Abstract
Neutrophil migration into injured tissues is invariably accompanied by pain. Bv8/prokineticin 2 (PK2), a chemokine characterized by a unique structural motif comprising five disulfide bonds, is highly expressed in inflamed tissues associated to infiltrating cells. Here, we demonstrate the fundamental role of granulocyte-derived PK2 (GrPK2) in initiating inflammatory pain and driving peripheral sensitization. In animal models of complete Freund's adjuvant-induced paw inflammation the development and duration of pain temporally correlated with the expression levels of PK2 in the inflamed sites. Such an increase in PK2 mRNA depends mainly on a marked up-regulation of PK2 gene transcription in granulocytes. A substantially lower up-regulation was also detected in macrophages. From a pool of peritoneal granulocytes, elicited in rats by oyster glycogen, we purified the GrPK2 protein, which displayed high affinity for the prokineticin receptors (PKRs) and, when injected into the rat paw, induced hypersensitivity to noxious stimuli as the amphibian prokineticin Bv8 did. Mice lacking PKR1 or PKR2 developed significantly less inflammation-induced hyperalgesia in comparison with WT mice, confirming the involvement of both PKRs in inflammatory pain. The inflammation-induced up-regulation of PK2 was significantly less in pkr1 null mice than in WT and pkr2 null mice, demonstrating a role of PKR1 in setting PK2 levels during inflammation. Pretreatment with a nonpeptide PKR antagonist, which preferentially binds PKR1, dose-dependently reduced and eventually abolished both prokineticin-induced hypernociception and inflammatory hyperalgesia. Inhibiting PK2 formation or antagonizing PKRs may represent another therapeutic approach for controlling inflammatory pain.
Keywords: hypernociception, inflammation, prokineticin receptors
In past years, several groups have generated convincing evidence that neutrophil-associated hyperalgesia results from the neutrophil release of proinflammatory pronociceptive factors, including products of arachidonic acid metabolism (1, 2) and cytokines such as IL-1β, IL-8, IL-12, TNF-α, and macrophage inflammatory proteins MIP-1a and MIP-1b (3, 4). Belonging to the chemokine family, the recently discovered prokineticins [Bv8, MIT, prokineticin 1 (PK1), and prokineticin 2 (PK2)] (5–9) activate two G protein-linked PK receptors (PKR1 and PKR2) (10–12) localized in the brain, dorsal root ganglia (DRG) neurons, granulocytes, macrophages, and endothelial cells. Bv8 was initially identified in the skin secretion of the frog Bombina variegata, as a secretory protein that induced hyperalgesia and gastrointestinal motility. Later on, the human orthologues of this highly conserved protein (PK1 or EG-VEGF and PK2 or mammalian Bv8) were shown to promote angiogenesis and modulate neurogenesis, circadian rhythms, hematopoiesis, and immune response (13). Activation of PKRs in primary afferent C fibers sensitizes nociceptors to thermal, mechanical, and chemical stimuli (14–16). In neutrophils, macrophages, and dendritic cells it promotes chemotaxis and cytokine release (17–20), and in capillary endothelial cells it stimulates angiogenesis (21). Testis, peripheral blood leukocytes, and macrophages express two mRNA transcripts of PK2, one coding for the canonical PK2 and the other for an elongated form containing additional 21 basic amino acids between Lys-47 and Val-48 of the mature PK2 protein, named PK2L (6, 19, 22). In inflamed tissues both PK2 and PK2L mRNA transcripts are overexpressed (15), but the mature proteins have not yet been isolated from inflammatory cells nor tested for their biological activity.
The goal of the present experiments is to demonstrate that the granulocyte-derived prokineticins may be major determinants in triggering inflammatory pain and PKRs may represent a therapeutic target for the development of novel peripherally acting antinociceptive drugs. First, we demonstrate, in animal models of inflammatory pain, that PK2 and PK2L expression levels timely correlate with the beginning and development of pain behavior. We identified the inflammatory cells that synthesize PK2 proteins and investigated whether PK2 protein released by these cells can produce hyperalgesia. Then, using pkr1- and pkr2-null mice, we evaluated the role of PKRs in modulating PK2 expression levels and inflammatory hyperalgesia. Finally, we studied the antinociceptive activity of a selective nonpeptide PKR antagonist (23) in acute inflammatory pain.
Results
PK2 Expression Levels Timely Correlate with Development and Duration of Inflammatory Pain.
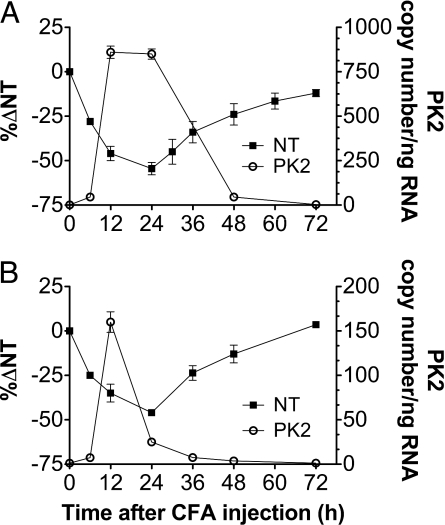
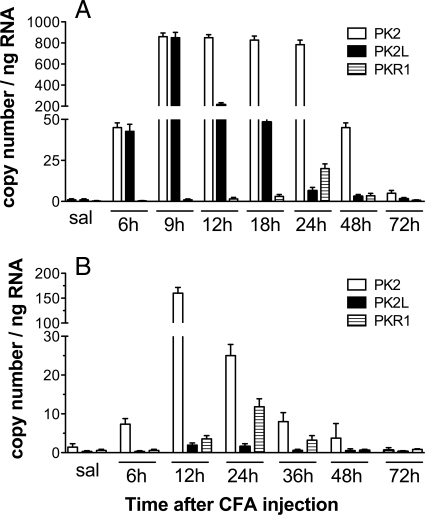
Intraplantar (ipl) injection of complete Freund's adjuvant (CFA) produces an inflammatory reaction with a concomitant swelling of the paw and hypernociception. In both rats and mice the hyperalgesia was already evident 6 h after injection, peaked at 24 h, and recovered to baseline levels in 72 h in mice, but lasted longer in rats. Significant edema formation occurred at the sixth hour. Swelling was maximal after ≈24 h, then began to decrease. Paw volume returned toward normal values in ≈3 days in mice, but in 1 week or more in rats. In both rats and mice PK2 mRNA expression levels in the paw tissue correlate with the time course of the CFA-induced hyperalgesia (Fig. 1). RT-PCR analysis performed on rat pad skin (Fig. 2A), collected at several time points after saline or CFA injection, revealed that expression levels of PK2 and PK2L in saline-injected paws were about equally low. In CFA-injected paws PK2 and PK2L expression strongly increased, reaching levels several hundred-fold higher than in the contralateral saline-injected paw (900 ± 105 copy number per ng RNA vs. 1 ± 1 copy number per ng RNA). PK2 expression was maximal from 9 to 24 h then began to decrease. At 72 h, it was still ≈10 times higher than in the control paw. PK2L peaked at 9 h and returned to normal levels in 72 h. In mice (Fig. 2B), in saline- and CFA-injected paws the expression levels of PK2L were significantly lower than those of PK2 (0.02 ± 0.01 and 1.3 ± 1 copy number per ng RNA vs. 1.8 ± 1 and 160 ± 25 copy number per ng RNA). PK2 was strongly up-regulated by CFA injection, reaching at 12 h an expression level 120-fold higher than in contralateral saline-injected paw. In both rats and mice PKR1 transcripts peaked 24 h after CFA injection, reaching levels 15- to 30-fold higher than those in the contralateral noninflamed paw (20 ± 3 vs. 0.6 ± 0.5 copy number per ng RNA in rats; 12.5 ± 3 vs. 0.65 ± 0.5 copy number per ng RNA in mice). PK1 and PKR2 transcript levels were very low and did not change after CFA injection.
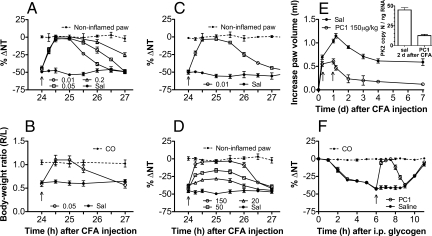
Fig. 1.
Correlation between the time course of PK2 mRNA expression in inflamed paws (right y axis) and hyperalgesia (left y axis) after ipl injection of CFA in rats (A) and mice (B).
Fig. 2.
Time course of PK2, PK2L, and PKR1 expression in rat and mouse paw tissue after ipl CFA, evaluated by real-time RT-PCR. (A) In rats expression levels of PK2 and PK2L were comparable. (B) In mice the expression levels of PK2L were significantly lower than those of PK2. In both rats and mice PKR1 transcripts peaked 24 h after CFA injection. Results are presented as input copy number of the gene of interest per ng of total RNA.
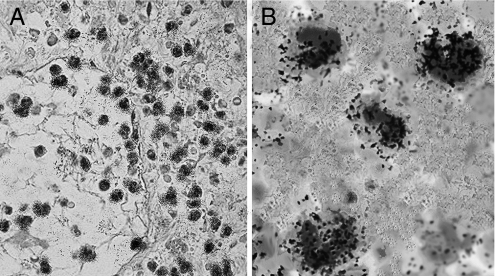
In situ hybridization with radiolabeled PK2 riboprobes was performed on sections of mouse paw 12 h after CFA or saline injection. Strong signal was evident only in CFA-injected paws, mainly around vessels, which probably was associated with infiltrating cells (Fig. 3).
Fig. 3.
Autoradiographic images of inflamed mouse paw section (12 h after CFA) hybridized with 35S-PK2 antisense riboprobe and counterstained with toluidine blue–light green solution. (A) PK2 riboprobe revealed PK2 mRNA (black granules) in PMNs along the capillary wall and into extravascular space of the derma (40×). (B) Higher magnification (100×) of PK2-expressing granulocytes.
PK2 and PKR1 mRNA in Cells Sorted from CFA-Injected Mouse Paw.
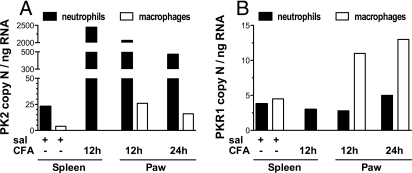
To identify the cells expressing PK2 and quantitate transcript levels of PK2 and PKR1 we prepared cell suspensions from mouse paws collected 12 and 24 h after CFA or saline injection. To obtain enough material we pooled inflamed paws of 20 mice for each point. Flow cytometric analysis indicated that neutrophils (Mac-1+Gr-1+) were more abundant than macrophages (Mac-1+Gr-1−) in the paws collected 12 h after CFA (18 ± 3% and 9 ± 3% of total isolated cells, respectively), but macrophages became as abundant as neutrophils 24 h after CFA injection (25 ± 4% and 22 ± 5%, respectively). CD3+ lymphocytes were undetectable. There were no detectable infiltrating cells in noninflamed paw; hence we used neutrophils and macrophages obtained from the spleen of control mice as a model of nonactivated immune cells. RT-PCR analysis performed on neutrophils and macrophages sorted from spleen of control mice demonstrated that neutrophils are the major source of PK2 mRNA (PK2 copy number per ng RNA: 23 in neutrophils vs. 3 in macrophages). The paw inflammation induced a marked PK2 up-regulation in both spleen (2,500 copy number per ng RNA) and paw (2,060 copy number per ng RNA) neutrophils. A substantially lower up-regulation (8- to 10-fold) was detected in macrophages (Fig. 4A). PKR1 transcript levels were comparable in neutrophils and macrophages from spleen of control mice. The inflammation did not change PKR1 levels in neutrophils but increased it ≈3 times in activated macrophages (Fig. 4B).
Fig. 4.
PK2 (A) and PKR1 (B) expression in immune cells purified by FACS from spleen of saline- or 12-h CFA-injected mice, and from paws of 12- and 24-h CFA-injected mice. Inflammation dramatically increased PK2 expression in neutrophils and slightly increased PKR1 expression in macrophages. PKR1 expression was 3-fold higher with respect to control cells. Results are presented as input copy number of the gene of interest per ng of total RNA.
Granulocyte-Derived PK2 (GrPK2) Induces Hyperalgesia.
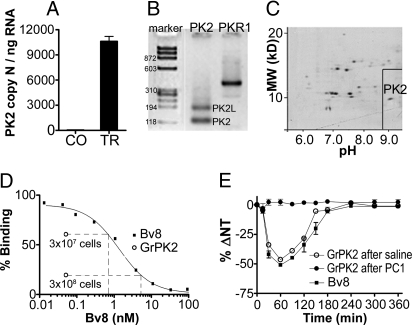
In rats, i.p. injection of oyster glycogen induced marked extravasation of granulocytes into the peritoneal cavity and decreased the systemic nociceptive threshold as assessed with the paw-pressure test. Hyperalgesia started 2 h after i.p. injection was maximal from 6 to 9 h, then decreased showing normal values 12 h after i.p. injection (see Fig. 8F). Control rats, i.p.-injected with saline, did not show any changes in the paw-pressure threshold over a 9-h observation period. RT-PCR analysis demonstrated that extravasated peritoneal granulocytes, collected 6 h after glycogen injection, express PK2 mRNA at levels enormously higher (10,500 ± 900 copy number per ng mRNA) than the peritoneal granulocytes collected 6 h after saline injections (2.3 ± 2 copy number per ng mRNA) (Fig. 5A). Gel electrophoresis of RT–PCR amplification products demonstrated that oyster glycogen-elicited peritoneal granulocytes express both PK2 isoforms, the canonical PK2 similar to the amphibian Bv8 and the long form, PK2L, already described in mouse and human testis (6, 22). Strong signal corresponding to PKR1 mRNA is also evident (Fig. 5B). 2D electrophoresis of peritoneal granulocyte proteins revealed a spot of ≈10 kDa and 8.9 pI that subsequent purification steps and biological tests allowed us to identify as authentic rat PK2 (Fig. 5C). We did not identify any PK2L-like protein. The PK2 protein purified from rat peritoneal granulocytes (GrPK2) concentration-dependently displaced 125I-MIT from membrane preparation of CHO cells stably transfected with the PKR1 (Fig. 5D) and, when injected ipl in rats, produced Bv8-like hyperalgesia that was antagonized by prior administration of the PKR1-preferring antagonist PC1 (0.01 μg/ipl) (Fig. 5E). Starting with 2 × 109 peritoneal granulocytes, the estimated yield in Bv8-like protein was ≈320 ng. Analysis of the N-terminal fragment (PRIMM) revealed the amino acid sequence AVITGACDKD that perfectly matches that of rat PK2 (Swiss Protein Bank accession no. NM_138852).
Fig. 8.
Antihyperalgesic and antinflammatory effect of ipl and s.c. injection of the PKR1-preferring antagonist PC1 in mice and rats. (A and B) In mice ipl injection of PC1 24 h after CFA reverted the thermal hyperalgesia (0.01, 0.05, and 0.2 μg) for 1–3 h (A) and abolished the CFA-induced reduction in the body weight-bearing of the inflamed paw (0.05 μg) for ≈1 h (B). (C) In rats, PC1 (0.01 μg, ipl) reverted CFA-induced mechanical hyperalgesia for ≈2 h. (D) In rats, PC1 dose-dependently antagonized the CFA-induced hyperalgesia also by systemic injections (20–150 μg/kg, s.c.), bringing the nociceptive threshold of inflamed paw toward that of contralateral noninflamed paw. (E) In rats, repeated administration of PC1 (150 μg/kg, s.c.), at 6 and 24 h after CFA injection, accelerated recovery of inflamed paw to normal paw volume and significantly reduced the inflammation-induced PK2 up-regulation (Inset). In rats, i.p. injection of oyster glycogen induced marked extravasation of granulocytes into peritoneal cavity and decreased the nociceptive threshold evaluated with the paw-pressure test. PC1 (150 μg/kg, s.c.) abolished the systemic hyperalgesia caused by GrPK2, released into circulation. (F) Intraperitoneal injection of saline did not modify the mechanical nociceptive threshold (CO).
Fig. 5.
Purification of GrPK2. (A) PK2 mRNA expression levels in peritoneal granulocytes collected 6 h after i.p. injection of saline (CO) or oyster glycogen (TR). (B) Gel electrophoresis of PK2, PK2L, and PKR1 transcripts, amplified by RT-PCR, in oyster glycogen-elicited peritoneal granulocytes. (C) 2D electrophoresis of peritoneal granulocyte proteins revealed a spot of ≈10 kDa and 8.9 pI. (D) Displacement curve of 125I-MIT from membrane preparations of PKR1-transfected CHO cells by graded concentrations of Bv8. Granulocyte extract (step C fractions) corresponding to 3 × 107 and 3 × 108 cells displaced 125I-MIT as ≈0.7 and 6 nM Bv8, respectively. (E) Time course of hyperalgesia induced in rats by ipl injection of Bv8 (0.5 ng) or GrPK2 corresponding to 3 × 106 cell lysate after pretreatment with saline or the PKR1-preferring antagonist PC1 (0.01 μg/ipl).
Involvement of the PKRs in Inflammation-Induced Hyperalgesia and PK2 Up-Regulation.
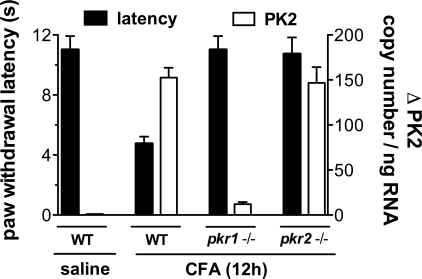
In WT mice CFA-induced inflammation reduced paw withdrawal latency to the noxious thermal stimulus and greatly up-regulated PK2 mRNA with respect to noninflamed paw (saline). In comparison with WT-mice, pkr1 (15) or pkr2 gene disruption significantly reduced CFA-induced inflammatory hypersensitivity, but only the pkr1 gene disruption reduced inflammatory PK2 mRNA up-regulation. Indeed, PK2 transcript levels 12 h after CFA injection were 15-fold lower in pkr1-null mice than in WT mice, whereas PK2 transcript levels in pkr2-null mice were similar to those in WT mice. These results indicate that, although both PKRs modulate inflammatory pain, PKR1 alone is involved in modulating in pkr1-null mice PK2 expression (Fig. 6).
Fig. 6.
In WT mice CFA-induced paw inflammation reduced the paw withdrawal latency (left y axis, paw immersion test, 48 °C) with respect to saline-injected paw (4 vs. 12 s). Withdrawal latency of inflamed paw was significantly longer in pkr1- and pkr2-null mice than in WT mice, whereas inflammation-induced PK2 up-regulation (right y axis) was significantly lower in pkr1-null mice than in WT and pkr2-null mice.
PKR Antagonism Is a Novel Pharmacological Strategy for the Therapy of Inflammatory Pain.
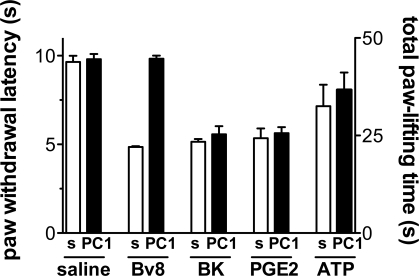
The nonpeptide PKR1-preferring antagonist PC1 (23), preinjected (0.01 μg per paw) into paws of mice (Fig. 7), antagonized the hyperalgesic effect of exogenous Bv8 (0.5 ng per paw) but was inefficacious against other proalgesic mediators such as bradykinin (2 μg per paw), prostaglandin E2 (PGE2) (1 μg per paw), and 2-methylthioATP (100 nmol per paw) (Fig. 7). PC1 local (0.01–0.05 μg per paw) and systemic (up to 150 μg/kg) injection did not change basal thermal and mechanical pain threshold in rats and mice. Moreover, in rats and mice, PC1 antagonized CFA-induced inflammatory pain. In mice, ipl injection of 0.01 μg of PC1 abolished the inflammation-induced thermal hypernociception (Fig. 8A) and 0.05 μg of PC1 ipl. abolished the reduction in the body weight-bearing of the inflamed paw as assessed by the incapacitance test (Fig. 8B) for ≈1 h. Indeed, CFA-induced inflammation caused a significant reduction in body weight distribution (ratio = 0.6) across the hind limbs, as the mice placed less weight through the ipsilateral limb. PC1 (0.05 μg, ipl) allowed the animals to distribute their body weight equally across both hind limbs (ratio = 1, as control mice).
Fig. 7.
Ipl injection of PC1 (0.05 μg) did not change the basal thermal nociceptive threshold of mice (paw-immersion test, 48 °C). Pretreatment with PC1 (0.01 μg, ipl) abolished the thermal hyperalgesia induced by Bv8 (0.5 ng, ipl), but was ineffective in antagonizing the hyperalgesia induced by ipl administration of BK (2 μg) and PGE2 (1 μg) (left y axis) and in reducing the paw-lifting time caused by ipl injection of ATP (100 nmol) (right y axis).
In rats, paw injection of 0.01 μg of PC1 abolished the inflammation-induced mechanical hypernociception for ≈2 h. The nociceptive threshold of the inflamed paw rapidly increased, reaching values near those of the contralateral noninflamed paw in ≈15 min (Fig. 8C). The longer duration of effect of PC1 in mechanical hyperalgesia in rats (Fig. 8C) compared with the effect in thermal hyperalgesia in mice is probably caused by different pharmacokinetics in the two species. Indeed increasing the PC1 doses in mice (0.05–0.2 μg ipl) prolongs the antihyperalgesic effect up to 2–3 h (Fig. 8A). Also by s.c. injection, PC1 (20, 50, and 150 μg/kg) raised the pressure pain threshold of the inflamed paw toward the threshold value of the control paw. The antihyperalgesic effect was dose-dependent and lasted for ≈2 h (Fig. 8D). Repeated systemic administration of PC1 (150 μg/kg at 6 and 24 h after CFA) also reduced the paw edema and accelerated the recovery to normal paw volume after the insult. The PK2 expression levels, assessed 2 days after CFA injection, were significantly lower (4-fold) in the inflamed paws of PC1-treated rats than in the inflamed paws of saline-treated rats (Fig. 8E). PC1 (150 μg/kg, s.c.) abolished the systemic mechanical hyperalgesia caused by oyster glycogen-induced peritonitis (Fig. 8F).
Discussion
We have previously demonstrated that systemic or local injections of very low doses (in the range of fmol) of the amphibian prokineticin Bv8 decrease nociceptive thresholds to thermal, mechanical, and chemical stimuli by activating PKR1 and PKR2 in the primary sensory neurons. This increase in nociceptor excitability resulted from functional cooperation between PKR1 and TRPV1, the two receptors being coexpressed in small sensory neurons of DRG (14–16).
Leukocytes of myeloid origin are a major source of endogenous mammalian Bv8, i.e., the PK2. Bv8/PK2 is overexpressed in inflamed tissue associated to infiltrating cells, hence Bv8/PK2 could be a link between polimorphonuclear cells (PMN) infiltrating the inflamed tissue and the development of inflammatory pain. Here, we demonstrate that the development and duration of CFA-induced hyperalgesia correlates with the expression level of PK2 in the inflamed paw, granulocytes infiltrating the tissue are the inflammatory cells overexpressing PK2, and the PK2 protein, isolated from peritoneal granulocytes, produces Bv8-like hyperalgesia by selectively binding the PKRs. We also demonstrate that blocking the PKRs by a nonpeptide selective PKR antagonist abolishes hypernociception in inflammatory models as well as the deletion of either PKR gene does.
The inflammation produced by paw injection of CFA was of higher intensity and longer duration in rats than in mice, mirroring the increase in PK2 expression that was 10 times higher in rats than in mice and lasted 12 h longer in rats. Granulocytes are the major source of PK2 in the inflamed paw skin; moreover the granulocytes sorted from the mouse paw 12 h after CFA injection are richer in PK2 mRNA than those sorted after 24 h (Fig. 4A), demonstrating a time dependency in CFA-induced up-regulation of PK2 in granulocytes. Nevertheless, granulocytes sorted from the spleen of inflamed mice showed elevated PK2 expression levels like those observed in granulocytes sorted from inflamed paws and were >100 times higher than PK2 levels in granulocytes sorted from the spleen of noninflamed mice. These results indicate that the inflammatory response induced by local CFA injection amplifies PK2 gene transcription in PMN not only locally in the paw but also systemically. The mechanism of inflammation-induced PK2 gene transcription in granulocytes is yet unknown. Inflammatory stimuli in mice and rats are known to produce an early and rapid increase in plasma levels of granulocyte colony-stimulating factor (G-CSF) (24), and, among the numerous cytokines released by inflammatory stimulation, G-CSF is the only cytokine able to activate PK2 transcription in CD11b+Gr1+ bone marrow-derived cells (25). G-CSF is a principal regulator of granulopoiesis and neutrophil mobilization from the bone marrow; thus, the early increase of G-CSF levels in plasma of CFA-inflamed animals could explain the enhanced PK2 transcription in spleen and paw granulocytes.
To support the hypothesis that granulocyte-released Bv8-like proteins directly modulate inflammatory pain, we isolated from granulocyte extracts a single protein that in vitro bound PKR1 and in vivo induced a Bv8-like hyperalgesia. This protein displayed molecular mass, isoelectric point, and N-terminal amino acid sequence identical to those reported for rat PK2. However, rat inflammatory granulocytes also express high levels of PK2L mRNA, a splice variant of Bv8/PK2 gene, encoding a 21-aa insert rich (19 of 21) in basic residues. The biological role of PK2L is still unknown. In functional assays and receptor binding assays the recombinant PK2L protein demonstrated very poor activity, whereas the hypothesized smaller peptide PK2Lβ, derived by proteolytic cleavage of PK2L, retains selective affinity for PKR1 (26, 27). However, these putative proteins were apparently absent in our extracts. These data may be interpreted as granulocytes being unable to translate PK2L transcript into proteins or release such a highly basic protein (pI ≈11) or our nociceptive and receptor-binding assays may not be sensitive enough to detect the very low biological activities of PK2L and PK2β (26, 27). Whatever the underlying reason, the 10 times higher PK2 expression level in rat inflamed paw than in mice inflamed paw might explain the stronger inflammatory response in rats than in mice.
Because prokineticins are potent chemoattractans for monocytes and macrophages both in vitro and in vivo and are able to stimulate the release of proinflammatory and proalgesic cytokines from macrophages (17–20) and monocytes (28) we can further hypothesize that PK2, released at the site of inflammation, represents a component of the cytokine–chemokine loop in inflammatory pain, which is initiated by the arrival of granulocyte and maintained by the subsequent recruitment of monocytes and macrophages. Inflammatory stimuli activate the release of the cytokine G-CSF that stimulates the synthesis and release of the chemokine PK2 by neutrophils, and this chemokine, in turn, stimulates macrophage chemotaxis and cytokine release by recruited macrophages. Although other leukocyte-derived chemokines are known to produce hypernociception in rats and mice (2, 29, 30) their pronociceptive activity and potency in activating G protein-coupled receptors is ≈100–500 times lower than that of PK2, and their receptors are far less abundant than PKRs in the primary sensory neurons under basal conditions (15, 16, 31, 32).
Producing CFA inflammation in mice lacking the pkr1 or pkr2 gene demonstrated that both PKR1 and PKR2 modulate pain perception, but only PKR1 modulates PK2 expression. Indeed, neither pkr1- nor pkr2-null mice developed inflammatory thermal hypernociception, confirming our hypothesis that CFA-induced hyperalgesia highly depends on the activation of PKRs of nociceptive terminals by granulocyte-released PK2. Conversely, pkr1- but not pkr2-null mice displayed significantly less inflammation-induced PK2 up-regulation than WT mice, demonstrating an involvement of PKR1 in setting the enhanced PK2 expression level in inflammation. We have already demonstrated the functional role of macrophage PKR1, which is responsible for chemotaxis and proinflammatory activity (19). Ferrara's group (17, 25) demonstrated that PK2 directly promotes survival and differentiation of granulocytic and monocytic lineages and, in vivo, increases the number of circulating leukocytes and synergizes with G-CSF in mobilization of CD11b+Gr1+ cells to the peripheral blood. Hence, GrPK2 released at the site of inflammation may activate the functional receptor, PKR1, present on granulocytes and macrophages, promoting their recruitment and survival by paracrine or autocrine mechanisms.
If Bv8/PK2 is a fundamental chemokine for the development of inflammatory pain, blocking the PKRs could potentially have two beneficial effects: an analgesic or, better, antihyperalgesic effect and a reduction of the inflammatory process as we demonstrated here by treating the animals with the nonpeptide PKR1-preferring antagonist PC1 (23). PC1, by local or systemic injection in both rats and mice, increased the nociceptive threshold of the inflamed paw toward the values of the control paw, leaving the pain threshold of control noninflamed paw unchanged. Furthermore, the mechanical hyperalgesia in the inflamed paw was so strongly reduced by PC1 that mice were enabled to bear body weight on the inflamed paw.
The rapid onset of PC1-induced antihyperalgesic effect suggests a direct antagonism at the PKRs of primary nociceptors. Indeed, PC1 antagonized the Bv8-induced hyperalgesia but not the hypernociception produced by proalgesic molecules such as PGE2, bradykinin, and ATP. Antihyperalgesic doses of PC1 also reduced paw edema in rats and accelerated the recovery of normal paw volume after the inflammatory insult. Two days after CFA injection, the paw volume of PC1-treated rats was approximately half that of saline-treated rats, and the PK2 expression level in the paw of PC1-treated rats was five times lower than in the paw of saline-treated rats, indicating that blocking PKR1 impairs granulocytes to up-regulate PK2, as pkr1 knockout did. Thus, our results indicate that a faster reduction of PK2 at the site of inflammation allows for faster healing. Moreover, s.c. injection of PC1 antagonized the systemic hyperalgesia produced by glycogen-induced peritonitis in rats, indicating that this inflammatory hypernociception may be linked to the release of PK2 from peritoneal granulocytes into circulation.
These results suggest a fundamental role of the Bv8/prokineticin system in inflammatory pain, at least in rodents. If this role can be confirmed in humans, the inhibition of Bv8/PK2 formation or antagonism at PKRs, as shown here, will constitute a promising strategy for a therapeutic approach to controlling inflammatory pain.
Materials and Methods
PK and PKR Gene Expression.
Total RNA was prepared from mouse or rat tissue samples by using a RNeasy Kit (Qiagen). cDNAs were amplified by real-time PCR (iCycler; Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). Rat primers were: PK2, 5′-CAAGGACTCTCAGTGTGGA-3′ and 5′-AAAATGGAACTTTCCGAGTC-3′; PK2L, 5′-AGGAAAGAAGAAGGGCGAAG-3′ and 5′-TCCTTAAACATGCCAAACCTG-3′. Rat PKR1 and PKR2 primers (16), mouse primers (15), and cycling conditions (15, 16) have been reported. For sorted cells, real-time RT-PCR was performed with the Quantitect SYBR one-step RT-PCR kit (Qiagen) as described (20). cDNA standards for each analyzed gene were generated, and serial dilutions ranging from 10 to 109 input copies were used as a standard curve in each PCR run. The expression level of the gene of interest was reported as input copy number per ng of total RNA.
In Situ Hybridization.
cDNA fragments for mouse PK2 (GenBank accession no. AF487280, nucleotides 24–504) were obtained by RT-PCR and subcloned into the pGEM-T easy vector (Promega). [35S]UTP and [35S]CTP double-labeled riboprobes were generated and tissues were processed as described (15).
Flow Cytometry and Cell Sorting.
Freshly isolated cells from mouse spleen were prepared and stained as described (33). Paw tissue samples were harvested 12 and 24 h after injection of CFA to obtain a single cell suspension as reported (34). Cells were counted and analyzed on a FACSCalibur with CellQuest software (BD Biosciences). PE-anti-Gr-1 (RB6–8C5) and FITC-anti-Mac-1 (M1/70) antibodies were purchased from BD Biosciences. For cell sorting, cells were appropriately stained, and Mac-1+Gr-1+ and Mac-1+Gr-1− populations were purified (purity ≥95%) with a FACSAria sorter (BD Biosciences).
Purification and Isolation of Prokineticins from Peritoneal Granulocytes.
Peritonitis was induced in male Sprague–Dawley rats (n = 100) by i.p. administration of oyster glycogen (1% in PBS), and the extravasated cells were collected 6 h later (35). The purity of granulocytes, as assessed by Wright-stained smears, was >95%. Harvested cells were washed, centrifuged at 4,000 × g at 4 °C, resuspended, and lysed with 2D Protein Extraction Buffer I (GE). After centrifugation, an aliquot of the supernatant (≈1/20 volume) was submitted to 2D electrophoresis to separate granulocyte proteins, and the remaining supernatant was added to 4 volumes of 0.2% TFA in acetonitril/isopropanol (4:1). The extract was centrifuged at 4,000 × g for 10 min at 4 °C, and the supernatant was collected and stored at −80 °C (step A).
First-dimension isoelectric focusing was performed by means of Bio-Rad Protean IEF Cell using 17-cm Bio-Rad ReadyStrip IPG strips, pH 3–10. Second-dimension SDS/PAGE was performed by means of Bio-Rad PROTEAN II XL cell using the Protean II Ready Gel precast gels in Tris/glycine/SDS running buffer, pH 8.5. In SDS/PAGE electrophoretic gels protein spots were revealed with the silver-staining method.
The extract (step A) was lyophilized, dissolved in 20 mM sodium phosphate buffer (pH 6.4), loaded on cationic exchanger Source 30S column (GE), and eluted by a gradient 0–100% of 0.4 M NaCl in 20 mM sodium phosphate buffer, pH 6.4 (step B). Eluates containing hyperalgesic activity (from 25 to 35% of 0.4 M NaCl) were reunited and further purified by gel filtration [Superdex 75 10/300 column (GE), eluting buffer 1 M acetic acid, 1 mL/min, UV detection 220 and 280 nm] to eliminate salts that interfere with binding assay (step C). Collected fractions were assayed, in vivo, for the presence of Bv8-like hyperalgesic activity and, in vitro, for PKR1 binding assay on PKR1-transfected CHO cells. The fractions containing bioactivity were reunited, lyophilized, dissolved in 0.2% TFA in water, loaded on a reversed-phase C8 column (Vydac), and eluted by a gradient 15–100% acetonitril containing 0.2% TFA (step D). The biological active fraction (PKR1 binding) was further chromatographed on a Grace-Vydac 238 EV 51 column before submitting to sequence analysis (PRIMM).
Receptor Binding Assay.
The fractions purified by gel filtration (step C) were evaluated for their ability to displace [125I]MIT binding from membrane preparations of PKR1-transfected CHO cells as described (36). The displacement curve of 125I-MIT by graded concentrations of Bv8 (0.01–100 nM) was used to generate a standard curve to determine the content of Bv8-like proteins in each tested fraction (PRISM; GraphPad).
Animals and Hypernociceptive Tests.
All experiments were performed under protocols approved by the Animal Care and Use Committee of the Italian Ministry of Health according to European Commission directives. Male Sprague–Dawley rats (250–300 g), C57BL/6 (25–30 g), pkr1(−/−) and pkr1(+/+), and pkr2(−/−) and pkr2(+/+) mice (Lexicon Genetics) were housed in temperature-controlled rooms (22–25 °C) with access to water and food ad libitum. Pain threshold was evaluated by the paw-pressure test in rats and the paw-immersion-in-hot-water test (48 °C) in mice as described (14, 15). Mice were tested for changes in mechanical hypersensitivity by using a body weight bearing averager (Incapacitance Tester; Biological Instruments). Results were expressed as mean ipsilateral/contralateral ratio. The data are presented as mean ± SE mean values.
Inflammatory Pain Models.
Peritonitis was induced in rats by i.p. administration of oyster glycogen as described above. Paw inflammation was induced by ipl injection of CFA (Sigma) in mice (20 μL per paw) and rats (100 μL per paw). Paw volume was measured with a 7140 plethysmometer (Ugo Basile).
Drug Injections.
Drugs were injected topically into the plantar region of hindpaws (ipl), in volumes of 20 μL in mice and 100 μL in rats and s.c. into the flank region in a volume of 2 mL/kg. GrPK2 (isolated from peritoneal granulocytes) and amphibian Bv8 (purified from skin secretion of B. variegata) (5) were injected ipl in mice and rats. PC1 synthesized by Balboni et al. (23) was injected ipl and s.c. in mice and rats. Bradykinin (Sigma–Aldrich), PGE2 (Sigma–Aldrich), and 2-methylthioATP (Tocris) were injected ipl in mice.
Acknowledgments.
This work was supported by grants from the Italian Ministry of University and Scientific Research and the University of Rome, “La Sapienza.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Levine JD, Gooding J, Donatoni P, Borden L, Goetzl EJ. The role of the polymorphonuclear leukocyte in hyperalgesia. J Neurosci. 1985;5:3025–3029. doi: 10.1523/JNEUROSCI.05-11-03025.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha TM, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukocyte Biol. 2008;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- 3.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd AR, Johnston J. Cytokines and cytokine receptors in health and disease: A summary of the National Heart, Lung, and Blood Institute Frontiers in Basic Sciences Symposium, December 2–3, 1992. Cytokine. 1993;5:399–406. doi: 10.1016/1043-4666(93)90029-5. [DOI] [PubMed] [Google Scholar]
- 5.Mollay C, et al. Bv8, a small protein from frog skin, and its homolog from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 6.Wechselberger C, et al. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 1999;462:177–181. doi: 10.1016/s0014-5793(99)01473-8. [DOI] [PubMed] [Google Scholar]
- 7.Schweitz H, Pacaud P, Diochot S, Moinier D, Lazduniski M. MIT-1, a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett. 1999;461:183–188. doi: 10.1016/s0014-5793(99)01459-3. [DOI] [PubMed] [Google Scholar]
- 8.LeCouter J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:876–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 10.Soga T, et al. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 11.Masuda Y, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 12.Lin DC, et al. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/EG-VEGF. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 13.Negri L, Lattanzi R, Giannini E, Melchiorri P. Bv8/prokineticin proteins and their receptors. Life Sci. 2007;81:1103–1116. doi: 10.1016/j.lfs.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Negri L, et al. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negri L, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: Focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vellani V, et al. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorsch M, et al. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukocyte Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 19.Martucci C, et al. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol. 2006;147:225–234. doi: 10.1038/sj.bjp.0706467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchi S, et al. The prokineticin receptor agonist Bv8 decreases IL-10 and IL-4 production in mice splenocytes by activating prokineticin receptor-1. BMC Immunol. 2008;9:60–73. doi: 10.1186/1471-2172-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeCouter J, Lin R, Ferrara N. Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nat Med. 2002;8:913–917. doi: 10.1038/nm0902-913. [DOI] [PubMed] [Google Scholar]
- 22.LeCouter J, et al. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balboni G, et al. Triazine compounds as antagonists at Bv8-prokineticin receptors. J Med Chem. 2008;51:7635–7639. doi: 10.1021/jm800854e. [DOI] [PubMed] [Google Scholar]
- 24.Bobrowski WF, et al. Comparative methods for multiplex analysis of cytokine protein expression in plasma of lipopolysaccharide-treated mice. Cytokine. 2005;32:194–198. doi: 10.1016/j.cyto.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Shojaei F, et al. Bv8 regulates myeloid cell-dependent tumor angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, et al. Identification and pharmacological characterization of prokineticin 2β as a selective ligand for prokineticin receptor 1. Mol Pharmacol. 2005;67:2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 27.Bullock CM, Li JD, Zhou QY. Structural determinants required for bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 28.Monnier J, Samson M. Cytokine properties of prokineticins. FEBS J. 2008;275:4014–4021. doi: 10.1111/j.1742-4658.2008.06559.x. [DOI] [PubMed] [Google Scholar]
- 29.Abbadie C. Chemokines, chemokine receptors, and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Cunha TM, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh SB, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5050. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang N, et al. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci USA. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maroder M, et al. Expression of TrκB neurotrophin receptor during T cell development: Role of brain-derived neurotrophic factor in immature thymocyte survival. J Immunol. 1996;157:2864–2872. [PubMed] [Google Scholar]
- 34.Rittner HL, et al. Opioid peptide-expressing leucocytes: Identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95:500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- 35.Cockrell A, et al. Role of nitric oxide synthase in leukocyte extravasation in vivo. Biochem Biophys Res Commun. 1999;257:684–686. doi: 10.1006/bbrc.1999.0484. [DOI] [PubMed] [Google Scholar]
- 36.Negri L, et al. Biological activities of Bv8 analogues. Br J Pharmacol. 2005;146:625–632. doi: 10.1038/sj.bjp.0706376. [DOI] [PMC free article] [PubMed] [Google Scholar]