Abstract
The purpose of this pictorial essay is to illustrate the multimodality imaging findings of a wide spectrum of radiation-induced complications of breast cancer in the sequence of occurrence. We have classified radiation-induced complications into three groups based on the time sequence of occurrence. Knowledge of these findings will allow for the early detection of complications as well as the ability to differentiate tumor recurrence.
Keywords: Breast, neoplasms; Radiation therapy, CT; Radiation therapy, US; Radiation therapy, PET; Radiation therapy, MRI
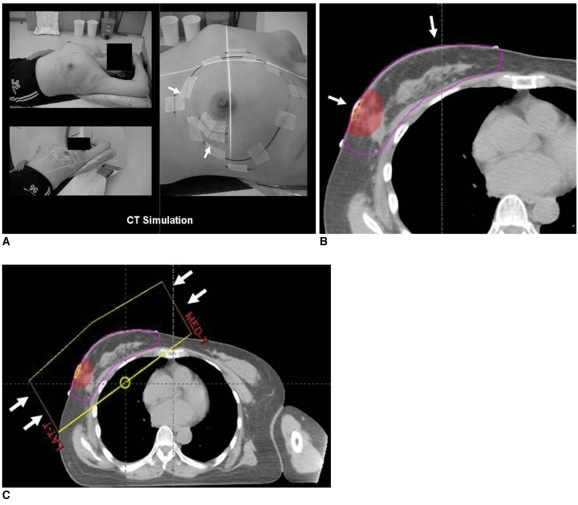
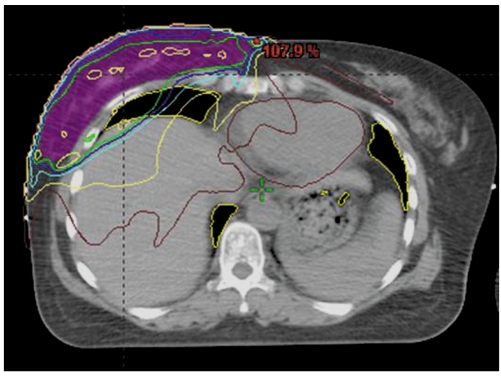
The primary target of radiation therapy is the eradication of microscopic residual disease adjacent to the original site of the tumor as well as the elimination of any evidence of multicentric disease. External beam radiation is the usual type of radiation therapy that is administered for the treatment of breast cancer. External beam radiation consists of three steps: computed tomography (CT) simulation, contouring of the targeted site and tangential two-field planning (Fig. 1). Exposure to radiation affects the adjacent structures as well as the operation bed within the entire breast (1) (Fig. 2).
Fig. 1.
External beam radiation is usual type of radiation therapy for breast cancer. Three steps of external beam radiation include (A) CT simulation (arrows), (B) contouring of targeted site (arrows) and (C) tangential two-field planning (arrows).
Fig. 2.
Radiation affects adjacent structures as well as operation bed with entire breast including following: target breast, overlying skin, chest wall, lung, heart, opposite breast and adjacent organs.
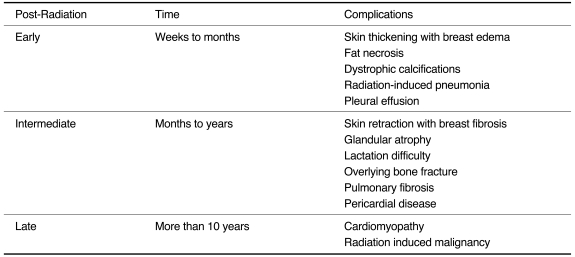
We have classified radiation-induced complications into three groups based on the time sequence of occurrence (2) (Table 1). The first group is composed of early complications that arise during the weeks to months following completion of radiation therapy including breast edema, fat necrosis, dystrophic calcifications, radiation-induced pneumonia and pleural effusion. The second group is composed of intermediate complications that arise during months to years following completion of radiation therapy including breast fibrosis, glandular atrophy, lactational difficulty, overlying bone fracture, pulmonary fibrosis and pericardial disease. The third group consists of late complications such as cardiomyopathy and radiation-induced malignancies that arise more than ten years following completion of radiation therapy.
Table 1.
Complications of Post-Radiation Therapy for Breast Cancer
EARLY COMPLICATIONS
Breast Edema
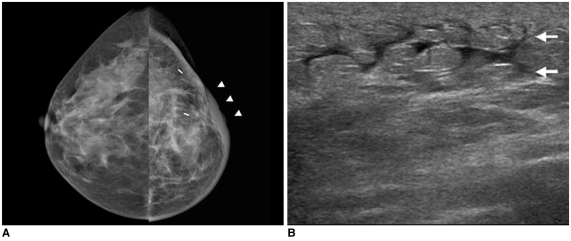
During the acute period after irradiation, inflammatory markers are induced and the expression of these proteins contributes to increased vascular permeability of breast tissue (3). Breast edema that manifests as skin and trabecular thickening appears generally in the first several weeks after completion of radiation therapy (Figs. 3, 4). Initially, there is engorgement of the dermal as well as of the intramammary lymphatics (trabecular thickening). This edema usually resolves over a period of weeks, months or sometimes years. Radiographically, an irradiated breast with skin and trabecular thickening appears denser as compared to the contralateral normal breast.
Fig. 3.
42-year-old female patient who had undergone radiation therapy in left breast six months ago are shown.
A. Mammography of left breast shows diffuse skin and trabecular thickening (arrowheads).
B. US of left breast shows diffuse skin thickening and interstitial edema (arrows).
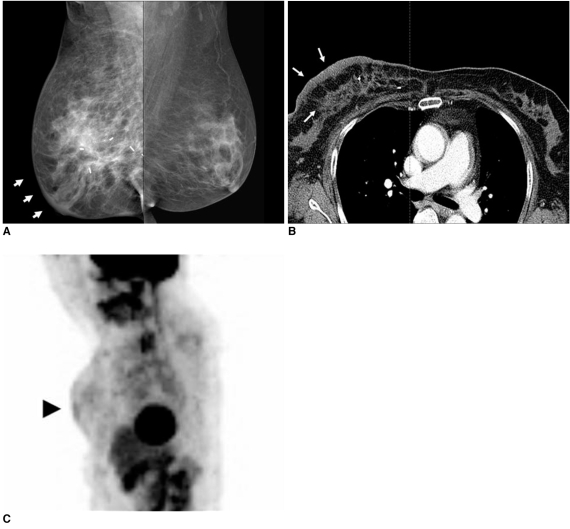
Fig. 4.
53-year-old female patient who had undergone radiation therapy in right breast six months ago are shown.
A. Mammography of right breast shows diffusely increased density due to skin and trabecular thickening (arrows).
B. Chest CT scan shows diffuse breast edema with skin and trabecular thickening (arrows).
C. Whole body PET image shows diffuse skin thickening and interstitial edema with mild hypermetabolic activity (max SUV = 1.8) (arrowhead) in irradiated right breast.
Fat Necrosis
Breast fat necrosis exhibits various profiles according to the process of nonpurulent inflammation and absorption. Intimal arterial damage caused by radiation exposure combined with surgical damage may result in tissue necrosis directly or indirectly (4).
Mammographically, the presence of a radiolucent oil cyst, round opacity, asymmetrical opacity, heterogeneity of the subcutaneous tissue, dystrophic calcification, clustered pleomorphic microcalcification or the presence of a spiculated mass is noted (Fig. 5). An ultrasound (US) examination can demonstrate the presence of a solid or anechoic mass with posterior acoustic shadowing or enhancement and can demonstrate the presence of a cyst with a mural nodule or internal echo or increased echogenicity of the subcutaneous tissue. On positron emission tomography (PET) imaging, fat necrosis may be seen with variable metabolic activities according to the process of inflammation. Therefore, a lesion with hypermetabolic activity can mimic tumor recurrence. As seen on magnetic resonance imaging (MRI), fat necrosis is characterized by the presence of a fatty signal intensity mass, often containing a fat-fluid level that exhibits variable enhancement following the administration of gadolinium contrast material. The presence of central fat signal intensity is the key to differentiate fat necrosis from tumor recurrence, as breast cancers do not contain central fat.
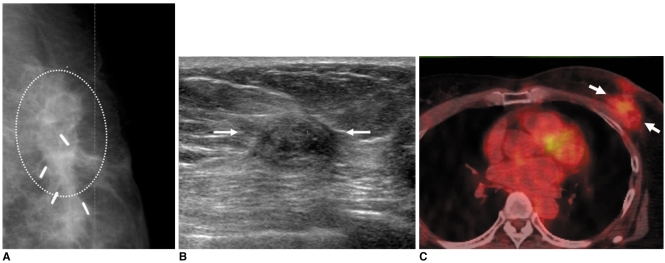
Fig. 5.
55-year-old female patient with hard palpable mass who had undergone radiation therapy in left breast two years ago are shown.
A. Mammography shows mass with spiculated margin, irregular shape and internal fat density (circle).
B. US study of left breast shows presence of isoechoic mass with spiculated margin and irregular shape (arrows).
C. Whole body PET/CT image shows mass with hypermetabolic activity (max SUV = 2.4) (arrows). Patient was diagnosed as having radiation-induced fat necrosis.
Radiation-Induced Calcifications
Radiation-induced calcifications are benign dystrophic calcifications that are due to a combination of surgical trauma and radiation exposure (Fig. 6). The calcifications are generally large and irregular in pattern, with central lucency and calcifications that always occur at the site of surgery. In addition, it is common for sutures to calcify after radiation therapy. Calcified sutures are usually characteristic as the calcified sutures are equally spaced along the suture line and the presence of calcified knots is frequently evident.
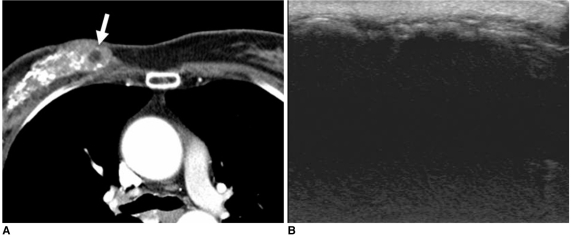
Fig. 6.
45-year-old female patient who had undergone radiation therapy in right breast one year ago are shown.
A. Chest CT scan shows diffusely scattered coarse calcifications and some internal fat density (arrow) in irradiated right breast.
B. US image of right breast shows diffuse acoustic shadowing due to dense calcification with diffuse skin thickening, suggesting presence of radiation-induced changes.
Radiation Pneumonitis
Radiation-induced lung injury typically presents with two distinct, subsequent clinical phases: pneumonitis and fibrosis (5). Radiation pneumonitis is consolidation or ground glass opacity that is localized in the radiation field due to acute exudation in the alveolar space and migration of inflammatory cells. Radiation pneumonitis occurs 4-12 weeks after completion of radiation therapy, and radiation pneumonitis can regress to complete restitution or evolve into fibrosis when present with a more severe grade. Lung alterations are scored according to a scoring system devised by Nishioka et al. (6) The scoring includes Grade 0, no significant changes in the radiation field; Grade 1, only pleural thickening in the radiation field; Grade 2, pulmonary changes (plaque-like or heterogeneous density) in < 50% of the area of the radiation field; Grade 3, pulmonary changes in > 50% of the area of the radiation field (Figs. 7, 8).
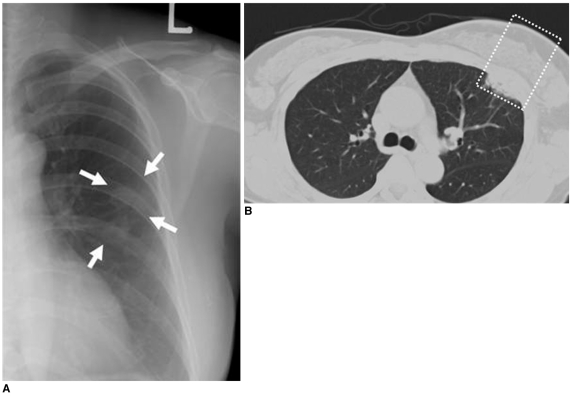
Fig. 7.
56-year-old female patient who had undergone radiation therapy in left breast eight months ago are shown.
A. Chest radiograph shows circumscribed patchy consolidation (arrows) in left mid lung field.
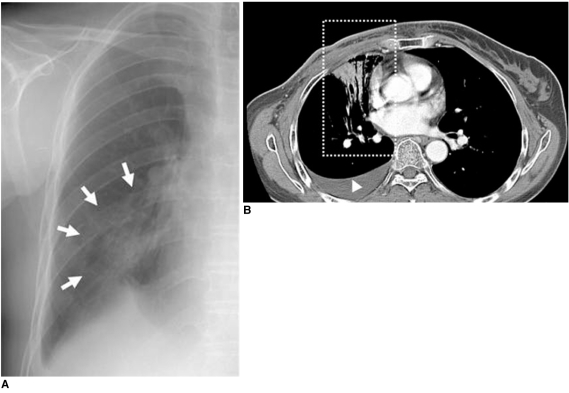
B. Chest CT scan demonstrates presence of sharply demarcated pneumonic consolidation in anterior aspect of left upper lobe along previous radiation field (box), suggesting presence of radiation-induced pneumonia.
Fig. 8.
52-year-old female patient who had undergone radiation therapy in right breast two weeks ago are shown.
A. Chest radiograph shows peribronchial consolidation in right mid lung field (arrows).
B. Chest CT scan shows air-space consolidation with air-bronchogram localized in medial portion of right lung (box) that was related to previous radiation field for internal mammary lymph nodes, suggesting presence of radiation-induced pneumonia. Associated right pleural effusion is also noted (arrowhead).
INTERMEDIATE COMPLICATIONS
Breast Fibrosis (Glandular Atrophy)
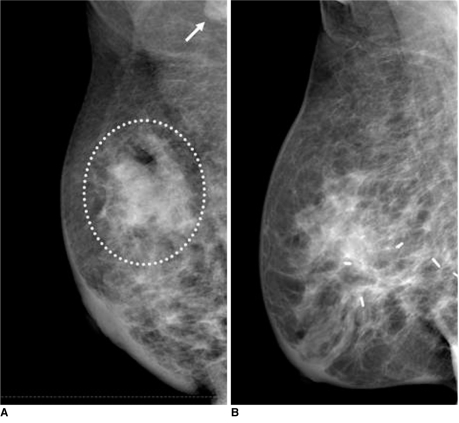
In some cases, radiation-induced breast edema progresses to become a permanent fibrotic change with glandular atrophy. As demonstrated in previous studies (7, 8), a high radiation dose, concurrent chemotherapy with radiation therapy and underlying collagen vascular disease have been shown to be significantly associated with an increased incidence of breast fibrosis. As seen on serial follow-up mammograms, the breast parenchyma gradually shrinks and is denser as compared with the contralateral normal breast (Fig. 9).
Fig. 9.
36-year-old female patient who had undergone radiation therapy in right breast four years ago are shown.
A. Initial postoperative mammogram obtained after six months shows diffuse skin and trabecular thickening, suggesting presence of radiation-induced breast edema.
B, C. Annual follow-up mammograms show gradually decreased breast volume with diffusely increased glandular density due to progressive glandular atrophy with fibrosis.
Lactation Difficulty
In the lactational period, it is possible to have poor glandular proliferation in the irradiated breast due to radiation-induced vascular injury, fibrotic change and glandular atrophy. For this condition, there is compensatory hyperstimulated glandular tissue in the contralateral breast (Fig. 10).
Fig. 10.
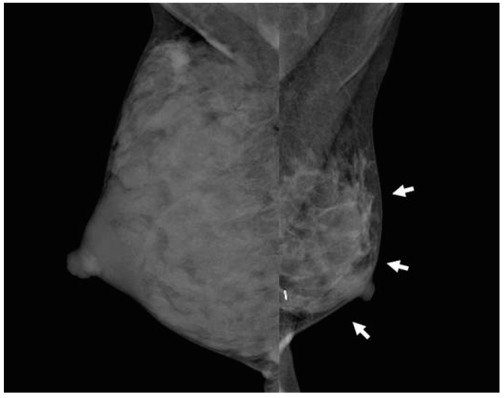
36-year-old female patient who had undergone radiation therapy in left breast three years ago are shown. Patient had breast-feeding difficulty in irradiated left breast. Mammography shows diffuse glandular atrophy (arrows) in irradiated left breast and compensatory glandular hypertrophy in right breast.
Pulmonary Fibrosis
Pulmonary fibrosis is a late injury due to interstitial damage involving the parenchyma as well as the pleura. Severity seems to be related to a number of factors, including the volume of the irradiated lung, radiation dose, fractionation or concomitant use of some chemotherapy regimens (9). It appears that relatively sharp marginated fibrosis is localized in the radiation field (Fig. 11).
Fig. 11.
49-year-old female patient who had undergone radiation therapy in right breast three years ago are shown.
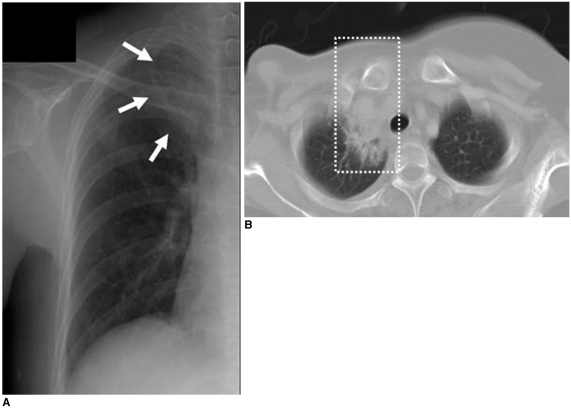
A. Chest radiograph shows presence of localized fibrotic lesion in apical portion of right upper lobe (arrows).
B. Chest CT scan shows fibrosis with bronchiectasis (box) localized in medial portion of right pulmonary apex that was associated with radiation therapy for right supraclavicular lymph nodes.
LATE COMPLICATIONS
Overlying Bone Fractures
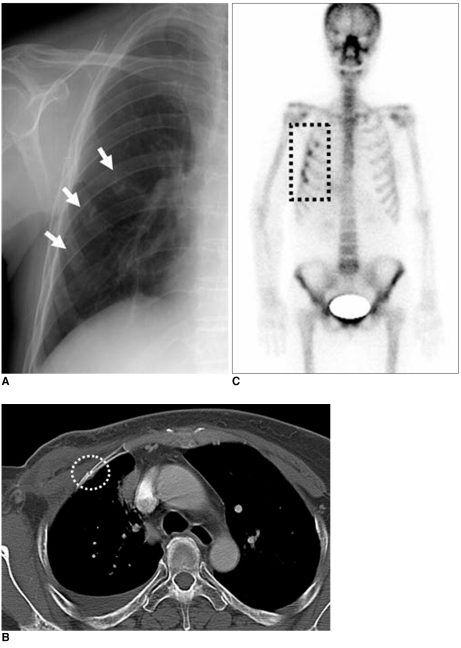
Radiation-induced osteonecrosis occurs due to vascular compromise with obliterative endarteritis and damage to osteoblasts and osteoclasts (10). To induce osteonecrosis, a dose greater than 6 Gy in adults is required and onset occurs more than one year after completion of radiation therapy. Findings include focal lucency, periostitis, sclerosis, insufficiency fractures and cortical thinning. Initially, a bone scan will show decreased uptake of radioactive material in the radiation field. In the late stage, radiation-induced bone fracture and increased radioactive material uptake are seen on a bone scan (Fig. 12). In addition, there is increased susceptibility of the irradiated bone to infection and an increased risk to develop bone sarcomas in the irradiated field (latency > five years).
Fig. 12.
59-year-old female patient who had undergone radiation therapy in right breast six years ago are shown.
A. Chest radiograph shows rib fractures involving right anterior chest wall (arrows).
B. Chest CT scan shows relatively sharply defined fracture line without adjacent mass formation (circle).
C. Bone scan shows multiple hot uptakes confined to anterior arcs of right ribs associated with radiation field (box).
Radiation-Induced Malignancy
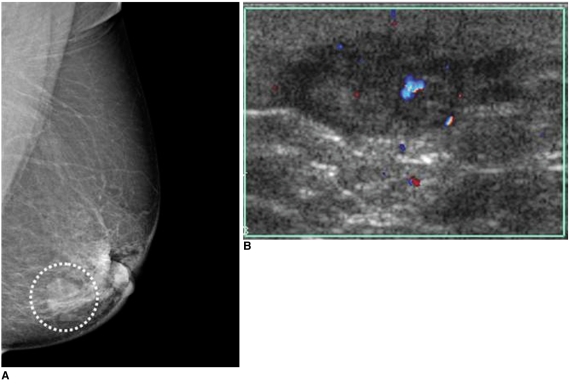
Radiation-induced malignancies such as invasive ductal carcinoma, lymphoma and angiosarcoma are infrequent and late complications (11). Radiation-induced angiosarcomas are very rare tumors of endovascular origin. Primary angiosarcoma accounts for only 0.04% of breast tumors and affects patients of younger age (20-40 years). Secondary angiosarcoma induced by radiotherapy occurs in older aged patients, with a mean age of 68 years. Secondary angiosarcoma is difficult to diagnose due to its rarity, benign appearance and difficulty in differentiation from radiation-induced changes in the skin (12) (Fig. 13). Therefore, it is very important that radiologists are aware of the possible presence of an angiosarcoma.
Fig. 13.
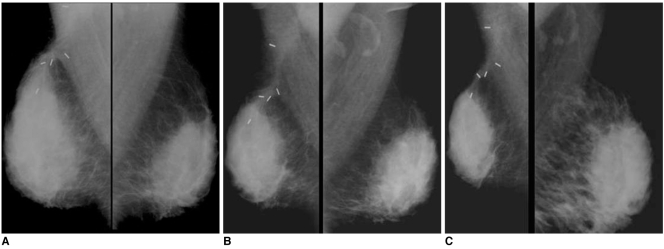
62-year-old female patient who had undergone left breast-conserving surgery that was followed by radiation therapy due to invasive ductal carcinoma six years ago are shown.
A. Mammography of left breast shows mass with obscured margin, relatively round shape and isodensity in previous operation site (circle).
B. Careful US study of left breast shows hypoechoic mass with microlobulated margin and irregular shape with increased vascularity, as seen on color Doppler US. Presence of radiation-induced angiosarcoma was confirmed after pathological examination. (Courtesy of Yeong-Mi Park, MD Inje University Pusan Paik Hospital and Ki-Seok Choo, MD, Pusan National University Hospital)
CASES AND PITFALLS
Fat Necrosis versus Local Recurrence
The use of MR imaging has some advantages in the differential diagnosis of fat necrosis and a recurred lesion. MR imaging with fat suppression can suggest the possibility of fat necrosis. On contrast-enhanced MR imaging, no enhancement, early enhancement and spiculated enhancement for a cystic lesion containing fat-fluid level can suggest the possibility of fat necrosis. The use of three-dimensional fat-suppressed dynamic MR imaging also appears to be efficacious for the specific detection of malignancies (2).
Radiation-Induced Dystrophic Calcifications versus Malignant Calcifications
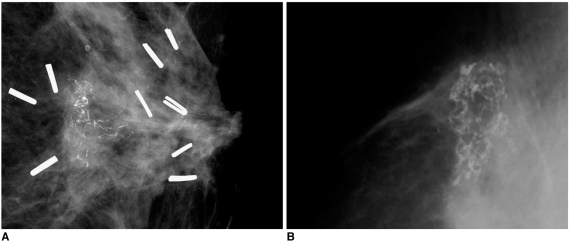
Analysis of calcifications that arise in the post-irradiated breast can be difficult. If tumor calcifications remain after surgery, the calcifications may be unaffected by radiation or may undergo resorption. In cases of neocalcifications that appear after radiotherapy, the neocalcifications represent benign calcifications associated with tumor necrosis rather than the presence of a recurrent tumor. In addition, neocalcifications during radiation therapy within two years following surgery are generally benign dystrophic calcifications. Benign radiation-induced calcifications are large and have an irregular margin, with central lucency. The calcifications always occur at the site of surgery. However, malignant calcifications show a fine linear or linear branching pattern and are commonly associated with the presence of a malignant mass lesion (13) (Fig. 14).
Fig. 14.
Examples of calcifications in previous operation bed are presented.
A. 53-year-old female patient who had undergone left breast-conserving surgery that was followed by radiation therapy due to invasive ductal carcinoma eight months ago are shown. Mammography of left breast shows clustered microcalcifications with linear branching pattern and pleomorphism. Presence of recurrent ductal carcinoma in situ was confirmed after pathological examination.
B. 68-year-old female patient who had undergone right breast-conserving surgery that was followed by radiation therapy due to invasive ductal carcinoma one year prior are shown. Mammography shows presence of lucent centered curvilinear calcifications without associated mass. Presence of fat necrosis was confirmed after pathological examination.
Radiation-Induced Breast Edema versus Inflammatory Breast Cancer
Radiation-induced breast edema shows similar imaging findings as the serious condition of inflammatory breast cancer, with a diffusely enlarged breast with trabecular and skin thickening as depicted on mammography and US. However, inflammatory breast cancer shows the presence of a malignant mass lesion within the edematous breast parenchyma and inflammatory breast cancer may be accompanied with a coexisting metastatic lymph node in the ipsilateral axillary area (Fig. 15). In contrast, radiation-induced breast edema does not show a coexisting malignant mass and metastatic axillary lymph node; radiation-induced breast edema slowly resolves and may progress to benign breast fibrosis.
Fig. 15.
Examples of breast edema with skin thickening are presented.
A. 45-year-old female patient who had diagnosed inflammatory breast cancer in right breast are shown. Mammography of right breast shows mass with indistinct margin, irregular shape and hyperdensity (circle). In addition, there is prominent metastatic lymph node located in right axilla (arrow).
B. 49-year-old female patient who had radiation-induced breast edema associated with previous radiation therapy are shown. Mammography shows diffuse skin and trabecular thickening without definite mass lesion.
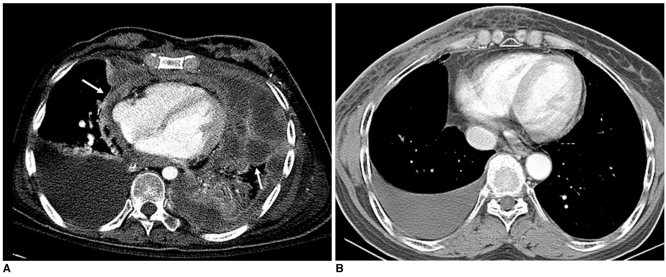
Radiation-Induced Pleural Effusion versus Malignant Pleural Effusion
Malignant effusion in the pleural and pericardial space have common seeding nodules or masses that appear with slow or rapid progression as seen on serial follow-up imaging. In contrast, radiation-induced effusion is a pure transudate associated with radiation-induced pneumonitis. There is no evidence for the presence of a seeding mass and the condition slowly resolves as seen on serial follow-up imaging with improvement of the condition (Fig. 16).
Fig. 16.
Examples of pleural effusion are presented.
A. 53-year-old female patient who had undergone left conservation surgery due to invasive ductal carcinoma two years ago are shown. Chest CT scans show nodular seeding lesions along pleuropericardial surface (arrows) with malignant pleural effusion.
B. Right pleural effusion without associated nodularity in 59-year-old female patient who was subjected to right breast irradiation two weeks prior is shown, suggesting presence of radiation-induced pleurisy.
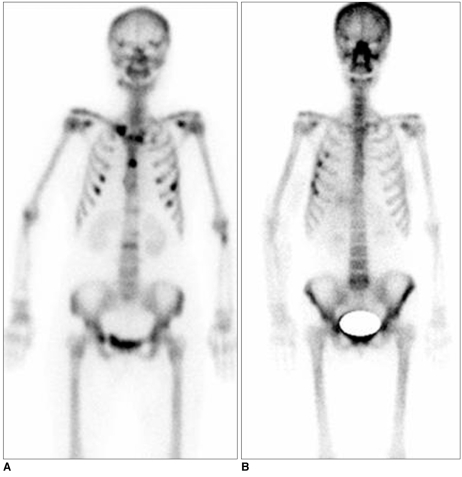
Overlying Bone Fracture versus a Bone Metastasis
Generally, for the postoperative breast cancer patient, hot (radioactive material) uptake lesions as seen on a bone scan highly suggest the presence of a bone metastasis. Metastatic bone lesions commonly show soft tissue mass formation and destructive bone change rather than a radiation-induced change. In addition, a hot uptake or decreased uptake that is sharply localized in bony structures of the radiation field may have the possibility to be a benign fracture associated with radiation-induced osteonecrosis (Fig. 17).
Fig. 17.
Examples of overlying bone lesions are presented.
A. Multiple bone metastases are seen as multifocal hot uptake lesions without association of radiation field on bone scan.
B. Radiation-induced bone fracture shows hot uptake lesions localized in overlying ribs associated with radiation field as depicted on bone scan.
CONCLUSION
It is necessary for a radiologist to know and to understand the expected complications for the interpretation of follow-up images in patients after radiation therapy for breast cancer. Familiarity with a spectrum of imaging features and pitfalls of these complications should allow for accurate diagnosis.
Acknowledgment
We thank Yeong-Mi Park, MD in Inje University Pusan Paik Hospital and Ki-Seok Choo, MD in Pusan National University Hospital, for their assistance in the preparation of this manuscript.
References
- 1.Sabin BM, Eric AS, Marsha DM, Thomas AB. Breast cancer. In: Levitt SH, Purdy JA, Perez CA, Vijayakumar S, editors. Technical basis of radiation therapy: practical clinical applications. 4th ed. Berlin: Baert AL; 2006. pp. 486–510. [Google Scholar]
- 2.Coles CE, Moody AM, Wilson CB, Burnet NG. Reduction of radiotherapy-induced late complications in early breast cancer: the role of intensity-modulated radiation therapy and partial breast irradiation. Part I--normal tissue complications. Clin Oncol (R Coll Radiol) 2005;17:16–24. doi: 10.1016/j.clon.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Moore AH, Olschowka JA, Williams JP, Paige SL, O'Banion MK. Radiation-induced edema is dependent on cyclooxygenase 2 activity in mouse brain. Radiat Res. 2004;161:153–160. doi: 10.1667/rr3116. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki H, Morimoto K, Koh M, Okamura T, Wakasa K, Wakasa T, et al. A case of fat necrosis after breast quadrantectomy in which preoperative diagnosis was enabled by MRI with fat suppression technique. Magn Reson Imaging. 2004;22:285–290. doi: 10.1016/j.mri.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Lind P. Clinical relevance of pulmonary toxicity in adjuvant breast cancer irradiation. Acta Oncol. 2006;54:13–15. doi: 10.1080/02841860500466632. [DOI] [PubMed] [Google Scholar]
- 6.Nishioka A, Ogawa Y, Hamada N, Terashima M, Inomata T, Yoshida S. Analysis of radiation pneumonitis and radiation-induced lung fibrosis in breast cancer patients after breast conservation treatment. Oncol Rep. 1999;6:513–517. doi: 10.3892/or.6.3.513. [DOI] [PubMed] [Google Scholar]
- 7.Toledano A, Garaud P, Serin D, Fourquet A, Bosset JF, Breteau N, et al. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys. 2006;65:324–332. doi: 10.1016/j.ijrobp.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JM, Clarke DH, Pevzner MM, Matter RC. Breast conservation therapy. Severe breast fibrosis after radiation therapy in patients with collagen vascular disease. Cancer. 1991;68:502–508. doi: 10.1002/1097-0142(19910801)68:3<502::aid-cncr2820680310>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Hardman PD, Tweeddale PM, Kerr GR, Anderson ED, Rodger A. The effect of pulmonary function of local and loco-regional irradiation for breast cancer. Radiother Oncol. 1994;30:33–42. doi: 10.1016/0167-8140(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 10.Ruggiero S, Gralow J, Marx RE, Hoff AO, Schubert MM, Huryn JM, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2:7–14. doi: 10.1200/jop.2006.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buatti JM, Harari PM, Leigh BR, Cassady JR. Radiation-induced angiosarcoma of the breast. Case report and review of the literature. Am J Clin Oncol. 1994;17:444–447. doi: 10.1097/00000421-199410000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Cafiero F, Gipponi M, Peressini A, Queirolo P, Bertoglio S, Comandini D, et al. Radiation-associated angiosarcoma: diagnostic and therapeutic implications--two case reports and a review of the literature. Cancer. 1996;77:2496–2502. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2496::AID-CNCR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Rebner M, Pennes DR, Adler DD, Helvie MA, Lichter AS. Breast microcalcifications after lumpectomy and radiation therapy. Radiology. 1989;170:691–693. doi: 10.1148/radiology.170.3.2492670. [DOI] [PubMed] [Google Scholar]