Abstract
S-adenosylmethionine decarboxylase (AdoMetDC) is an essential enzyme of polyamine (PA) biosynthesis, and both AdoMetDC and PA levels are often up-regulated in cancer cells. The second generation inhibitor SAM486A inhibits AdoMetDC enzyme activity and has been evaluated in phase II clinical cancer trials. However, little is known about the mechanism of action and potential use of this therapeutic drug in the treatment of the pediatric cancer neuroblastoma (NB). Here we show that p53-wild type NB cells are highly sensitive to SAM486A treatment. Most notably, SAM486A treatment resulted in the rapid accumulation of pro-apoptotic proteins p53 and Mdm2. Concomitant with the increase of proteins at endogenous levels, the in vivo phosphorylation of p53 at residues Ser46/Ser392 and Mdm2 at residue Ser166 was observed. Moreover, the anti-apoptotic protein Akt/PKB was down-regulated and also dephosphorylated at residue Ser473 in a dose- and time-dependent manner and NB cells entered apoptotic cell death. The results presented in this study highlight the importance of PA homeostasis and provide a direct link between PA metabolism and apoptotic cell signaling pathways in p53-wild type NB cells. PA inhibitors such as SAM486A may be effective alternative agents for the treatment of NBs with or without MYCN amplification.
Keywords: apoptosis, AdoMetDC, neuroblastoma, polyamine inhibitor, SAM486A, p53-Mdm2-Akt/PKB
Introduction
Elevated polyamine (PA) levels are sustained in rapidly proliferating cells and suppression of PA biosynthesis provides an attractive therapeutic target for many cancers (1-6). S-adenosylmethionine decarboxylase (AdoMetDC), a key enzyme in PA biosynthesis, provides the aminopropyl donor, decarboxylated S-adenosylmethionine (dcAdoMet), which is required for the sequential conversions of PAs putrescine (Put) to spermidine (Spd) and then spermine (Spm). Ornithine decarboxylase (ODC) is a second rate-limiting enzyme in PA biosynthesis and catalyzes the conversion of ornithine (Orn) to Put.
Both biosynthetic enzymes can be inhibited with specific inhibitors. SAM486A (also known as CGP48664), a derivative of the first generation AdoMetDC inhibitor mitoguazone (MGBG), exerts potent and specific inhibition of AdoMetDC (7-10). The efficacy of SAM486A has been assessed in various cancer cells and animal systems (7, 8, 10-13), and the inhibitor has been evaluated in phase II human clinical trials (14, 15). Alpha-difluoromethylornithine (DFMO) (5) is a specific suicide inhibitor of ODC that has been intensively studied in human clinical trials as an anticancer or chemopreventive agent (2, 5, 16).
Despite a plethora of information concerning the effect of these inhibitors in various cancer systems, very little is known about their role and therapeutic use in neuroblastoma (NB), an aggressive cancer of childhood (17). While ~ 50% of human tumors harbor p53 mutations, the majority of primary NB tumors are tumor suppressor protein p53-wild type with intact downstream p53 signaling pathways (18, 19). Our previous work showed that DFMO and DFMO combined with SAM486A (but not SAM486A alone) caused the down-regulation of MYCN protein and p27/Rb-regulated G1 cell cycle arrest without the induction of apoptosis in MYCN-amplified and p53-mutant NB cells (20). In this study, we report that p53-wild type NB cells, independent of their MYCN amplification status, are highly sensitive to SAM486A. This targeted PA inhibitor induces the rapid accumulation and phosphorylation of p53 and Mdm2, down-regulates Akt/PKB, and induces apoptotic cell death.
Materials and Methods
Chemicals
The AdoMetDC inhibitor SAM486A was provided by Novartis (Basel, Switzerland) and dissolved in water. Aliquots at 10 mM concentration were sterile-filtered and stored frozen at -20 °C until used in the experiment. Spd, aminoguanidine, and doxycycline (Doxy) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cell lines and Treatment of Cultured Cells
The human NB cell lines SK-N-SH, IMR-32, and SH-SY5Y were obtained from the American Type Culture Collection (Manassas, VA, USA). MYCN-2, a doxycycline-inducible, MYCN-expressing cell line was kindly provided by Jason Shohet (Houston, TX, USA) (21). Cells were maintained in RPMI 1640 (Biosource, Rockeville, MD, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), penicillin (100 IU/ml) and streptomycin (100 μg/ml). If cells were treated with Spd (10 μM), aminoguanidine (1 mM) was included as an inhibitor of serum PA oxidation. Cells in early log-phase were seeded 2-3 hours before treatment with 0.1-10 μM SAM486A and analyzed after designated time periods. NB cells were cultured at 37 °C, in a humidified atmosphere containing 5% CO2. The number of viable cells was determined using a hemacytometer in the presence of trypan blue.
Flow Cytometry
For the detection of apoptosis, NB cells were seeded and treated with equal volumes of either sterile water (control) or 10 μM SAM486A. Where indicated, cells were treated with Doxy (1μg/ml) or Spd (10 μM) and aminoguanidine (1mM). Cells were trypsinized, washed twice in phosphate-buffered saline (PBS), counted, and 1-2 × 105 cells suspended in 0.1 ml of 1× assay buffer according to the manufacturer's instructions (BD Biosciences, Palo Alto, CA, USA). Cells were stained with annexin V-FITC (5 μl) and propidium iodide (PI) (5 μl) for 15 min in the dark at room temperature. Assay buffer (0.4 ml) was added, and 5,000 cells analyzed using a FACScan flow cytometry instrument (Becton Dickinson, San Jose, CA, USA). The CellQuest program or the FlowJo software (Tree Star, Inc., Ashland, OR, USA) were used for data analysis. For the detection of cell cycle phase distribution, SAM486A-treated cells were stained with PI as previously described (20), and cell cycle distribution was determined using the ModFit software.
Western Blot Analysis
Cell lysates were prepared in RIPA buffer (20 mM Tris-HCl, pH 7.5, 0.1% (w/v) sodium lauryl sulfate, 0.5% (w/v) sodium deoxycholate, 135 mM NaCl, 1% (v/v) Triton X-100, 10% (v/v) glycerol, 2 mM EDTA, supplemented with Complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA), and phosphatase inhibitors sodium fluoride (NaF) (20 mM) and sodium vanadate (Na3VO4) (0.27mM). Western blot analysis was performed as previously described (20). The total protein concentration was determined using the protein assay dye reagent from Bio-Rad Laboratories (Hercules, CA). Cell lysates in SDS-sample buffer were boiled for 5 min and equal amounts of total protein analyzed by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. The antibodies used in this study are: mouse monoclonal p53 (sc-126) (1:250) from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit polyclonal phospho-p53 (Ser46) (1:1,000), rabbit polyclonal phospho-p53 (Ser392) (1:1,000), rabbit polyclonal Akt/PKB (#9272) (1:1,000), and rabbit polyclonal phospho-Akt/PKB (Ser473) (1:1,000) from Cell Signaling Technology, Inc. (Beverly, MA, USA); mouse monoclonal Mdm2 (Ab-4) (1:1,000) and rabbit polyclonal phospho-Mdm2 (Ser166) (1:1,000) from EMD Biosciences (La Jolla, CA, USA); mouse monoclonal β-actin (A5316) (1:5,000) from Sigma Chemical Co. (St. Louis, MO, USA), and mouse monoclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (6C5) (1:5,000) from Ambion, Inc. (Austin, TX, USA). Secondary anti-mouse (1:5,000) and anti-rabbit (1:5,000) antibodies coupled to horseradish peroxidase (HRP) were from Amersham Biosciences (Piscataway, NJ, USA). Proteins were detected using the ECL Plus reagents (Amersham Biosciences, Piscataway, NJ, USA) and Kodak BioMax XAR film (Fisher Scientific, Pittsburgh, PA, USA). Membranes were stripped at 50 °C for 30 min with ECL stripping buffer (62.5 mM Tris-HCL, pH 6.7, 2% SDS, 100 mM 2-Mercaptoethanol) and sequentially probed. Quantification was performed as described previously using a Bio-Rad Fluor-S Multi Imager and Quantity One Quantitation Software, Version 4 (Bio-Rad Laboratories, Hercules, CA, USA).
Microscopy
NB cells were grown in cell culture dishes, treated with inhibitors as described above, and light micrographs were taken after 72 hours using an inverted microscope (Nikon Diaphot, Nikon Corporation, Japan or Leica DM IL, Leica Microsystems, Deerfield, IL, USA). To visualize p53 using immunofluorescence microscopy, SAM486A-treated or untreated cells were grown on cover slips (1 × 105 cells/ml) in 12-well plates for 72 hours. Washed cells were blocked in BSA, washed again, incubated with p53 antibody, followed by incubation with secondary Alexa-488 Fluor antibody. To visualize the actin cytoskeleton, NB cells were stained with Texas Red-X phalloidin according to the manufacturer's instructions (Molecular Probes, Eugene, OR). Cells were washed, fixed in 3% p-formaldehyde for 10 min, and exposed to 0.1% Triton X-100 for 5 min. Fixed cells were washed again, blocked in 1% BSA/PBS for 30 min and incubated with phalloidin for 20 min in the dark at room temperature. Washed cover slips were mounted using Vectashield® Mounting Medium with 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA) and samples analyzed with an epifluorescence microscope (Zeiss Axioplan, Carl Zeiss MicroImaging GmbH, Germany).
Polyamine Pool Analysis
Intracellular PA pools were measured in human SK-N-SH cells treated with SAM486A as previously described (13). The samples were normalized in 0.2 N sodium hydroxide and the amount of total protein per sample was measured using the Bio-Rad assay. Data from the PA measurements are represented as nmol per mg of total cellular protein.
MTS Assay
The CellTiter 96® AQueous One Solution Cell Proliferation Assay is a colorimetric method for determining the number of viable cells in proliferation or cytotoxicity (Promega, San Luis Obispo, CA, USA). The CellTiter 96® AQueous One Solution Reagent contains a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and was used to determine the proliferation rate of NB cell lines. SK-N-SH, IMR-32, SH-SY5Y, and MYCN-2 (± Doxy) cells were treated with increasing concentrations of SAM486A (0-10 μM) for 72 hours or treated with SAM486A (10 μM) ± Spd (10 μM) for 24-72 hours, and the proliferation rate was compared to untreated control cells. Briefly, cells were seeded at a density of 750-1000 cells/well on a transparent, flat-bottom, 96-well plate, in a total volume of 100 μL. After cell treatments, 20 μL of CellTiter 96® AQueous One Solution Reagent was added to wells, and incubated for 1-4 hours at 37 °C. The absorbance was measured at 490 nm using a 96-well microplate reader.
Results
SAM486A Induces Apoptosis and Inhibits Cellular Proliferation
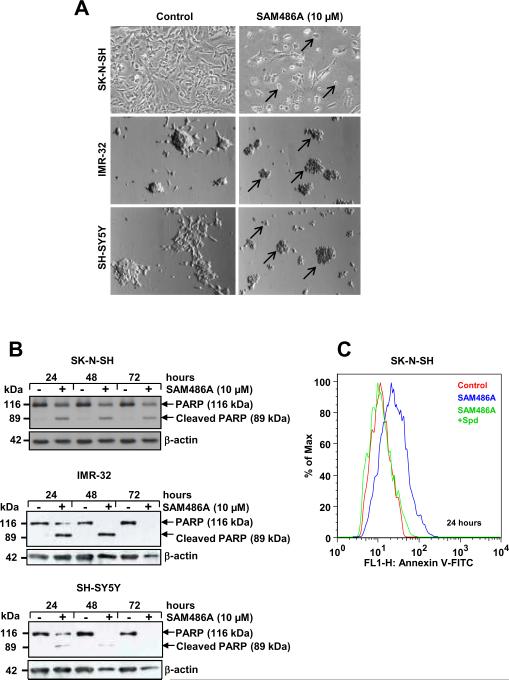
To examine whether the AdoMetDC inhibitor SAM486A induces apoptosis, we tested the effect of SAM486A in NB cells with wild type p53: SK-N-SH and SH-SY5Y (MYCN non-amplified), IMR-32 (MYCN-amplified), and MYCN-2 (MYCN non-amplified, Doxy-inducible MYCN). First, we examined the effects of SAM486A using an inverted light microscope and noticed that SAM486A affects the morphology of NB cells as evidenced by profound cell shrinkage and rounding of cells, membrane blebbing, and cell detachment, all of which are typical signs of apoptosis (Fig. 1A). The onset of apoptosis was confirmed by PARP cleavage (Fig. 1B) and annexin V staining (Fig. 1C) as early as 24 hours after treatment with SAM486A, using Western blot and flow cytometry analyses, respectively. Spd supplementation reversed the SAM486A-induced apoptosis in SK-N-SH cells (Fig. 1C). Comparable effects were observed with MYCN-2 cells and were independent of MYCN expression (Supplemental Fig. 1).
Figure 1.
SAM486A induces apoptosis in p53-wild type NB Cells. SK-N-SH, IMR-32, and SH-SY5Y cells were exposed to 10 μM SAM486A or left untreated for 24, 48, and/or 72 hours. A, representative light micrographs demonstrate the effects of SAM486A on the cell morphology (black arrows) after 72 hours. B, whole cell lysates were analyzed by Western blot for PARP cleavage, a marker for late apoptosis, after 24, 48, and 72 hours. C, SK-N-SH cells were analyzed with flow cytometry and annexin V staining, an early apoptosis marker, to confirm the induction of apoptotic cell death after 24 hours. Spd (10μM) reversed SAM486A-induced apoptosis. Similar results (without Spd control) were obtained after 48 and 72 hours (not shown). Data are representative of three independent experiments (n=3). Spd control in C (n=2).
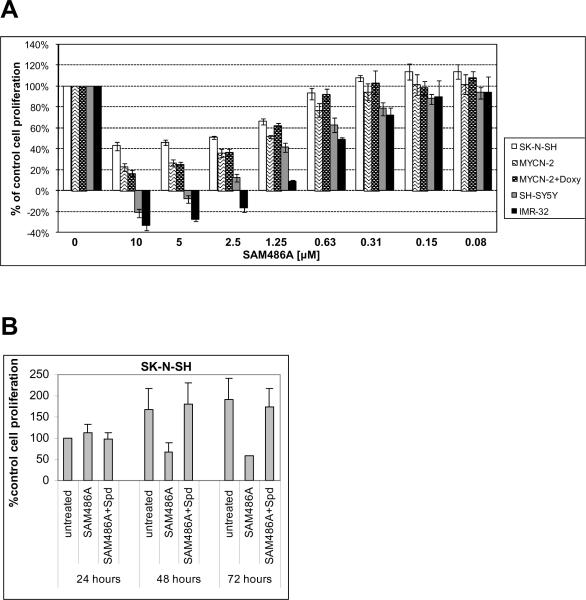
To examine whether SAM486A inhibits cell proliferation, cells were analyzed by MTS assay. The treatment of cell lines SK-N-SH, SH-SY5Y, IMR-32, and MYCN-2 (± Doxy) with SAM486A at various concentrations significantly reduced the number of viable cells in a dose-dependent manner (Fig. 2A), an effect that was reversible with Spd supplementation (Fig. 2B). At doses ≥ 2.5 μM, SAM486A exhibited cytotoxic effects in cell lines SH-SY5Y and IMR-32. The induction of MYCN expression with Doxy in MYCN-2 cells did not have an impact on SAM486A-induced growth inhibition (Fig. 2A). Interestingly, the treatment with SAM486A did not significantly disturb cell cycle progression of SK-N-SH cells, indicating that the observed induction of apoptosis is not a consequence of cell cycle arrest (Supplemental Table 1). Together, these results confirm that SAM486A induces apoptosis and inhibits cell proliferation in several p53-wild type NB cell lines independent of their MYCN amplification status.
Figure 2.
SAM486A inhibits cell proliferation in p53-wild type NB cells. Cell proliferation rate was determined by tetrazolium-based MTS assay. A, the dose-dependent effect of SAM486A was determined in SK-N-SH, SH-SY5Y, IMR-32, and MYCN-2 cells (± doxycycline; Doxy), at 72 hours. B, cells were left untreated or exposed to 10 μM SAM486A, in the presence or absence of 10 μM Spd and 1 mM aminoguanidine, for 24, 48, and 72 hours. Data represent the mean (± SE) of three independent experiments (n=3).
SAM486A Modulates Protein Levels and In Vivo Phosphorylation of p53, Mdm2, and Akt/PKB
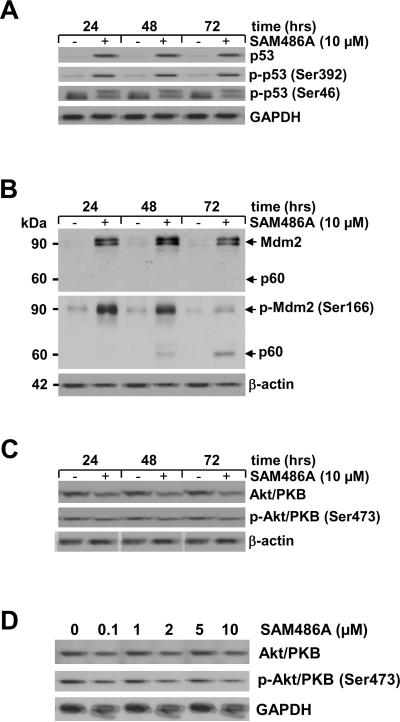
The tumor suppressor protein p53 plays an important role in regulatory processes including apoptosis and cell cycle progression. To examine the potential effect of SAM486A on p53 at endogenous levels, we determined the protein content in SK-N-SH cells. As shown in Fig. 3A, strong accumulation of p53 was evident 24 hours after cell treatment with 10 μM SAM486A. Extended treatments (48 and 72 hours) under identical conditions did not lead to further accumulation of p53 (Fig. 3A). To examine the phosphorylation status of p53, antibodies specifically recognizing phosphorylated p53 at residues Ser392 and Ser46 were used. SAM486A increased p53 phosphorylation at Ser392 and Ser46 after 24 hours (Fig. 3A). Extended treatments (48 and 72 hours) under identical conditions did not increase p53 phosphorylation at either phosphorylation site.
Figure 3.
Analysis of apoptosis-regulating proteins in SAM486A-treated NB cells. Whole cell lysates were obtained from SK-N-SH cells exposed to 10 μM SAM486A for 24, 48, and 72 hours or exposed to increasing concentrations of SAM486A (0, 0.1, 1, 2, 5, and 10 μM) for 72 hours, and analyzed by Western blot. Resolved proteins were transferred to PVDF membranes and sequentially probed for (A) total p53, phospho-p53 (Ser392), and phospho-p53 (Ser46), (B) total Mdm2 and phospho-Mdm2 (Ser 166), (C-D) total Akt/PKB and phospho-Akt/PKB (Ser473). SAM486A treatment increased total p53, phospho-p53 (Ser392), phopho-p53 (Ser46), total Mdm2, phospho-Mdm2 (Ser166) and decreased total Akt/PKB and phospho-Akt/PKB (Ser473). Total Akt/PKB and phospho-Akt/PKB (Ser473) also decreased in a dose-dependent manner. GAPDH or β-actin served as loading markers. Data are representative of three independent experiments (n=3).
Since p53 regulation and its proteasomal degradation are dependent on its association with Mdm2, we performed an identical experiment as described in Fig. 3A and measured the total amount of Mdm2 protein. As shown in Fig. 3B, control SK-N-SH cells expressed basal levels of Mdm2, while SAM486A-treated cells accumulated large amounts of Mdm2. Concomitant with the increased protein levels, a large increase in Mdm2 phosphorylation at Ser166 was observed (Fig. 3B). In addition to the Mdm2 protein at 90 kDa, we noticed a previously reported p60 fragment of Mdm2 at 48 hours which increased at 72 hours.
The anti-apoptotic protein Akt/PKB is a cell survival factor and a key regulator in apoptosis. We therefore tested whether SAM486A treatment of SK-N-SH cells modulates Akt/PKB levels and alters its phosphorylation status. We found that Akt/PKB protein levels as well as its phosphorylation at Ser473 were strongly reduced after 48 hours of treatment, and less so at the beginning of the experiment at 24 hours (Fig. 3C). This observation was confirmed by a decrease of Akt/PKB levels and its phosphorylation in response to increasing concentrations of SAM486A (Fig. 3D).
SAM486A Induces Rapid In Vivo Phosphorylation of p53
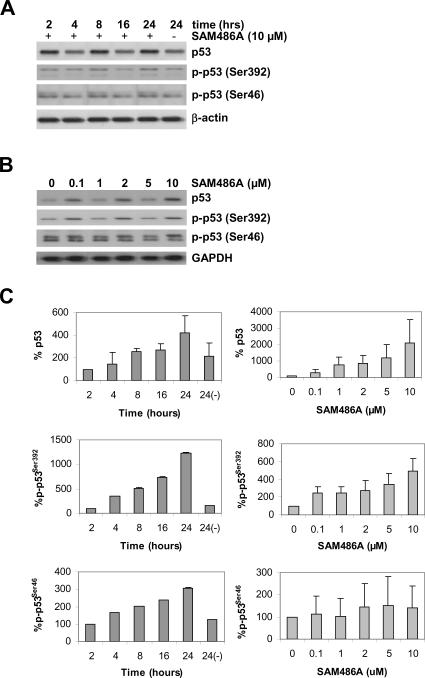
Since we observed a strong accumulation of p53 at 24 hours (Fig. 3A), we decided to determine the earliest time point at which p53 increases in SAM486A-treated NB cells. To accomplish this, we performed an identical time-course experiment but within a shorter time frame (0-24 hours). The accumulation of p53 occurred as early as 8 hours after SK-N-SH cell treatment with SAM486A (Fig. 4A). In addition, we also showed that the effect of SAM486A is dose-dependent, thus confirming the linear relation between SAM486A and p53 (Fig. 4B). SAM486A increased the phosphorylation of p53 at Ser392 and Ser46 in a time- and dose-dependent manner (Fig. 4A-B). While some basal phosphorylation of p53 at Ser392 and Ser46 was observed in all cells, SAM486A treatment clearly resulted in stronger phosphorylation compared to untreated cells (Fig. 4A-B). In addition, a second, larger band immediately above the p-p53 (Ser46) band was observed, suggesting that SAM486A treatment leads to the phosphorylation of multiple phosphosites (hyperphosphorylation) of p53. Quantifications of total p53, p-p53 (Ser392) and p-p53 (Ser46) are shown in Fig. 4C.
Figure 4.
Rapid response of p53 in SAM486A-treated NB cells. Whole cell lysates were obtained from SK-N-SH cells exposed to (A) 10 μM SAM486A for 2, 4, 8, 16, and 24 hours or (B) exposed to increasing concentrations of SAM486A (0, 0.1, 1, 2, 5, and 10 μM) for 72 hours, and analyzed by Western blot. Resolved proteins were transferred to PVDF membranes and sequentially probed for total p53, phospho-p53 (Ser392), and phospho-p53 (Ser46). SAM486A treatment increased total p53, phospho-p53 (Ser392) and phospho-p53 (Ser46) in a time-and dose-dependent manner. These effects were observed as early as 8 hours after treatment with 10 μM SAM486A, and at concentrations as low as 1-2 μM SAM486A. GAPDH or β-actin served as loading markers. C, the bands were quantified using a Bio-Rad Multi Imager and Quantity One Quantitation Software (Bio-Rad), and normalized to the untreated control group. Western blot data are representative of three independent experiments (n=3).
To examine whether SAM486A treatment causes the translocation of p53, we performed immunofluorescence-based microscopy. SAM486A-treated and untreated cells were stained with p53 antibody and visualized. As shown in Supplemental Fig. 2, p53 primarily localized in the nucleus of SK-N-SH cells, and SAM486A did not induce a subcellular translocation, for example, to the cytoplasm of the cells. However, the staining intensity of SAM486A-treated cells was slightly enhanced in all samples examined, which is in agreement with the increase in p53 protein levels observed in Fig. 3 and Fig. 4.
Polyamine Levels in SAM486A-Treated Cells
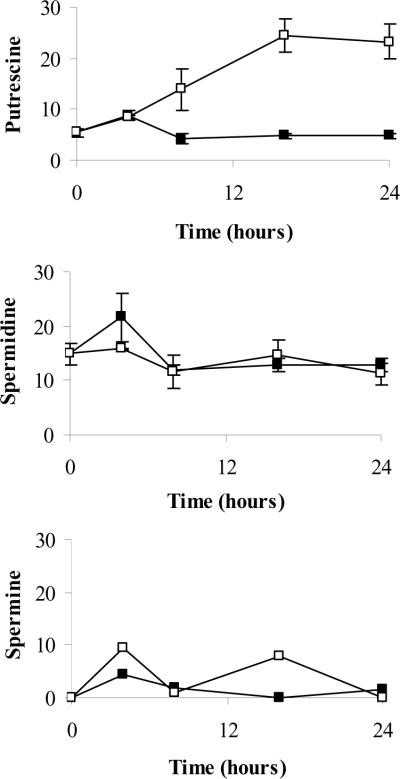
Many of the effects observed with SAM486A in the present study occurred within the first 24 hours post treatment. Therefore, we examined the changes in intracellular PA content in SAM486A-treated and untreated cells over a 24 hour time course. SAM486A exposure led to a large and progressive increase in Put content whereas Put levels in control cells changed very little (Fig. 5). The increase in Put was observed as early as 8 hours. Spd and Spm levels were not significantly altered in response to SAM486A treatments at these early time points. Spm levels were very low and not detectable in some samples. Of note, this early increase in Put content in SAM486A-treated cells directly correlates with the rapid increase of p53 levels at 8 hours.
Figure 5.
Intracellular PA pool determination in SAM486A-treated NB cells. SK-N-SH cells were exposed to 10 μM SAM486A or left untreated. Intracellular Put, Spd, and Spm pools were measured at 0, 4, 8, 16, and 24 hours. At 8 hours, SAM486A-treated cells had a significantly larger Put content compared to untreated cells. Put levels continued to rise in later time points. SAM486A did not have any significant effects on Spd and Spm throughout the 24 hour time course. Filled squares (■) and open squares (□) represent data collected from untreated and SAM486A-treated NB cells, respectively. Data are expressed as nmol/mg protein and represent the mean (± SE) of three independent experiments (n=3).
Discussion
Our previous work showed that DFMO and DFMO/SAM486A combinations effectively inhibit cell cycle progression (without apoptosis) in MYCN-amplified, p53-mutant NB cells (20). In those NB cell lines, SAM486A alone decreased cell viability, but otherwise had little or even opposing effects. In a more recent study, we further showed that DFMO activates two opposing pathways in MYCN-amplified and p53-mutant NB cells, one inducing cell survival via the PI3K-Akt/PKB signaling pathway, another inducing cell cycle arrest through a mechanism that involves p27Kip1 phosphorylation and accumulation (20, 22). In contrast, the present study shows that SAM486A rapidly induces apoptosis in p53-wild type NB cells with or without MYCN amplification. SAM486A treatment in NB cells resulted in cell blebbing, cell shrinking, and eventually detachment of cells. These morphological changes are typical signs of apoptosis, which were confirmed by annexin V staining (an early apoptosis marker) and PARP cleavage (a late apoptosis marker), via flow cytometry and Western blot analyses, respectively. This suggests that SAM486A is an apoptosis-inducing agent.
We also observed a decrease of the anti-apoptotic protein Akt/PKB 48 hours post treatment. However, due to the fact that annexin V staining and PARP cleavage occurred already within 24 hours of SAM486A treatment, SAM486A may exert its apoptotic effects independent of Akt/PKB in these NB cells. Interestingly, we also found that total PARP protein levels decreased after 48 and 72 hours of SAM486A treatment, suggesting that SAM486A disrupts the synthesis of PARP or increases its degradation (Fig. 1B).
Next, we examined the mechanism by which SAM486A induces apoptosis in these NB cells. SAM486A induced rapid accumulation of native p53 protein and phosphorylation of p53 at Ser392 and Ser46 in a time- and dose-dependent manner. The phosphorylation of p53 at Ser392 occurred within 8 hours of SAM486A treatment, and was represented by a single band in the Western blot analysis. In contrast, the phosphorylation of p53 at Ser46 was represented by two bands, one immediately above the other. These results suggest that the mechanism by which SAM486A induces p53-mediated cell death may involve post-translational phosphorylation of p53 at Ser392 which stabilizes p53, and subsequent phosphorylation at Ser46 which is a signal for the induction of apoptosis in response to DNA damage, a process that might be mediated by DNA-damage induced ATM. Previous studies on stress-induced, p53-mediated cell death in MCF-7 breast cancer cells resulted in similar findings (23). Furthermore, a recent study reported that p53 may perform different functions under conditions that result in DNA damage (camptothecin treatment) compared to conditions that induce PA depletion (DFMO treatment) (24). Both treatments led to the phosphorylation and stabilization of p53. However, while camptothecin-induced DNA damage led to p53-dependent apoptosis, DFMO-induced PA depletion protected the cells from undergoing apoptosis by modulating cell cycle checkpoint proteins and decreasing transcription of genes that are involved in apoptosis.
To further elucidate the mechanism of SAM486A-induced p53 accumulation, we examined the effects of SAM486A on Mdm2, an E3 ubiquitin ligase that mediates p53 ubiquitination and subsequent proteasomal degradation. The increase in native Mdm2 protein levels and phosphorylation of Mdm2 at Ser166 may occur as a consequence of p53 accumulation, as Mdm2 is a transcriptional target of p53 (25). However, this increase does not appear to play an antagonistic role in the SAM486A-induced effects observed in the present study. Furthermore, the increase in Mdm2 p60 fragments has been previously shown to occur during p53-mediated apoptosis, as Mdm2 cleavage is mediated by caspases or caspase-like proteases (26).
We previously showed that the inhibition of AdoMetDC by SAM486A in MYCN-amplified, p53-mutant NB cells led to strong accumulation of Put levels and depleted Spd and Spm levels after 48-72 hours of treatment (20). However, based on the findings of the present study, SAM486A induced p53 levels very rapidly and as early as 8 hours post treatment (Fig. 4A). Therefore, we were curious to determine whether the intracellular PA pools alter accordingly, thus suggesting that the change in p53 levels is linked to PA levels and modulated by SAM486A. Remarkably, we found that SAM486A-treated SK-N-SH cells indeed accumulated Put as early as 8 hours after treatment (Fig. 5). To our knowledge, this is the first time that a significant increase in Put pools has been observed within such a short time period and suggests that the early increase in p53 could be due to the SAM486A-induced changes of intracellular Put levels. The fact that Spd and Spm levels were not significantly altered at these early time points was expected, as the depletion of higher PAs in response to PA inhibitors is usually observed after 48 hours in NB cells (20). In contrast, accumulation of Put may occur more rapidly as SAM486A-mediated inhibition of AdoMetDC reduces dcAdoMet, the immediate precursor necessary for the conversion of Put to Spd and Spm. In addition, the rapid accumulation of Put may be accounted for by the fact that cells in the early log-phase of growth exhibit highest ODC enzyme activities and, consequently, produce particularly high levels of Put. Finally, SAM486A has been shown to inhibit diamine oxidase and increase ODC activity, possibly further contributing to an increase in Put levels (8).
We also attempted to simulate these conditions by first depleting PAs with DFMO and then supplementing with exogenous Put. However, the intracellular Put content did not increase to the same extent as with SAM486A treatment, and therefore, it was not surprising that we did not observe the same effect on p53 accumulation and phosphorylation (data not shown). An explanation for the observed difference may be that due to modulations in the PA uptake and/or export system, exogenous Put is not able to effectively enter DFMO-treated NB cells and accumulate to high intracellular levels such as detected in SAM486A-treated cells. Furthermore, the inhibition of AdoMetDC and diamine oxidase as well as the activation of ODC enzyme activity by SAM486A (8) may elevate cellular Put levels to a degree much higher than detected in DFMO- treated cells supplemented with exogenous Put.
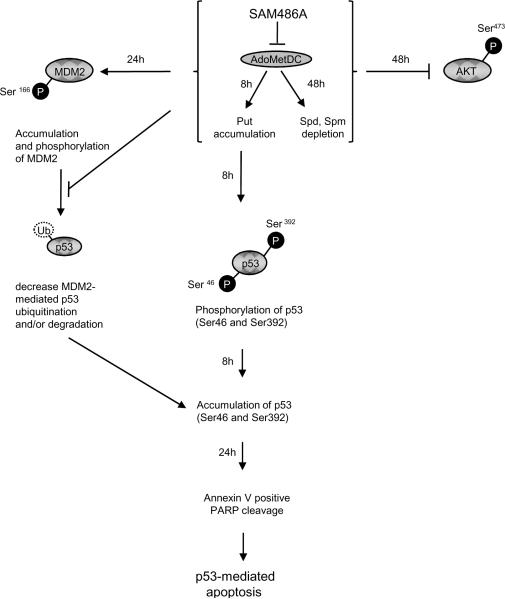
As depicted in the schematic diagram of Fig. 6, our study proposes that Put levels increase within 8 hours of SAM486A treatment in NB cells with wild type p53. In addition, we observed an increase in activation of p53 within 8 hours of SAM486A treatment, followed by the onset of apoptosis (annexin V staining and PARP cleavage) within 24 hours of treatment. In summary, our results suggest that the rapid Put accumulation induced by SAM486A may play a role in p53- mediated apoptosis. However, it should be noted that SAM486A may be able to induce the effects observed in the current study through non-PA mediated mechanisms (11).
Figure 6.
Schematic diagram showing proposed interplay between PAs and apoptosis-regulating proteins in p53-wild type NB cells. AdoMetDC is a key enzyme necessary for the production of dcAdoMet, a precursor in PA biosynthesis. Inhibition of AdoMetDC by SAM486A leads to the accumulation of Put as early as 8 hours after treatment. Remarkably, within the same time period, a rapid increase of native p53 protein levels was observed, suggesting that Put may be linked to p53-mediated apoptosis in these NB cells. The observed apoptosis-inducing properties of the clinically-tested SAM486A may be most effective in the treatment of NB patients with p53-wild type status, independent of their MYCN amplification status.
While PA inhibitors such as SAM486A and DFMO have been used in a number of human cancer trials (2, 3, 15), their efficacy as a treatment for NBs (individually or combined) has never been explored. Based on available clinical data from human trials, both inhibitors are relatively well tolerated, even at higher doses, with the occasional occurrence of temporary ototoxicity (with DFMO), nausea, and mild neutropenia. DFMO is water soluble and may be administered in drinking water which is a particular advantage for pediatric patients. Based on our findings in this study as well as previous studies (20, 22), PA inhibitors such as SAM486A are effective in killing NB cells, namely those with intact p53 signaling, thus providing further evidence that these targeted PA inhibitors are possible alternative agents for the treatment of NBs. Both SAM486A and DFMO should be tested in combination with other chemotherapeutics (possibly at lower doses) to achieve an improved therapeutic outcome with hopefully reduced side effects.
Supplementary Material
Acknowledgements
We are grateful to Shannon Wilson and Craig Byus (University of California, Riverside) for their help with preliminary PA analyses, and Suzanne Sass-Kuhn (Pennsylvania State University) for technical support in PA analysis experiments. Jason Shohet (Texas Children's Hospital, Houston, TX) is gratefully acknowledged for providing inducible MYCN-2 NB cells. Alan Lau, Joe Ramos, Bonnie Warn-Cramer, Darren Park, and Patricia Lorenzo (Cancer Research Center of Hawaii) are thanked for their support, advice, and stimulating discussions during the course of this work. Kelsie Takasaki, Jennifer Seki, Noah Yuen, and Risha Mishima are acknowledged for technical support at the beginning or ending of this project. We give special thanks to Novartis (Basel, Switzerland) for providing the AdoMetDC inhibitor SAM486A.
This work was supported by NIH grants from the National Cancer Institute R01 CA-111419 and R01 CA-111419-S1 (André S. Bachmann) and R01 CA-018138 (Anthony E. Pegg), the Hawaii Community Foundation, grants 20022113 and 20041684 (André S. Bachmann), and a grant from the Cancer Research Center of Hawaii Developmental Funds (André S. Bachmann).
References
- 1.Davidson NE, Hahm HA, McCloskey DE, Woster PM, Casero RA., Jr Clinical aspects of cell death in breast cancer: the polyamine pathway as a new target for treatment. Endocr Relat Cancer. 1999;6:69–73. doi: 10.1677/erc.0.0060069. [DOI] [PubMed] [Google Scholar]
- 2.Levin VA, Hess KR, Choucair A, et al. Phase III randomized study of postradiotherapy chemotherapy with combination alpha-difluoromethylornithine-PCV versus PCV for anaplastic gliomas. Clin Cancer Res. 2003;9:981–90. [PubMed] [Google Scholar]
- 3.Levin VA, Uhm JH, Jaeckle KA, et al. Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res. 2000;6:3878–84. [PubMed] [Google Scholar]
- 4.McCann PP, Pegg AE. Ornithine decarboxylase as an enzyme target for therapy. Pharmacol Ther. 1992;54:195–215. doi: 10.1016/0163-7258(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 5.Meyskens FL, Jr, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–51. [PubMed] [Google Scholar]
- 6.Takahashi Y, Mai M, Nishioka K. Alpha-difluoromethylornithine induces apoptosis as well as anti-angiogenesis in the inhibition of tumor growth and metastasis in a human gastric cancer model. Int J Cancer. 2000;85:243–7. [PubMed] [Google Scholar]
- 7.Regenass U, Caravatti G, Mett H, et al. New S-adenosylmethionine decarboxylase inhibitors with potent antitumor activity. Cancer Res. 1992;52:4712–8. [PubMed] [Google Scholar]
- 8.Regenass U, Mett H, Stanek J, Mueller M, Kramer D, Porter CW. CGP 48664, a new S-adenosylmethionine decarboxylase inhibitor with broad spectrum antiproliferative and antitumor activity. Cancer Res. 1994;54:3210–7. [PubMed] [Google Scholar]
- 9.Stanek J, Caravatti G, Frei J, et al. 4-amidinoindan-1-one 2'-amidinohydrazone: a new potent and selective inhibitor of S-adenosylmethionine decarboxylase. J Med Chem. 1993;36:2168–71. doi: 10.1021/jm00067a014. [DOI] [PubMed] [Google Scholar]
- 10.Svensson F, Mett H, Persson L. CGP 48664, a potent and specific S-adenosylmethionine decarboxylase inhibitor: effects on regulation and stability of the enzyme. Biochem J. 1997;322:297–302. doi: 10.1042/bj3220297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorhout B, Odink MF, de Hoog E, Kingma AW, van der Veer E, Muskiet FA. 4-Amidinoindan-1-one 2'-amidinohydrazone (CGP 48664A) exerts in vitro growth inhibitory effects that are not only related to S-adenosylmethionine decarboxylase (SAMdc) inhibition. Biochim Biophys Acta. 1997;1335:144–52. doi: 10.1016/s0304-4165(96)00134-1. [DOI] [PubMed] [Google Scholar]
- 12.Dorhout B, te Velde RJ, Ferwerda H, Kingma AW, de Hoog E, Muskiet FA. In vivo effects of 4-amidinoindan-1-one 2'-amidinohydrazone (CGP 48664A) and alpha-difluoromethylornithine (DFMO) on L1210 growth, cell-cycle phase distribution and polyamine contents. Int J Cancer. 1995;62:738–42. doi: 10.1002/ijc.2910620615. [DOI] [PubMed] [Google Scholar]
- 13.Seiler N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 1. Selective enzyme inhibitors. Curr Drug Targets. 2003;4:537–64. doi: 10.2174/1389450033490885. [DOI] [PubMed] [Google Scholar]
- 14.Eskens FA, Greim GA, van Zuylen C, et al. Phase I and pharmacological study of weekly administration of the polyamine synthesis inhibitor SAM 486A (CGP 48 664) in patients with solid tumors. European Organization for Research and Treatment of Cancer Early Clinical Studies Group. Clin Cancer Res. 2000;6:1736–43. [PubMed] [Google Scholar]
- 15.Pless M, Belhadj K, Menssen HD, et al. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin's lymphoma: results from a phase II multicenter study. Clin Cancer Res. 2004;10:1299–305. doi: 10.1158/1078-0432.ccr-0977-03. [DOI] [PubMed] [Google Scholar]
- 16.Meyskens FL, Jr, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 18.Goldman SC, Chen CY, Lansing TJ, Gilmer TM, Kastan MB. The p53 signal transduction pathway is intact in human neuroblastoma despite cytoplasmic localization. Am J Pathol. 1996;148:1381–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Hosoi G, Hara J, Okamura T, et al. Low frequency of the p53 gene mutations in neuroblastoma. Cancer. 1994;73:3087–93. doi: 10.1002/1097-0142(19940615)73:12<3087::aid-cncr2820731230>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Wallick CJ, Gamper I, Thorne M, et al. Key role for p27Kip1, retinoblastoma protein Rb, and MYCN in polyamine inhibitor-induced G1 cell cycle arrest in MYCN-amplified human neuroblastoma cells. Oncogene. 2005;24:5606–18. doi: 10.1038/sj.onc.1208808. [DOI] [PubMed] [Google Scholar]
- 21.Slack A, Chen Z, Tonelli R, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci U S A. 2005;102:731–6. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koomoa DL, Yco LP, Borsics T, Wallick CJ, Bachmann AS. Ornithine decarboxylase inhibition by alpha-difluoromethylornithine activates opposing signaling pathways via phosphorylation of both Akt/protein kinase B and p27Kip1 in neuroblastoma. Cancer Res. 2008;68:9825–31. doi: 10.1158/0008-5472.CAN-08-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tampio M, Loikkanen J, Myllynen P, Mertanen A, Vahakangas KH. Benzo(a)pyrene increases phosphorylation of p53 at serine 392 in relation to p53 induction and cell death in MCF-7 cells. Toxicol Lett. 2008;178:152–9. doi: 10.1016/j.toxlet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya S, Ray RM, Johnson LR. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell Signal. 2009;21:509–22. doi: 10.1016/j.cellsig.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Haines DS. The mdm2 proto-oncogene. Leuk Lymphoma. 1997;26:227–38. doi: 10.3109/10428199709051772. [DOI] [PubMed] [Google Scholar]
- 26.Erhardt P, Tomaselli KJ, Cooper GM. Identification of the MDM2 oncoprotein as a substrate for CPP32-like apoptotic proteases. J Biol Chem. 1997;272:15049–52. doi: 10.1074/jbc.272.24.15049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.