates asymmetrically into one of the resulting daughter cells. In this daughter cell, Numb might inhibit Notch activation by linking Notch to transport vesicles that target it for accelerated degradation [47] and/or by sequestering and inactivating the positive Notch effector Sanpodo within endocytic vesicles [48–50]. In another mechanism that leads to asymmetric Notch signaling activity in the SOP lineages, the E3 ubiquitin ligase Neuralized is also asymmetrically partitioned between daughter cells and, by virtue of its action in promoting ligand endocytosis, enhances the signaling potency of ligand in one daughter cell relative to the other [51]. This asymmetry in ligand potency is reinforced by a Numb- and Neuralized-independent mechanism whereby preferential entry of the ligand Delta into a Rab11-positive, Sec15-dependent recycling compartment in one daughter cells increases its signaling activity [52,53].
Remarkably, in addition to these mechanisms that increase Delta signaling and negatively regulate Notch activity in one SOP daughter cell, a new study reveals that asymmetric segregation of Notch- and Delta-containing endosomes that are already present in the parental SOP cell prior to cell division also contributes to the Notch signaling bias between daughter cells [54]. In the Drosophila SOP cell that is to undergo mitosis, specialized endosomes are found that contain Notch, Delta, and the protein Sara, and they segregate into one daughter cell in preference to the other. These Sara-positive endosomes contain γ-secretase activity and generate the active Notch signaling fragment, and hence they confer an increased level of Notch signaling to the daughter cell that inherits them. This mechanism is a striking example of selective transmission of not just a single protein or asymmetric determinant, but of an intact endosomal organelle from a parental cell to one of two daughter cells. The asymmetric sorting of these Sara endosomes illustrates the great potential for exploiting the flexibility of the endosomal trafficking system in the fine-tuning of Notch signaling.
Future prospects
While much still remains to be learned about the endocytic trafficking of Notch and its ligands, it is already abundantly clear that both the early and late endocytic compartments exert important regulatory controls on productive signaling and signal attenuation. The importance of receptor internalization and intracellular trafficking is now appreciated for many different signaling pathways, raising the possibility that some trafficking mechanisms that influence Notch activity might play even more general roles in biological signal transduction.
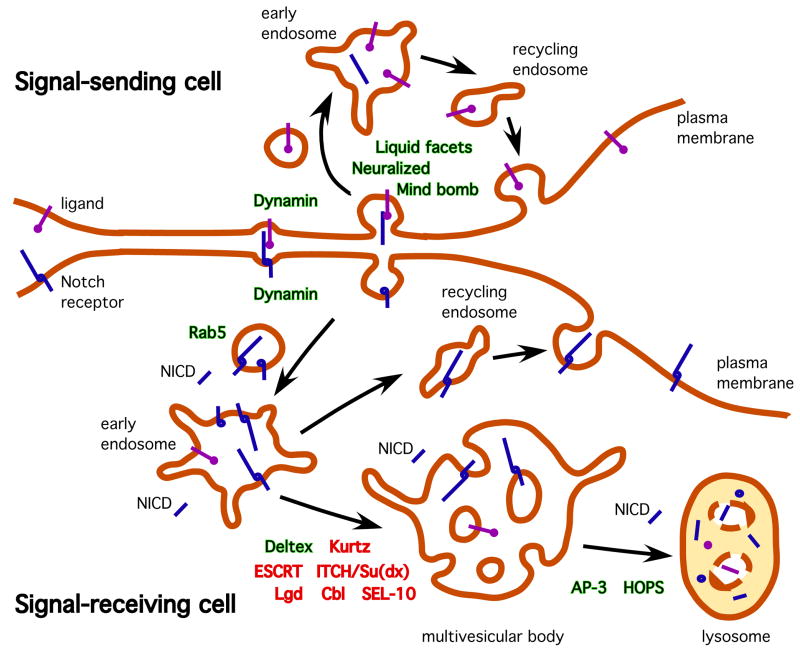
Figure 1.
Overview of ligand and receptor endocytosis in the Notch signaling pathway. In the signal-sending cell (top), endocytosis of Notch ligands (violet) is needed for their productive signaling activity. Ligand endocytosis generates the physical forces needed to dissociate and activate Notch receptors in nascent invaginating endocytic vesicles, and it might also concentrate ligand molecules through their trafficking and/or post-translational modification in the recycling compartment. In the signal-receiving cell (bottom), Notch receptors (blue) are internalized into endocytic vesicles and subsequently routed to early endosomes. Ligand-activated Notch is sorted into multivesicular bodies and thence to lysosomes for degradation; non-ligand-activated Notch can undergo trafficking to the cell surface via recycling endosomes. The identities of various endocytic factors involved in these trafficking steps are shown near their approximate sites of activity; those that promote signaling are indicated in green whereas those involved in signaling down-regulation are indicated in red. The γ-secretase-mediated cleavage of Notch that generates the active signaling fragment NICD can occur at different points in the endosomal trafficking pathway (see text for details).
Acknowledgments
We apologize to all our colleagues whose studies could not be covered due to space limitations. M.E.F. is supported by National Institutes of Health grant 1R01GM087650 and funds from the Department of Biochemistry and Molecular Biology, Kimmel Cancer Center, Thomas Jefferson University; D.B. is supported by National Institutes of Health grant R01GM068675 and American Cancer Society grant RSG-07-040-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Weinmaster G. Notch signal transduction: a real rip and more. Curr Opin Genet Dev. 2000;10:363–369. doi: 10.1016/s0959-437x(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 3.Kopan R, Goate A. A common enzyme connects Notch signaling and Alzheimer’s disease. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- 4.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 5.Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol. 2006;18:213–222. doi: 10.1016/j.ceb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol. 2003;4:786–797. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- 7.Tien A-C, Rajan A, Bellen HJ. A Notch updated. J Cell Biol. 2009;184:621–629. doi: 10.1083/jcb.200811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. Classic study that first revealed the requirement for dynamin-dependent endocytosis in Notch signaling. The observation of autonomous and non-autonomous effects was an early indication that both the ligand and the receptor must undergo endocytosis for productive signaling. [DOI] [PubMed] [Google Scholar]
- 9•.Parks AL, Klueg KM, Stout JR, Muskavitch MAT. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. The first study to propose that ligand endocytosis generates the forces that induce conformational changes in the Notch receptor, triggering dissociation of the Notch heterodimer and its activation, a mechanism that is now widely recognized as a key step in Notch signaling. [DOI] [PubMed] [Google Scholar]
- 10.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Isräel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 11.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 12••.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. This structural analysis of Notch receptor inhibition reveals that a specific extracellular domain of Notch, termed the NRR, is responsible for shielding a key cleavage site, thereby protecting Notch from inappropriate and potentially oncogenic activation. [DOI] [PubMed] [Google Scholar]
- 13.Ahimou F, Mok LP, Bardot B, Wesley C. The adhesion force of Notch with Delta and the rate of Notch signaling. J Cell Biol. 2004;167:1217–1229. doi: 10.1083/jcb.200407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 15.Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 17.Nichols JT, Miyamoto A, Olsen SL, D’Souza B, Yao C, Weinmaster G. DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol. 2007;176:445–458. doi: 10.1083/jcb.200609014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 19•.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. This paper presents key genetic evidence that Notch receptor activation and γ-secretase-mediated Notch cleavage require entry of Notch into endosomes in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Thompson BJ, Mathieu J, Sung H-H, Loeser E, Rørth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating Notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Childress JL, Acar M, Tao C, Halder G. Lethal giant discs, a novel C2-domain protein, restricts Notch activation during endocytosis. Curr Biol. 2006;16:2228–2233. doi: 10.1016/j.cub.2006.09.031. Together with references 25 and 26, this study demonstrates that the endosomal factor Lethal giant discs is needed to prevent inappropriate Notch activation during its endosomal trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Gallagher CM, Knoblich JA. The conserved C2 domain protein Lethal(2) Giant Discs regulates protein trafficking in Drosophila. Dev Cell. 2006;11:641–653. doi: 10.1016/j.devcel.2006.09.014. Together with references 24 and 26, this study demonstrates that the endosomal factor Lethal giant discs is needed to prevent inappropriate Notch activation during its endosomal trafficking. [DOI] [PubMed] [Google Scholar]
- 26•.Jaekel R, Klein T. The Drosophila Notch inhibitor and tumor suppressor gene lethal(2) giant discs encodes a conserved regulator of endosomal trafficking. Dev Cell. 2006;11:655–669. doi: 10.1016/j.devcel.2006.09.019. Together with references 24 and 25, this study demonstrates that the endosomal factor Lethal giant discs is needed to prevent inappropriate Notch activation during its endosomal trafficking. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 28.Jékely G, Rørth P. Hrs mediates downregulation of multiple signalling receptors in Drosophila. EMBO Rep. 2003;4:1163–1168. doi: 10.1038/sj.embor.7400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard EJ, Wu G, Kitejewski J, Greenwald I. sel-10, a negative regulator of lin-12 activity in Caenorhabditis elegans, encodes a member of the CDC4 family of proteins. Genes Dev. 1997;11:3182–3193. doi: 10.1101/gad.11.23.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jehn BM, Dittert I, Beyer S, von der Mark K, Bielke W. c-Cbl binding and ubiquitin-dependent lysosomal degradation of membrane-associated Notch1. J Biol Chem. 2002;277:8033–8040. doi: 10.1074/jbc.M108552200. [DOI] [PubMed] [Google Scholar]
- 31.Sakata T, Sakaguchi H, Tsuda L, Higashitani A, Aigaki T, Matsuno K, Hayashi S. Drosophila Nedd4 regulates endocytosis of Notch and suppresses its ligand-independent activation. Curr Biol. 2004;14:2228–2236. doi: 10.1016/j.cub.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 32.Wilkin MB, Carbery AM, Fostier M, Aslam H, Mazaleyrat SL, Higgs J, Myat A, Evans DA, Cornell M, Baron M. Regulation of Notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol. 2004;14:2237–2244. doi: 10.1016/j.cub.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Fuwa TJ, Hori K, Sasamura T, Higgs J, Baron M, Matsuno K. The first deltex null mutant indicates tissue-specific Deltex-dependent Notch signaling in Drosophila. Mol Genet Genomics. 2006;275:251–263. doi: 10.1007/s00438-005-0087-3. [DOI] [PubMed] [Google Scholar]
- 34.Hori K, Fostier M, Ito M, Fuwa TJ, Go MJ, Okano H, Baron M, Matsuno K. Drosophila Deltex mediates Suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development. 2004;131:5527–5537. doi: 10.1242/dev.01448. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual β-arrestin. Nature Cell Biol. 2005;7:1191–1201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- 36••.Wilkin MB, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, Hori K, Taniguchi-Kanai M, Franklin E, Matsuno K, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of Notch in the endosomal trafficking pathway. Dev Cell. 2008;15:762–772. doi: 10.1016/j.devcel.2008.09.002. This study provides important insights into a mechanism that routes Notch to the limiting membrane of the lysosome, where a basal level of Notch signaling is generated that might be important for dampening signaling noise or boosting the responsiveness of ligand-induced Notch signaling. [DOI] [PubMed] [Google Scholar]
- 37.Lah JJ, Levey AI. Endogenous presenilin-1 targets to endocytic rather than biosynthetic compartments. Mol Cell Neurosci. 2000;16:111–126. doi: 10.1006/mcne.2000.0861. [DOI] [PubMed] [Google Scholar]
- 38.Kaether C, Lammich S, Edbauer D, Ertl M, Rietsdorf J, Capell A, Steiner H, Haass C. Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol. 2002;158:551–561. doi: 10.1083/jcb.200201123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasternak SH, Bagshaw RD, Guiral M, Zhang S, Ackerley CA, Pak BJ, Callahan JW, Mahuran DJ. Presenilin-1, Nicastrin, Amyloid Precursor Protein, an γ-secretase activity are co-localized in the lysosomal membrane. J Biol Chem. 2003;278:26687–26694. doi: 10.1074/jbc.m304009200. [DOI] [PubMed] [Google Scholar]
- 40.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 41••.Tagami S, Okochi M, Yanagida K, Ikuta A, Fukumori A, Matsumoto N, Ishizuka-Katsura Y, Nakayama T, Itoh N, Jiang J, et al. Regulation of Notch signaling by dynamic changes in the precision in S3 cleavage of Notch-1. Mol Cell Biol. 2008;28:165–176. doi: 10.1128/MCB.00863-07. This study re-examines Notch receptor mutations that were previously interpreted as preventing the γ-secretase cleavage of Notch, reaching the surprising conclusion that they instead shift the cleavage position and lead to unstable NICD fragments. These findings emphasize the importance of precise γ-secretase cleavage site positioning in generating alternative NICD species with widely differing biological potencies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber U, Eroglu C, Mlodzik M. Phospholipid membrane composition affects EGF receptor and Notch signaling through effects on endocytosis during Drosophila development. Dev Cell. 2003;5:559–570. doi: 10.1016/s1534-5807(03)00273-9. [DOI] [PubMed] [Google Scholar]
- 43.Doherty D, Jan LY, Jan YN. The Drosophila neurogenic gene big brain, which encodes a membrane-associated protein, acts cell autonomously and can act synergistically with Notch and Delta. Development. 1997;124:3881–3893. doi: 10.1242/dev.124.19.3881. [DOI] [PubMed] [Google Scholar]
- 44.Rao Y, Jan LY, Jan YN. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990;345:163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- 45.Kanwar R, Fortini ME. The Big brain aquaporin is required for endosome maturation and Notch receptor trafficking. Cell. 2008;133:852–863. doi: 10.1016/j.cell.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. Journal of Experimental Biology. 1992;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- 47.McGill MA, McGlade CJ. Mammalian Numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278:23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor-Giles KM, Skeath JB. Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev Cell. 2003;5:231–243. doi: 10.1016/s1534-5807(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 49.Hutterer A, Knoblich JA. Numb and α-adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep. 2005;6:836–842. doi: 10.1038/sj.embor.7400500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roegiers F, Jan LY, Jan YN. Regulation of membrane localizaiton of Sanpodo by lethal giant larvae and neuralized in asymmetrically dividing cells of Drosophila sensory organs. Mol Biol Cell. 2005;16:3480–3487. doi: 10.1091/mbc.E05-03-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Borgne R, Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev Cell. 2003;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 52.Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gonzalez Gaitan M, Knoblich JA. Asymmetric Rab11 endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes Notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 54••.Coumailleau F, Fürthauer M, Knoblich JA, González-Gaitán M. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature. 2009 doi: 10.1038/nature07854. in press. An intriguing mechanism is uncovered in this study, whereby Notch- and Delta-containing endosomes that are actively contributing to γ-secretase-mediated Notch activation and signaling are asymmetrically transmitted from a cell to one of its daughters during mitosis, leading to a biased outcome in Notch signaling between the two daughter cells. [DOI] [PubMed] [Google Scholar]