Abstract
Fat loss in response to exercise training varies between individuals, even when differences in compliance to the exercise program are accounted for. The purpose of this study was to investigate whether individual variation in change in fasting respiratory quotient (RQ) after exercise training contributes to this interindividual variability. Fifty-five premenopausal women participated in a 7-week endurance-type exercise training program; and fitness, body composition, and resting substrate utilization and metabolic rate in the fasted state were assessed at baseline and postintervention. Total net energy expenditure of the exercise intervention (exEE) was determined from heart rate obtained in all exercise sessions and individualized calibration of the heart rate vs oxygen uptake relationship. Dietary intake and physical activity (by constant heart rate monitoring) were assessed at baseline and during the final week of the intervention. Mean change in fat mass for the group was −0.97 kg (range, +2.1 to −5.3 kg). The strongest correlate of change in fat mass was exEE (r = 0.60, P < .0005). Change in fasting RQ correlated significantly (r = −0.26, P = .05) with the residual for change in fat mass after adjusting for the effects of both exEE and change in energy intake, explaining 7% of the variance. In multiple regression analysis, exEE (P < .0005) and change in fasting RQ (P = .02) were the only statistically significant independent predictors of change in fat mass, together explaining 40.2% of the variance. Thus, fat loss in response to exercise training depends not only on exercise energy expenditure but also on exercise training–induced changes in RQ at rest. This suggests that development of strategies to maximize the change in resting fat oxidation in response to an exercise training program may help individuals to maximize exercise-induced fat loss.
1. Introduction
It is well established that the extent of weight or fat loss in response to exercise training varies between individuals [1-3]. The extent of compliance to the exercise intervention clearly contributes to this variation [2,4,5]; but even when this is accounted for, differences in weight and fat loss between individuals are observed [1,3]. It has been suggested that individual differences in compensatory adjustments to the increased exercise energy expenditure are responsible for this variability [6]. Indeed, it has recently been reported that those who lost less weight than predicted in response to an exercise intervention increased their energy intake over the course of the intervention, whereas those who lost more weight than predicted decreased energy intake, although there was no overall change in energy intake before and after the intervention for the group as a whole [1]. Furthermore, compensatory decreases in energy expended in spontaneous activity have been shown to lead to smaller than expected increases in total energy expenditure in response to exercise interventions [7,8]; and a wide variation in the extent of change in nonintervention energy expenditure when individuals undergo an exercise training intervention has been reported [7]. The extent to which this contributes to individual differences in weight or fat loss is unclear; but it has been shown that individuals with the greatest increases in nonexercise activity thermogenesis in response to overfeeding are protected from weight gain [9], which suggests that individual differences in activity compensation could conceivably play a role in determining responsiveness to exercise-induced weight loss.
However, differences in behavioral compensation probably cannot explain all of the variability in responsiveness to exercise-induced weight and fat loss. For example, Bouchard and colleagues [10] reported that, in men residing at an isolated experimental station in a highly controlled environment, imposition of an exercise-induced energy deficit of 4.2 MJ/d for 84 days, with constant energy intake, led to reductions in body weight ranging from 3 to 12 kg, a range which is unlikely to be fully explained by differences in compensatory activity between subjects. Thus, nonbehavioral metabolic factors are also likely to contribute to individual responsiveness to exercise-induced weight loss. One metabolic factor that could contribute is resting metabolic rate; however, a recent report found no significant difference in the change in resting metabolic rate between the start and end of an exercise intervention between subjects who lost more weight than expected and those who lost less weight than expected, suggesting that this does not play a major role [1]. A further metabolic factor that might play a role is the magnitude of the exercise-induced change in resting fat oxidation. Both fasting fat oxidation and postprandial fat oxidation have been shown to increase for at least 24 hours after an exercise session [11-13], even in the absence of an exercise-induced energy deficit [12]; and studies have reported greater fat oxidation at rest in endurance-trained vs untrained individuals [14]. The exercise-induced increase in resting fat oxidation varies between individuals [12,15,16]; and indeed, the extent of this increase has been demonstrated to be a strong predictor of other exercise-induced metabolic changes, such as the magnitude of change to postprandial lipid metabolism [12] and insulin sensitivity [16]. Furthermore, a number of studies have shown that a high respiratory quotient (RQ) (indicating high carbohydrate and low fat oxidation) measured fasting [17,18] or over 24 hours [19] is a significant predictor of long-term weight gain, independent of metabolic rate [17-19]. Thus, effects of exercise on resting fat oxidation may contribute to fat loss in response to an exercise intervention. We therefore hypothesized that variability in the up-regulation of resting fat oxidation after an exercise training intervention would contribute to the interindividual variability in exercise-induced fat loss and that this effect would be independent of energy expended during exercise training sessions and the effects of behavioral compensatory responses. In addition, we sought to determine whether any baseline physiologic, metabolic, anthropometric, or behavioral characteristics could predict responsiveness to exercise-induced fat loss.
2. Methods
2.1. Subjects
Subjects for this study participated in an intervention trial to determine the effects of exercise training on insulin sensitivity in women with and without a family history of diabetes [20]. They were recruited via newspaper articles, a study Web site, posters, the University newsletter, and personal contacts. Sixty-two women completed the intervention, and complete data sets for the variables of interest were available in 55 subjects. These 55 women were included in the data analysis, and their characteristics are presented in Table 1. All subjects were in general good health, were premenopausal with a regular menstrual cycle, were nonsmokers, had a sedentary lifestyle (<1 hour of planned physical activity per week and a sedentary job), had fasting plasma glucose less than 7 mmol/L, and had blood pressure less than 160/90 mm Hg. Twenty-seven of the women were offspring of patients with type 2 diabetes mellitus, and 28 had no family history of the disease. All participants gave written informed consent before inclusion in this trial that was approved by the Research Ethics Committee of the North Glasgow University Hospitals National Health Service Trust and registered with ClinicalTrials.gov (trial identifier: NCT00268541). We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Table 1.
Subject characteristics at baseline and changes in response to exercise training
| Baseline | Change with exercise training | |
|---|---|---|
| Age (y) | 34.7 ± 6.4 | – |
| BMI (kg/m2) | 27.5 ± 4.7 | −0.2 ± 0.7† |
| Lean mass (kg) | 41.8 ± 5.8 | 0.3 ± 1.4 |
| Fat mass (kg) | 29.9 ± 9.6 | −1.0 ± 1.5† |
| Trunk fat mass (kg) | 15.2 ± 5.3 | −0.5 ± 1.2† |
| Upper body fat mass (kg) | 19.0 ± 6.9 | −0.7 ± 1.3† |
| Leg fat mass (kg) | 10.2 ± 3.1 | −0.2 ± 0.6† |
| % Body fat | 39.3 ± 6.0 | −1.0 ± 1.5† |
| Waist circumference (cm) | 85.9 ± 11.9 | −1.2 ± 2.3† |
| Vo2max (mL/[kg min]) | 31.3 ± 5.1 | 4.2 ± 4.0† |
| Resting metabolic rate (kJ/d) | 6250 ± 918 | −12 ± 410 |
| Fasting RQ | 0.85 ± 0.05 | −0.03 ± 0.06† |
| Energy intake (kJ/d) | 7904 ± 1787 | −500 ± 1530⁎ |
| Fat intake (g/d) | 66.9 ± 20.4 | −1.8 ± 19 |
| Carbohydrate intake (g/d) | 247.3 ± 72.0 | −18.9 ± 53.4† |
| Protein intake (g/d) | 71.6 ± 17.5 | −6.3 ± 14.1† |
| Resting heart rate (beat/min) | 68.1 ± 8.3 | −3.4 ± 5.7 |
| Average daily heart rate minus resting heart rate (beat/min) | 18.1 ± 6.0 | 4.2 ± 6.9† |
| Time >1.5 times resting heart rate including exercise training (min/d) | – | 40 ± 85 |
| Time >2 times resting heart rate including exercise training (min/d) | – | 23 ± 24 |
| Time >1.5 times resting heart rate excluding exercise training (min/d) | 106 ± 91 | 9 ± 85 |
| Time >2 times resting heart rate excluding exercise training (min/d) | 11 ± 15 | 4 ± 17 |
| No. of exercise training sessions completed | – | 28.3 ± 6.3 |
| Total duration of exercise training completed (min) | – | 1402 ± 469 |
| Mean heart rate during exercise (beat/min) | – | 142.7 ± 9.3 |
| Total net exercise energy expenditure (MJ) | – | 36.9 ± 17.0 |
N = 55; values are mean ± SD.
P < .05 for change with exercise training.
P < .01.
2.2. Study design
The overall study design has been described previously [20]; but, in brief, subjects all underwent a metabolic assessment, body composition measurements (by dual x-ray absorptiometry [DEXA]), and a cardiorespiratory fitness test, including individualized calibration of the heart rate vs oxygen uptake relationship, at baseline and after a controlled 7-week endurance-type exercise training program. Dietary intake and physical activity were monitored during the week preceding baseline metabolic assessment and during the final week of the exercise training program.
2.3. Metabolic assessment
Subjects reported to the metabolic suite after 12-hour overnight fast; and after a 10-minute rest lying on a couch, a 20-minute expired air sample was collected using a ventilated hood system (Deltatrac Metabolic Monitor, Datex Engstrom, Kent, United Kingdom) to determine resting oxygen uptake (Vo2), carbon dioxide production (Vco2), RQ (ie, Vco2/Vo2), and metabolic rate. Resting heart rate was recorded immediately after the ventilated hood measurement (ie, after subjects had been lying supine for at least 30 minutes) using an automated device (Complior; Artech Medical, Pantin, France). Volunteers completed their final exercise session 15 to 24 hours before the postintervention measurements.
2.4. Body composition assessment
The DEXA scans (LUNAR Prodigy DEXA scanner; GE Healthcare Diagnostic Imaging, Slough, Berkshire, United Kingdom) were used to determine body composition and fat distribution. Height, body mass, and waist circumferences were also determined using standard protocols [21].
2.5. Fitness test and calibration of the heart rate vs Vo2 relationship
Subjects attended for this test at least 2 hours after eating. After a 10-minute rest, 5-minute expired air samples were collected via a mouthpiece into Douglas bags for the determination of Vo2 and Vco2 during 3 sedentary activities, that is, sitting, standing, and standing with arms swaying. The mean Vo2 of these 3 activities was taken as representative of Vo2 during sedentary activities. After this, subjects performed an incremental, submaximal treadmill walking test to determine their heart rate vs Vo2 relationship and to estimate maximal oxygen uptake (Vo2max) [22]. Treadmill speed was set at 5 km/h, with gradient increasing by 2% per 5-minute stage. Expired air samples for the determination of oxygen uptake and carbon dioxide production and heart rates were taken throughout. Tests were terminated once subjects achieved approximately 85% of their age-predicted maximum heart rate, and the oxygen uptake vs heart rate relationship was extrapolated to age-predicted maximum (220 − age) to estimate Vo2max.
2.6. Physical activity and dietary assessment
For 7 days preceding each metabolic assessment day, subjects wore a heart rate monitor for all waking hours (Polar 610i; Polar Electro, Kempele, Finland); and minute-by-minute heart rates were recorded. Average daily heart rate minus resting heart rate (ie, the time-averaged area under the heart rate vs time curve, using resting heart rate as baseline) was used as a surrogate measure of total activity. Time spent more than 1.5 and 2 times resting heart rate (determined during the metabolic assessment) were used as surrogate indices of time spent engaging in activities of at least light and at least moderate intensity, respectively. During this time, subjects also completed 7-day weighed food diaries that were analyzed using a computerized version of food composition tables (CompEat Pro; Nutrition Systems, Banbury, United Kingdom).
2.7. Exercise intervention
Subjects underwent a progressive 7-week endurance-type exercise training program starting with 3 × 30 minutes of exercise in the first week, building progressively to 5 × 60 minutes of exercise in weeks 6 and 7 of the intervention. All subjects were given free access to the University Sports Centre, were provided with a downloadable heart rate monitor (Polar 610i), and were instructed to exercise at 65% to 80% of their predicted maximum heart rate for all exercise sessions. Subjects could use whichever cardiovascular exercise equipment they preferred (eg, treadmill, stepper, cycle ergometer, rowing ergometer) or attend scheduled aerobic exercise classes. Alternatively, subjects could run, cycle, or perform other modes of exercise at other locations if they preferred, provided that they completed the required duration and intensity of exercise. One exercise session per week was supervised by an investigator. At this session, heart rate data from the previous week's sessions were downloaded to verify compliance; and the exercise plan for the following week was agreed. A total of 32 exercise sessions were prescribed over the course of the intervention. Other than participating in the exercise intervention, subjects were requested to make no changes to their lifestyle for the duration of the study.
For each exercise training session, the mean heart rate of the session was converted into an equivalent Vo2 based on the individualized heart rate vs Vo2 relationship. The individual's Vo2 associated with sedentary activity was subtracted from this value to determine the net exercise Vo2 (ie, the oxygen cost of exercise over and above that of sedentary daily activities). The net exercise Vo2 was then multiplied by the duration of exercise to determine the total net oxygen cost of the exercise session, and the net energy cost of the session (in kilojoules) was obtained by multiplying the net oxygen cost by 20.3 [23]. For exercise sessions performed during the first 3.5 weeks of the program, energy expenditure values were determined using the heart rate vs Vo2 relationship from the baseline fitness test; for sessions during the second 3.5 weeks of the program, values obtained during the postintervention fitness test were used in the energy expenditure calculations. The net energy expenditures of all exercise sessions were summed to determine the total net energy expenditure of the exercise training program.
2.8. Reproducibility and statistical power
To determine the reproducibility of the resting RQ measurements in a free-living situation, 28 of the women in this study underwent 2 ventilated hood measurements after a 12-hour fast, at an interval of 8 weeks, with no specific guidelines other than to maintain their usual diet and physical activity habits throughout the interval between measurements. Fasting RQ was 0.84 ± 0.04 on the first visit and 0.85 ± 0.05 on the second visit (not significant), and the SD for the difference in RQ between visits was 0.05. Based on these data, the present study, with 55 participants, had sufficient statistical power to detect a difference in RQ with exercise training of 0.02 with 90% power. This number of volunteers also enables detection of a correlation between variables of 0.26 at the P equal to .05 level.
2.9. Statistical analysis
Data were analyzed using Statistica (version 6.0; StatSoft, Tulsa, OK) and Minitab (version 13.1; Minitab, State College, PA). Univariate linear regression analyses were performed to determine the relationship between change in total and regional (trunk, upper body [ie, trunk plus arm], and leg) fat mass over the course of the intervention and the following: total net energy expenditure of the exercise intervention; behavioral compensation variables (ie, change in energy, fat, carbohydrate, and protein intake; change in average daily heart rate minus resting heart rate; and change in time spent more than 1.5 and 2 times resting heart rate excluding exercise training); changes in physiologic variables (ie, change in Vo2max, change in resting metabolic rate, change in fasting RQ); and baseline physiologic and behavioral characteristics (age; body mass index [BMI]; total and regional fat mass; Vo2max; resting metabolic rate; fasting RQ; energy, fat, carbohydrate, and protein intake; average daily heart rate minus resting heart rate; time spent more than 1.5 and 2 times resting heart rate). To determine the extent to which variables were related to change in fat mass independently of exercise energy expenditure, univariate linear regressions were then performed between the residuals for change in fat mass of the regression between change in fat mass and net total energy expenditure of exercise and the other variables. This classic statistical approach has been used previously to determine the independent effects of individual variables within multifactorial systems on biological outcomes [24,25] and, in effect, provides the correlations between change in fat mass and other variables, adjusted for the effect of net total energy expenditure of exercise. To further adjust for the effect of change in energy intake on change in fat mass, the residual for change in fat mass after regression with net total energy expenditure of exercise and change in energy intake was used in univariate linear regressions with the other variables. Finally, to determine which variables were independent predictors of the change in fat mass, multiple regression analysis was performed. Statistical significance was accepted at the P equal to .05 level.
3. Results
Baseline characteristics and group changes in response to the exercise intervention are shown in Table 1. In response to the exercise training intervention, the group as a whole significantly reduced BMI (by 0.9%), total fat mass (by 3.3%), trunk fat mass (by 3.0%), upper body fat mass (by 3.8%), leg fat mass (by 2.5%), percentage body fat (by 2.6%), and waist circumference (by 1.4%); but lean body mass was not significantly changed. Maximal oxygen uptake (by 13.6%) was significantly increased. Resting metabolic rate was not changed; but fasting RQ was significantly reduced, indicating a shift in substrate utilization toward fat oxidation. Although subjects were asked not to alter their diets over the course of the intervention, energy (by 6.3%), carbohydrate (by 7.6%), and protein (by 8.8%) intakes were significantly lower at the end of the intervention than at baseline; but there were no significant difference in fat intake between the start and end of the intervention. Average daily heart rate minus resting heart rate and total time spent more than 1.5 and 2 times resting heart rate (including time spent in the exercise sessions) were all significantly higher at the end of the intervention than at baseline (P < .01); but the latter 2 factors did not differ between baseline and the end of the intervention when the time spent during the intervention exercise sessions was excluded.
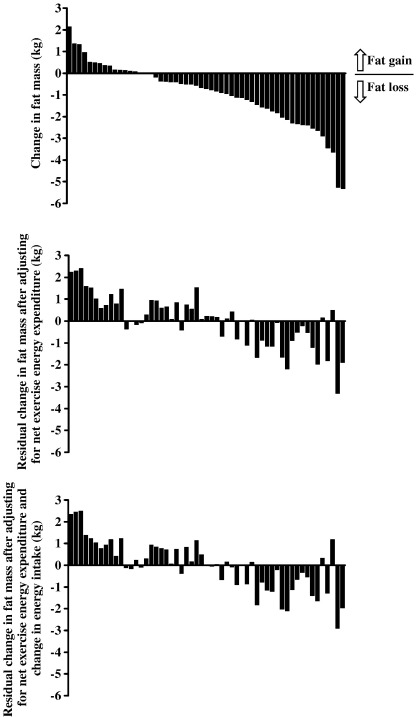
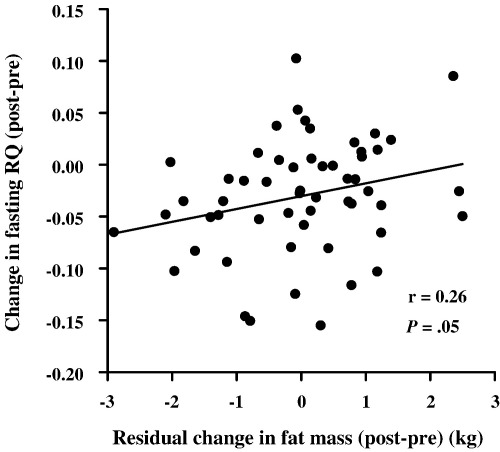
Across the group, there was a wide individual variation in change in total fat mass over the intervention, with individual changes in total fat mass ranging from a 2.1-kg gain to a 5.3-kg loss (Fig. 1, top panel). Correlates of the change in total fat mass over the intervention are shown in Table 2. The strongest correlate of change in total fat mass during simple univariate regression analysis was total net energy expenditure of exercise (r = 0.60, P < .0005), explaining 36% of the variance in this factor. Change in average daily heart rate minus resting heart rate (which incorporates heart rate elevations from the exercise intervention) also significantly correlated with change in total fat mass (r = −0.31, P = .02); but change in time spent more than 1.5 and 2 times resting heart rate (excluding exercise training) did not. Change in fat intake (r = −0.25, P = .06) and change in fasting RQ (r = −0.25, P = .06) had borderline significant correlations with change in total fat mass in simple univariate regression analysis. The middle panel of Fig. 1 shows the individual residual changes in total fat mass after adjustment for the net total energy expenditure of exercise, and the bottom panel shows the individual residual changes in total fat mass after adjustment for the net total energy expenditure of exercise and changes in energy intake. In other words, these panels show the variation in change in total fat mass that cannot be explained by changes in exercise energy expenditure and energy intake over the intervention. The relationship between change in total fat mass and change in fasting RQ was strengthened after adjustment for the total net energy expenditure of the exercise (r = −0.31, P = .02) and remained significant after further adjustment for change in energy intake (r = -0.26, P = .05) (Fig. 2). Thus, change in fasting RQ explained approximately 7% of the variance in the change in total fat mass over the course of the intervention after adjustment for effects of the total net energy expenditure of exercise and change in energy intake. Adjusting for exercise energy expenditure and change in energy intake both weakened the relationship between change in total fat mass and change in fat intake. None of the included baseline factors correlated significantly with change in total fat mass in any of these analyses (data not shown). In multiple regression analysis including all of the variables described in the “Statistical analysis” section in the model, net total energy expenditure of exercise (P < .0005) and change in fasting RQ (P = .02) were the only statistically significant independent predictors of change in total fat mass. Together, these 2 variables explained 40.2% of the variance in change in total fat mass (adjusted R2).
Fig. 1.
Changes in fat mass for each of the 55 women in the study (top panel). Residual change in fat mass after adjustment for the net total energy expenditure of exercise (middle panel). Residual change in fat mass after adjustment for the net total energy expenditure of exercise and change in energy intake over the intervention (bottom panel). For all 3 panels, subjects ranked in order of change in unadjusted fat mass.
Table 2.
Correlations between intervention, behavioral compensation, and physiologic variables, and change in fat mass in response to exercise training
| Correlation with change in fat mass |
||||
|---|---|---|---|---|
| No adjustment | After adjustment for total net exercise energy expenditure | After adjustment for total net exercise energy expenditure and change in energy intake | ||
| Intervention variables | Total net energy expenditure of exercise | −0.60 (<.0005) | – | – |
| Behavioral compensation variables | Change in energy intake | 0.20 (.14) | 0.20 (.14) | – |
| Change in fat intake | 0.25 (.06) | 0.24 (.07) | 0.10 (.48) | |
| Change in carbohydrate intake | 0.11 (.42) | 0.17 (.22) | 0.00 (.99) | |
| Change in protein intake | 0.05 (.73) | 0.05 (.71) | −0.06 (.65) | |
| Change in average daily heart rate minus resting heart rate | −0.31 (.02) | 0.15 (.27) | −0.16 (.25) | |
| Change in time spent >1.5 times resting heart rate excluding exercise training | 0.06 (.64) | −0.05 (.73) | −0.03 (.85) | |
| Change in time spent >2 times resting heart rate excluding exercise training | −0.04 (.75) | −0.10 (.48) | −0.07 (.60) | |
| Physiologic variables | Change in Vo2max | 0.03 (.82) | 0.20 (.14) | 0.15 (.26) |
| Change in resting metabolic rate | 0.13 (.33) | 0.15 (.28) | 0.15 (.28) | |
| Change in fasting RQ | 0.25 (.06) | 0.31 (.02) | 0.26 (.05) | |
N = 55; values are correlation coefficients, with P values in parentheses. Statistically significant correlations shown in bold.
Fig. 2.
Scattergram showing the relationship between change in fasting RQ and residual change in fat mass, adjusted for the effects of net energy expenditure of exercise and change in energy intake (N = 55; r and P values for Pearson product-moment correlations).
In univariate regression, changes in trunk (r = 0.87, P < .0005), upper body (r = 0.92, P < .0005), and leg (r = 0.52, P < .0005) fat were strongly associated with changes in total fat mass. In addition, changes in trunk (r = −0.43, P = .001), upper body (r = −0.49, P < .0005), and leg (r = −0.45, P = .001) fat all significantly correlated with total net energy expenditure of exercise. Change in trunk fat also significantly correlated with change in average daily heart rate minus resting heart rate (r = −0.29, P = .03), and change in upper body fat correlated significantly with fat intake at baseline (r = −0.28, P = .04) (ie, those with highest baseline fat intake lost the greatest amount of upper body fat). Changes in trunk, upper body, or leg fat were not significantly associated with change in fasting RQ.
Because the exercise training–induced change in fasting RQ was a significant predictor of change in total fat mass, univariate and multivariate regression analyses were performed to determine whether any of the other measured variables could predict the exercise-induced change in RQ. Change in fasting RQ was significantly associated with diabetes family history (with 0 and 1 included as dummy variables for negative and positive diabetes family history, respectively) (r = −0.347, P = .009) (ie, those with a positive diabetes family history had a bigger exercise-induced reduction in fasting RQ); baseline BMI (r = 0.29, P = .035) and total (r = 0.29, P = .033) and leg (r = 0.36, P = .006) fat mass (ie, those with lower baseline BMI and fat mass had bigger reductions in RQ); baseline fasting RQ (r = −0.67, P < .0005) (ie, those with higher RQ at baseline had bigger reductions in RQ); and changes in energy (r = 0.29, P = .035), carbohydrate (r = 0.28, P = .40), and fat (r = 0.27, P = .047) intake (ie, those with bigger decreases in energy, carbohydrate, and fat intake had bigger reductions in RQ). In multiple regression analysis, baseline fasting RQ (P < .0005), diabetes family history (P = .001), and change in energy intake (P = .049) were independent significant predictors of the change in fasting RQ, together explaining 55.9% of the variance. Baseline fasting RQ alone explained 44.2% of the variance in the change in fasting RQ.
4. Discussion
In this study, 55 women underwent a 7-week exercise training program, which induced a mean total fat loss for the group of 0.97 kg. The mean net total energy expenditure of the exercise program was 36.9 MJ; so, assuming that fat loss requires a negative energy balance of 39.4 MJ/kg [26], fat loss in the group as a whole was broadly at the expected level. However, considering the change in fat mass at group level obscures the wide interindividual variability in change in fat mass with the intervention (Fig. 1). The most important correlate of change in total fat mass was net total energy expenditure of exercise, which explained 36% of the variance; but even after adjusting for this and for changes in energy intake over the intervention, a wide variation for the residual change in fat mass was evident, ranging from +2.5 to −2.9 kg. The main novel finding of the study was that the residual change in total fat mass in response to an exercise intervention, after adjustment for the energy expended during the exercise intervention and for changes in energy intake, was related to the change in fasting RQ between the start and end of the intervention. Change in fasting RQ explained approximately 7% of the variance of the residual change in fat mass. Indeed, the relationship between change in fasting RQ and change in total fat mass was independent of all other physiologic and behavioral variables included in a multivariate analysis model; and in this model, change in RQ and net total energy expenditure of exercise were the only significant independent predictors of change in total fat mass.
It is not possible from the design of this study to determine the direction of causality between change in fasting RQ and change in fat mass conclusively. However, the case that exercise-induced reduction in RQ was a significant predictor of exercise-induced fat loss fits well with the body of data indicating that a high RQ predicts long-term weight gain [17-19]; in other words, good fat oxidizers were protected from future weight gain. Our data build on this work, indicating that individuals who had the largest shifts in resting substrate utilization toward fat oxidation in response to exercise training experienced the greatest losses in fat mass, independent of exercise energy expenditure and change in energy intake. The studies of RQ and long-term weight gain found this effect to be independent of metabolic rate [17-19], and our findings are consistent with this. Indeed, in agreement with the study of King et al [1], we found that changes in resting metabolic rate between the start and end of the intervention were not associated with change in fat mass.
On the other hand, it is well established that negative energy balance leads to increased fat oxidation [27]; and the women with the greatest fat losses in this study would, by definition, have incurred the greatest negative energy balances over the course of the intervention. However, this shift toward fat oxidation appears to occur largely in response to acute negative energy balance over the relatively short term; and over the longer term, a number of studies have reported that weight loss (ie, incurring a large cumulative negative energy balance over a number of weeks or months, with consequent changes in body composition) is often associated with no change [28] or even an increase [29-31] in fasting and 24-hour RQ. Indeed, an increase in RQ in response to weight loss is thought to be one of the factors predisposing to weight regain after weight loss: a number of reports have indicated that the individuals with the highest RQs after weight loss are those who are most susceptible to weight regain [28,32]. Furthermore, the change in fat oxidation in response to the same degree of short-term negative energy balance [12] and long-term change in body weight [30] is highly variable between individuals. For example, we have reported individual changes in whole-body postprandial fat oxidation ranging from a decrease of 4 g to an increase of 16 g over an 8.5-hour observation on the day after an exercise session inducing an identical energy deficit of 27 kJ/kg in all subjects [12]. It has been demonstrated that this variability has metabolic consequences beyond the regulation of body weight: individuals who up-regulate postexercise fat oxidation to the greatest extent in response to a given energy deficit also experience the largest changes to postprandial lipid metabolism [12] and insulin sensitivity [16]. In addition, we have shown that exercise increases subsequent fat oxidation for at least 24 hours even in the absence of an associated energy deficit and that the extent of this increase also varies markedly between individuals [12]. As the posttraining metabolic assessment was undertaken 15 to 24 hours after an exercise session, it is likely that the variation in change in RQ in the present study at least partly reflects interindividual differences in the acute postexercise increase in fat oxidation. Thus, the evidence from the literature indicates that exercise-induced changes in RQ exhibit a large degree of interindividual variability and are evident in the absence of energy deficit, and that RQ is not consistently reduced after weight loss; so it does not support the rationale that larger reductions in RQ are simply the consequence of larger incurred negative energy balances and greater weight losses.
As the change in fasting RQ was significantly and independently associated with change in total fat mass, we sought to determine what factors influenced change in RQ. By far, the most important predictor of the change in fasting RQ was its baseline value: those with the highest baseline values for RQ experienced the greatest reductions. Thus, it appears that individuals who are “carbohydrate oxidizers” when sedentary experience the greatest shift toward fat oxidation in response to exercise training. This is an exciting possibility that suggests that those who are most susceptible to weight gain due to their high RQ [17-19] experience the greatest benefits in terms of increase in resting fat oxidation from exercise. However, baseline fasting RQ was not significantly associated with change in total fat mass; a degree of caution is advised when interpreting this observation. Change in fasting RQ was also significantly associated with other “innate” baseline factors, namely, BMI, total and leg fat mass, and diabetes family history; however, change in components of dietary intake was also implicated, albeit to a much lesser degree than baseline RQ, suggesting that “behavioral” and “metabolic” factors influencing change in fat mass are not completely independent of each other.
The present study has a number of strengths. With 55 participants, it is the largest study to date attempting to address the issue of factors influencing individual responsiveness to exercise-induced fat loss, which provides statistically high power to detect associations between variables, making the statistical findings of this study robust. In addition, both metabolic and behavioral variables were included in the analyses, enabling the relative importance of each to be determined; and energy expenditure of the exercise intervention was objectively quantified on an individual-by-individual basis. Furthermore, baseline and postintervention testing was performed at an interval of 8 weeks to ensure that, as far as possible, women were in the same phase of menstrual cycle for baseline and postintervention testing, thus limiting the confounding effects of cyclical hormonal changes on the results.
The main limitations to this study, which are common to most reports in this field, relate to the measurement of behavioral compensation variables. Firstly, it is well established that underreporting is a common problem incurred in measurement of dietary intake and that this problem is greater in obese than lean individuals [33,34]. However, it appears that the extent of underreporting is relatively consistent within an individual [34], implying that differences in dietary intake between 2 observation points (eg, baseline and postintervention) are likely to be determined with greater accuracy than absolute dietary intakes at a single time point. Thus, the repeated-measures design in the present study may attenuate this potential error. Furthermore, the effect of inaccuracy in measurement of dietary intake would act to diminish any association with exercise-induced change in fat mass (a regression dilution bias effect [35]): thus, the finding that there was a borderline significant relationship between change in fat intake and exercise-induced change in fat mass in the present study, despite potential errors in the assessment on dietary intake, suggests that this association was likely to be real. This would be in agreement with the recent work of King and colleagues [1] who reported that dietary compensation influenced the extent of weight loss in response to exercise training. In contrast, we found no evidence to suggest that individual differences in compensation by reducing nonexercise activities influenced the extent of fat loss with the heart rate monitoring methodology used in the present study. However, because factors other than activity (eg, excitement and stress) can influence heart rate, particularly when heart rates are relatively low (as they are over most of the day), this approach may have missed subtle changes in activity that could have contributed to responsiveness to exercise-induced fat loss. Thus, it is conceivable that individual differences in compensatory changes in spontaneous physical activity in response to an exercise intervention could have an effect on the extent of exercise-induced changes in fat mass that we were unable to detect in the present study.
In conclusion, this study found that the extent of exercise-induced fat loss is associated not only with exercise energy expenditure but also with changes in RQ at rest. Thus, development of strategies to maximize the shift in resting substrate utilization toward fat oxidation in response to an exercise training program may help individuals to maximize exercise-induced fat loss.
Acknowledgment
This work was supported by a project grant from the British Heart Foundation (PG/03/145). None of the authors has any conflict of interest relevant to this work.
References
- 1.King N.A., Hopkins M., Caudwell P., Stubbs R.J., Blundell J.E. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes (Lond) 2008;32:177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- 2.Byrne N.M., Meerkin J.D., Laukkanen R., Ross R., Fogelholm M., Hills A.P. Weight loss strategies for obese adults: personalized weight management program vs. standard care. Obesity. 2006;14:1777–1788. doi: 10.1038/oby.2006.205. [DOI] [PubMed] [Google Scholar]
- 3.Snyder K.A., Donnelly J.E., Jabobsen D.J., Hertner G., Jakicic J.M. The effects of long-term, moderate intensity, intermittent exercise on aerobic capacity, body composition, blood lipids, insulin and glucose in overweight females. Int J Obes Relat Metab Disord. 1997;21:1180–1189. doi: 10.1038/sj.ijo.0800533. [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A., Sorensen B., Irwin M.L., Morgan A., Yasui Y., Rudolph R.E. Exercise effect on weight and body fat in men and women. Obesity. 2007;15:1496–1512. doi: 10.1038/oby.2007.178. [DOI] [PubMed] [Google Scholar]
- 5.Colley R.C., Hills A.P., O'Moore-Sullivan T.M., Hickman I.J., Prins J.B., Byrne N.M. Variability in adherence to an unsupervised exercise prescription in obese women. Int J Obes (Lond) 2008;32:837–844. doi: 10.1038/sj.ijo.0803799. [DOI] [PubMed] [Google Scholar]
- 6.King N.A., Caudwell P., Hopkins M., Byrne N.M., Colley R., Hills A.P. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15:1373–1383. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 7.Goran M.I., Poehlman E.T. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol. 1992;263:E950–E957. doi: 10.1152/ajpendo.1992.263.5.E950. [DOI] [PubMed] [Google Scholar]
- 8.Kempen K.P., Saris W.H., Westerterp K.R. Energy balance during an 8-wk energy-restricted diet with and without exercise in obese women. Am J Clin Nutr. 1995;62:722–729. doi: 10.1093/ajcn/62.4.722. [DOI] [PubMed] [Google Scholar]
- 9.Levine J.A., Eberhardt N.L., Jensen M.D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard C., Tremblay A., Nadeau A., Dussault J., Despres J.P., Theriault G. Long-term exercise training with constant energy intake. 1: effect on body composition and selected metabolic variables. Int J Obes. 1990;14:57–73. [PubMed] [Google Scholar]
- 11.Votruba S.B., Atkinson R.L., Hirvonen M.D., Schoeller D.A. Prior exercise increases subsequent utilization of dietary fat. Med Sci Sports Exerc. 2002;34:1757–1765. doi: 10.1097/00005768-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Burton F.L., Malkova D., Caslake M.J., Gill J.M.R. Energy replacement attenuates the effects of prior moderate exercise on postprandial metabolism in overweight/obese men. Int J Obes (Lond) 2008;32:481–489. doi: 10.1038/sj.ijo.0803754. [DOI] [PubMed] [Google Scholar]
- 13.Hansen K., Shriver T., Schoeller D. The effects of exercise on the storage and oxidation of dietary fat. Sports Med. 2005;35:363–373. doi: 10.2165/00007256-200535050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Romijn J.A., Klein S., Coyle E.F., Sidossis L.S., Wolfe R.R. Strenuous endurance training increases lipolysis and triglyceride–fatty acid cycling at rest. J Appl Physiol. 1993;75:108–113. doi: 10.1152/jappl.1993.75.1.108. [DOI] [PubMed] [Google Scholar]
- 15.Blaak E.E., Saris W.H. Substrate oxidation, obesity and exercise training. Best Pract Res Clin Endocrinol Metab. 2002;16:667–678. doi: 10.1053/beem.2002.0226. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster B.H., Katsiaras A., Kelley D.E. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 17.Seidell J.C., Muller D.C., Sorkin J.D., Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16:667–674. [PubMed] [Google Scholar]
- 18.Marra M., Scalfi L., Covino A., Esposito-Del Puente A., Contaldo F. Fasting respiratory quotient as a predictor of weight changes in non-obese women. Int J Obes Relat Metab Disord. 1998;22:601–603. doi: 10.1038/sj.ijo.0800612. [DOI] [PubMed] [Google Scholar]
- 19.Zurlo F., Lillioja S., Esposito-Del Puente A., Nyomba B.L., Raz I., Saad M.F. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–E657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 20.Barwell N.D., Malkova D., Moran C.N., Cleland S.J., Packard C.J., Zammit V.A. Exercise training has greater effects on insulin sensitivity in daughters of patients with type 2 diabetes than in women with no family history of diabetes. Diabetologia. 2008;51:1912–1919. doi: 10.1007/s00125-008-1097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marfell-Jones M., Olds T., Stewart A. International standards for anthropometric assessment. Potchefstroom; South Africa, ISAK: 2006. [Google Scholar]
- 22.American College of Sports Medicine . Williams and Wilkins; Baltimore: 1995. Guidelines for exercise testing and prescription (5th ed) [Google Scholar]
- 23.Frayn K.N., Macdonald I.A. Assessment of substrate and energy metabolism in vivo. In: Draznin B, Rizza R, editors. Clinical research in diabetes and obesity part I: methods, assessment, and metabolic regulation. Humana Press; Totowa NJ: 1997. pp. 101–124. [Google Scholar]
- 24.Speakman J.R., Selman C., McLaren J.S., Harper E.J. Living fast, dying when? The link between aging and energetics. J Nutr. 2002;132:1583S–1597S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- 25.Westerterp K.R., Speakman J.R. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes (Lond) 2008;32:1256–1263. doi: 10.1038/ijo.2008.74. [DOI] [PubMed] [Google Scholar]
- 26.Elia M., Stratton R., Stubbs J. Techniques for the study of energy balance in man. Proc Nutr Soc. 2003;62:529–537. doi: 10.1079/pns2003255. [DOI] [PubMed] [Google Scholar]
- 27.Pagliassotti M.J., Gayles E.C., Hill J.O. Fat and energy balance. Ann N Y Acad Sci. 1997;827:431–448. doi: 10.1111/j.1749-6632.1997.tb51853.x. [DOI] [PubMed] [Google Scholar]
- 28.Valtuena S., Sola R., Salas-Salvado J. A study of the prognostic respiratory markers of sustained weight loss in obese subjects after 28 days on VLCD. Int J Obes.Relat Metab Disord. 1997;21:267–273. doi: 10.1038/sj.ijo.0800398. [DOI] [PubMed] [Google Scholar]
- 29.Luscombe N.D., Clifton P.M., Noakes M., Farnsworth E., Wittert G. Effect of a high-protein, energy-restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2003;27:582–590. doi: 10.1038/sj.ijo.0802270. [DOI] [PubMed] [Google Scholar]
- 30.Weyer C., Pratley R.E., Salbe A.D., Bogardus C., Ravussin E., Tataranni P.A. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85:1087–1094. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- 31.Pasman W.J., Westerterp M.S., Saris W.H. The effect of body weight changes and endurance training on 24h substrate oxidation. Int J Obes Relat Metab Disord. 1999;23:1223–1232. doi: 10.1038/sj.ijo.0801073. [DOI] [PubMed] [Google Scholar]
- 32.Froidevaux F., Schutz Y., Christin L., Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57:35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 33.Westerterp K.R., Goris A.H. Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care. 2002;5:489–493. doi: 10.1097/00075197-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Black A.E., Cole T.J. Biased over- or under-reporting is characteristic of individuals whether over time or by different assessment methods. J Am Diet Assoc. 2001;101:70–80. doi: 10.1016/S0002-8223(01)00018-9. [DOI] [PubMed] [Google Scholar]
- 35.Frost C., Thompson S.G. Correcting for regression dilution bias: comparison of methods for a single predictor variable. J R Stat Soc A. 2000;163:173–189. [Google Scholar]