Abstract
Introduction
Several studies have confirmed the increasing rate of type 1 diabetes mellitus (T1DM) in children and the link with increasing BMI at diagnosis termed the ‘accelerator hypothesis’. Our objective was to assess whether changing incidence of type 1 diabetes in a group of children and adolescent from the Midwest United States was associated with changes in BMI.
Methods
Data from 1618 (52.1% M/47.9% F) newly-diagnosed children and adolescents (<19 years) with T1DM, admitted to Children's Hospital of Wisconsin (CHW) between January 1995 and December 2004, was analyzed in relationship to body mass index (BMI) standard deviation score (SDS).
Results
An overall, 10-year cumulative incidence of 27.92 per 100,000 (19.12 to 41.72/100,000) was observed, with an average yearly cumulative incidence of 2.39%. The increase was largest in the younger age groups, 0–4, 5–9, and 10–14 having an average yearly increase of 2.4, 2.3, and 3.0%, respectively, corresponding to a relative 10-year increase of 25.3, 33.8, and 38.0%, respectively. Age at diagnosis was inversely correlated with BMI SDS (p<0.001) and remained significant for both males and females.
Conclusions
Annual incidence of T1DM increased two-fold at CHW over the 10-year study period. The majority of the increase was observed in the youngest age groups, which also appeared to be the heaviest. This research adds to the growing literature supporting the hypothesis that excess weight gain during childhood may be a risk factor for early manifestation of T1DM.
Introduction
Type 1A diabetes mellitus (T1DM), an autoimmune disorder, accounts for 10% of diabetes diagnoses, affecting approximately 1.4 million people in the United States (US) and 10–20 million worldwide [1]–[3]. In the United States, 30,000 new cases occur annually and 40% of patients diagnosed are under the age of 20 [2]–[4]. Recently, studies suggest that the incidence of T1DM may be on the rise and increasing incidence in younger children is of the greatest concern.
Incidence rates of pediatric T1DM vary widely throughout the world. Onkamo et al., (1999) reviewed pooled data from 37 studies (from 1960 to 1996), and observed an overall 2.8% to 3.0% per year global increase in incidence of T1DM [5]. However, only one study, by Kostraba et al., (1992) suggested a slightly negative, but not significant, trend in T1DM incidence in children and adolescents [6]. The World Health Organization's DIAMOND study reported incidence rates from over 100 Centers ranging from 0.1/100,000 per year in China and Venezuela to 37.8/100,000 per year in Sardinia and 42.9/100,000 per year in Finland [2]. A large number of studies have been published supporting the rising incidence of T1DM, especially in the younger age groups [7]–[19]. One of the most notable and recent, in the United States, includes a population-based study of incidence rates of T1DM from 10 study locations by The SEARCH for Diabetes in Youth Study. The Search Group found an overall incidence of T1DM in children 0–19 of 24.3 per 100,000 person years with the highest rates observed among the 5–9 and 10–14 age groups with rates of 22.9 and 33.9 per 100,000 respectively [14]. There is still some speculation as to whether there is also an increase in incidence in the older adolescent groups.
While the autoimmune nature of T1DM continues to be under investigation [20], [21], the underlying mechanisms responsible for the rise of T1DM, especially in the younger age groups, remain unknown. However, the “accelerator hypothesis” proposed by Wilkin, is one of the more compelling theories [22]–[24]. This investigator suggested that increasing body weight in younger children acts as an accelerator mechanism for an increased risk of developing T1DM. In fact, an inverse relationship was found between age at diagnosis and body mass index (BMI) at diagnosis and at 12 months after diagnosis, as well as weight at diagnosis and weight change since birth. Essentially, the age at diagnosis becomes younger as children become heavier; suggesting that being overweight accelerates insulin resistance, leading to the development of T1DM in genetically-predisposed individuals. Thereafter a number of papers have been published supporting Wilkin's accelerator hypothesis' [24]–[30]. A study by Libman et al., (2003) in the United States showed an overall significant increase in the prevalence of being overweight in children with T1DM from 12.6% (1979–1989) to 36.8% (1990–1998). However, the older adolescent population (>11 years) was more overweight than the younger children [31]. To date, the role of increasing body weight in very young children as a risk factor for early development of T1DM remains inconclusive.
The increase in incidence of T1DM in younger age groups in relation to increasing BMI has not been confirmed in the literature. Therefore, we set out to determine the changing burden of T1DM in specific age cohorts in relationship to BMI and body weight at diagnosis in Southeastern Wisconsin.
Methods
Ethics Statement
This study was approved by the institutional review board (IRB) of Children's Hospital Wisconsin for the retrospective review of patients' clinic charts and, therefore, no informed consent was required. Details that might disclose the identity of the subjects were omitted from data collection.
Subjects
Data from 1618 children and adolescents with newly diagnosed T1DM, who were evaluated (inpatients and outpatients) at Children's Hospital of Wisconsin (CHW) Diabetes Center (affiliated with Medical College of Wisconsin) between January 1995 and December 2004, were included in the study. Children's Hospital of Wisconsin is the primary source for pediatric diabetes care in the region of Southeastern Wisconsin. No more than 2.8% of all pediatric admissions for any disease are seen at the other 10 area hospitals. Referral rates for new onset patients with diabetes from primary care physicians remained the same throughout the 10-year study period [32]. Therefore, we ascertain that we identified the majority of pediatric patients with new-onset T1DM.
The diagnosis of T1DM was ascertained based on physician diagnosis extracted from chart review. Patients with a physician-diagnosis of Type 2 Diabetes Mellitus or other endocrinology disorder were excluded from the study. We cross-referenced the hospital medical records with clinic medical records to make sure that we had captured all the diabetes patients. The date of admission (date of diagnosis), date of birth, gender, race, diabetic ketoacidosis status at diagnosis, weight, height, BMI, and medical identification number for each patient were extracted from the patients medical record. Growth parameters were assessed at initial evaluation (admission to hospital), including height, weight, and body mass index (BMI, kg/m2). BMI was calculated by using weight and height, which were normalized for age and sex by calculating standard deviation scores (SDS) (z-scores) using 2000 Centers for Disease Control and Prevention (CDC) growth charts as a reference standard. Body mass index (BMI) and SDS calculations were determined using the Epi Info nutrition calculator [33].
Subjects were divided into four age cohorts according to age at diagnosis: 0–4, 5–9, 10–14, and 15–19 years and further divided into BMI percentile categories: underweight (≤5th), normal (6th–85th), overweight (86th–95th), obese (≥96th) [34]. The denominator for the analysis was the number of children 0–19 years of age who were located within the 8-county (population: 586,080) study region of SE Wisconsin [35]. This region was chosen by mapping the T1DM patients by zip code. The overall age- and sex-adjusted incidence rates were calculated using the yearly US census data [36].
Statistical Analysis
Initial descriptive analysis was conducted to summarize data. Data is expressed as mean±SE when appropriate. Rates were calculated using yearly census data. BMI and weight were converted into SDS. SDS is a type of z-score that normalizes age and sex of the research population based on 2000 CDC growth charts. Linear and logistic multivariate regression was used to determine which variables were associated with BMI for age SDS. Variables introduced into the regression analysis included sex, age at diagnosis, year of diagnosis, and presence of ketoacidosis. A p-value of less than or equal to 0.05 was considered significant. Statistical analysis was conducted by using NCSS [37].
Results
A total of 1618 (52.1% male; 47.9% female) new cases of T1DM were identified among children aged 0–19 during the study period. The ethnic distribution was 80.1% Caucasians (n = 1296), 10.8% African Americans (n = 175), 4.2% Hispanic (n = 68), and 4.9% other ethnicities (Asians/Inuit Indians) (n = 79). The overall cumulative incidence from 1995 to 2004 was 27.92/100,000 (95% CI: 262.8–289.8) (from 19.12 to 41.72/100,000; p = 0.006) for SE Wisconsin. Males have a slightly higher overall 10-year incidence than females. A significant increase in the second half of the study period, between 2000 and 2004, from 23.54 per 100,000 a year to 41.72 per 100,000 per year was found, which corresponds with an increase of 20% for this five-year period (p<0.001) as seen in table 1.
Table 1. Yearly incidence (0–19 yrs) of Type 1 DM from 1995 to 2004 in Southeastern Wisconsin.
| Year | ALL Cases | ALL Incidence | Males Cases | Males Incidence | Female Cases | Female Incidence |
| 1995 | 110 | 18.8 | 57 | 19.02 | 53 | 18.51 |
| 1996 | 126 | 21.5 | 65 | 21.69 | 61 | 21.3 |
| 1997 | 117 | 20.0 | 65 | 21.69 | 52 | 18.16 |
| 1998 | 120 | 20.5 | 55 | 18.35 | 65 | 22.7 |
| 1999 | 135 | 23.0 | 73 | 24.35 | 62 | 21.65 |
| 2000 | 138 | 23.5 | 78 | 26.02 | 60 | 20.95 |
| 2001 | 181 | 30.9 | 86 | 28.69 | 95 | 33.18 |
| 2002 | 214 | 36.5 | 117 | 39.03 | 97 | 33.88 |
| 2003 | 241 | 41.1 | 120 | 40.04 | 121 | 42.26 |
| 2004 | 236 | 40.3 | 127 | 42.37 | 109 | 38.07 |
| 1995–1999 | 608 | 103.7 | 315 | 105.1 | 293 | 102.3 |
| 2000–2004 | 1010 | 172.3 | 528 | 176.2 | 482 | 168.3 |
| 1995–2004 | 1618 | 276.0 | 843 | 281.3 | 775 | 270.6 |
Incidence calculated by using yearly U.S census data for 8 county metro area [35].
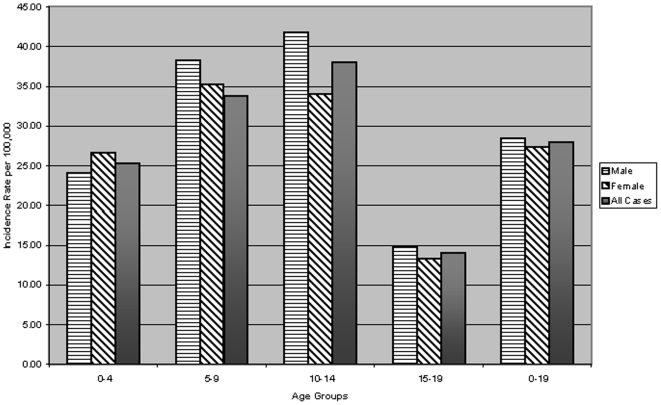
The increase in incidence was largest in the younger age groups. The children aged 0–4, 5–9, and 10–14 years, had an increase of 2.4, 2.3, and 3.0% per year, respectively and an overall 10-year relative increase of 25.3, 33.8, and 38.0%, respectively. In the older children (15–19 years), the increase was 1.8% per year with an overall 10-year relative increase of 14.0% as seen in figure 1.
Figure 1. Overall 10-year age (0–19 years) and gender specific incidence.
Incidence calculated by using yearly U.S census data for 8 county metro area [35].
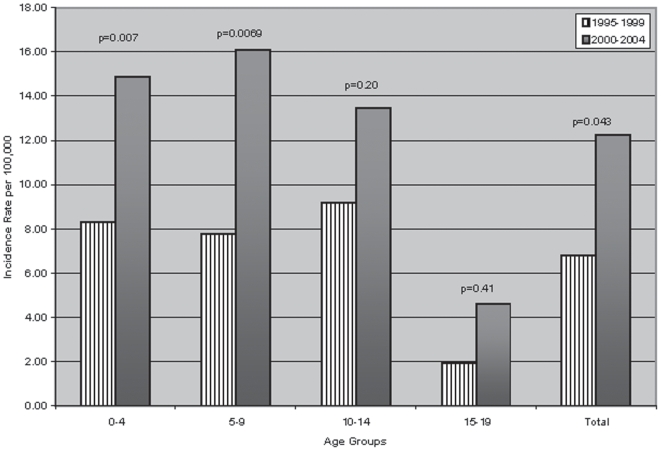
A similar increase was seen in both males and females. Females had a slightly higher mean incidence in the younger age groups (0–4 and 5–9 years), and males had a higher mean incidence in the older age groups (10–14 and 15–19 years). Additionally, we calculated the proportion of children and adolescents with BMI SDS above 86th (overweight) and 96th (obese) percentiles from 1999 to 2004. Twenty-eight point four were considered either overweight or obese in this cohort with children ≤9 years, accounting for 60.3 (BMI SDS) and 55.2% (weight SDS) of newly-diagnosed T1DM patients in this category. There also appeared to be a sharp increase in the number of children both overweight and obese during the second five-year period. In fact, the combined rate of overweight (>86th percentile for age) and obese (>96th percentile for age) was significantly increased in the period 2000–2004 for 0–4 year (p = 0.007) and 5–9 year (p = 0.007) age groups, but not for the remainder of the cohort as seen in figure 2.
Figure 2. Rates of BMI SDS above 85th (overweight & obese) percentiles for each 5-year period (1995–1999 and 2000–2004) by age.
Incidence calculated by using yearly U.S census data for 8 county metro area [35].
In the entire cohort, BMI SDS at diagnosis and weight for age were highly correlated (r = 0.61, p<0.001). There were inverse and statistically significant relationships for BMI SDS (p<0.001) at diagnosis in relation to age at diagnosis, based on F-statistic one-way ANOVA. BMI SDS remained significant when separating males and females (males' p = 0.03; females' p = 0.03). A stepwise regression analysis was performed, and BMI SDS was selected (BMI SDS, p = 0.037). In the multiple regression models when using age at diagnosis as the dependent variable, BMI SDS remained significant (BMI r = 0.19, p = 0.04). Age at diagnosis remained significant when using weight SDS as the dependent variable (p<0.001). Although when BMI SDS was used as the dependent variable, age at diagnosis was no longer significant (p = 0.15). penultimate.
Discussion
Our study results suggest that the overall incidence of T1DM in SE Wisconsin increased two-fold from 1995 to 2004. The largest overall increase in incidence occurred among the youngest age groups of 0–4, 5–9 and 10–14 years, which is consistent with other studies published included the SEARCH group [7]–[19]. Similar to previous studies, females had a slightly higher mean incidence in the younger age groups (0–4 and 5–9 years) than males, whereas males had higher mean incidence than females in the older age groups (10–14 and 15–19 years) [7]–[19]. Indeed, the incidence of T1DM in our cohort in 2004 appeared to be similar to that observed in Finland [9] and the SEARCH Group [14]. Furthermore, the youngest age groups seem to also be the heaviest, in relation to BMI SDS. This relationship seemed to become more prevalent throughout the 10-year study period. Our cohort had a slightly higher number of children and adolescents considered overweight (19.7, BMI SDS; 21.8, weight SDS) and obese (8.53, BMI SDS; 12.67, weight SDS) as compared to the State of Wisconsin's averages of 14 overweight and 10 percent obese [38].
Wilkin (2001) suggested that three accelerators might be responsible: an impending β-cell death, insulin resistance, and a genetic propensity to develop β-cell autoimmunity [22]. Recently, Knerr et al., (2005) also reported that higher BMI was associated with a younger age at diabetes onset in a large cohort of German and Austrian children with T1DM [28]. In our cohort, there was not only an inverse relationship between the age of diagnosis and BMI SDS, but also a two-fold increase in BMI SDS from our second five-year period (2000–2004) in all of the age cohorts as compared with the first five-year period (1995–1999). This increase corresponded with a 20% increase in the proportion of newly-diagnosed children with T1DM during the same period (2000–2004).
There were a number of limitations to this study. First, case ascertainment methods were limited. Cross-reference of all patients diagnosed with diabetes in the hospital medical records was compared with clinic “shadow” records to ascertain that we had captured all the diabetes patients and to verify the T1DM diagnosis. Children's Hospital of Wisconsin (CHW) provides in-patient care to 86% to 90% of pediatric population in the SE Wisconsin region [35]. Consequently, CHW Diabetes Center is believed to capture similar percentages of children and adolescents with T1DM, therefore we believe that our results can be generalizable to other pediatric populations. The referral rates to this hospital for “all treatments” and specifically for “diabetes” remained constant during the 10-year study period. In order to verify that the children in our cohort corresponded with the 8-county metro area we mapped the zip codes recorded in their medical records. Secondly, we were only able to collect one point of height and weight data at diagnosis, which doesn't take into account the possibility of weight loss due to symptoms related to the onset of the diabetes. Therefore, it would have been more meaningful if weight and height data from six months before or after diagnosis were available to evaluate the relationship between age at onset and body weight. Thirdly, we were not able to evaluate β-cell function and number of diabetes antibodies in relationship to age at diagnosis and body weight. Indeed, obesity-induced insulin resistance is believed to up-regulate the β-cells, which become susceptible to an autoimmune attack [39]. These limitations would suggest that our data may be an under-representation of the number of children newly diagnosed with T1DM.
Our study was the first 10-year retrospective cohort in the Midwest, looking at the burden of T1DM and the relationship with BMI. Our findings suggest that the overall incidence of T1DM is increasing in Southeastern Wisconsin, with the largest increase seen in children ≤14 years of age. A likely mechanism for this increase is an increase in weight gain at diagnosis, namely “accelerator hypothesis” [22].
Our research adds to the growing literature that emphasizes the importance of maintaining a healthy weight throughout childhood; which could delay the onset on T1DM into adolescence. Indeed, our data reaffirms that excess weight during early childhood could be an important mechanism in understanding the etiology of T1DM.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.The DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 3.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 4.LaPorte R, Matsushima M, Chang Y. Bethesda, MD: National Institute of Health; 1995. Diabetes in America. Chapter 3. Prevalence and Incidence of Insulin-Dependent Diabetes. [Google Scholar]
- 5.Onkamo P, Vaananen S, Karvonen M, Tuomileho J. Worldwide Increase in Incidence of Type 1 Diabetes- the Analysis of the Data on Published Incidence Trends. Diabetologia. 1999;42:1395–1403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 6.Kostraba JN, Gay EC, Cai Y, Cruickshanks KJ, Rewers MJ, et al. Incidence of insulin-dependent diabetes mellitus in Colorado. Epidemiology. 1992;3:232–238. doi: 10.1097/00001648-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kavonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, et al. Incidence of childhood type 1 diabetes worldwide: Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23:1516–1526. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 8.Ehehalt S, Blumenstock G, Willasch AM, Hub R, Ranke MB, et al. DIARY-study Group Baden-Württemberg. Continuous rise in incidence of childhood Type 1 diabetes in Germany. Diabet Med. 2008;25(6):755–7. doi: 10.1111/j.1464-5491.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 9.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;24;371(9626):1777–82. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 10.Weets I, Rooman R, Coeckelberghs M, De Block C, Van Gaal L, et al. Belgian Diabetes Registry. The age at diagnosis of type 1 diabetes continues to decrease in Belgian boys but not in girls: a 15-year survey. Diabetes Metab Res Rev. 2007;23(8):637–43. doi: 10.1002/dmrr.758. [DOI] [PubMed] [Google Scholar]
- 11.Green A, Patterson C EURODIAB TIGER study group. Trends in the Incidence of Childhood-Onset Diabetes in Europe 1989–1998. Diabetologia. 2001;44(Suppl 3):B3–8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 12.Schoenle EJ, Lang-Muritano M, Gschwend S, Laimbacher J, Mullis PE, et al. Epidemiology of Type 1 Diabetes Mellitus in Switzerland: Steep Rise in Incidence in Under 5 Year Old Children in the Past Decade. Diabetologia. 2001;44:286–289. doi: 10.1007/s001250051615. [DOI] [PubMed] [Google Scholar]
- 13.Charkaluk ML, Czernichow P, Levy-Marchal C. Incidence Data of Childhood-Onset Type 1 Diabetes in France During 1988–1997: The Case for a Shift Toward Younger Age at Onset. Pediatr Res. 2002;52:859–862. doi: 10.1203/00006450-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 14.The SEARCH Study Group. Incidence of Diabetes in Youth in the United State. JAMA. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 15.Cotellessa M, Barbieri P, Mazzella M, Bonassi S, Minicucci L, et al. High Incidence of Childhood Type 1 Diabetes in Liguria, Italy, from 1989 to 1998. Diabetes Care. 2003;26:1786–1789. doi: 10.2337/diacare.26.6.1786. [DOI] [PubMed] [Google Scholar]
- 16.Schober E, Rami B, Waldhoer T. Austrian Diabetes Incidence Study Group. Steep increase of incidence of childhood diabetes since 1999 in Austria. Time trend analysis 1979–2005. A nationwide study. Eur J Pediatr. 2008;167(3):293–7. doi: 10.1007/s00431-007-0480-5. [DOI] [PubMed] [Google Scholar]
- 17.Rangasami JJ, Greenwood DC, McSporran B, Smail PJ, Patterson CC, et al. Rising Incidence of Type 1 Diabetes in Scottish Children, 1984–1993. Archives of Disease in Childhood. 1997;77:210–213. doi: 10.1136/adc.77.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner SG, Bingley PJ, Sawtell PA, Weeks S, Gale EA. Rising Incidence of Insulin Dependent Diabetes in Children Aged Under 5 Years in the Oxford Region: Time Trend Analysis. BMJ. 1997;315:713–717. doi: 10.1136/bmj.315.7110.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karvonen M, Pitkaniemi J, Tuomilehto J. The Onset Age of Type 1 Diabetes in Finnish Children Has Become Younger. Diabetes Care. 1999;22:1066–1070. doi: 10.2337/diacare.22.7.1066. [DOI] [PubMed] [Google Scholar]
- 20.Sabbah E, Savola K, Ebeling T, Kumala P, Vahasalo P, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care. 2000;23:1326–1332. doi: 10.2337/diacare.23.9.1326. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 22.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001;44:914–922. doi: 10.1007/s001250100548. [DOI] [PubMed] [Google Scholar]
- 23.Wilkin TJ. The great weight gain experiment, accelerators, and their implications for autoantibodies in diabetes. Arch. Dis. Child. 2006;91(6):456–458. doi: 10.1136/adc.2006.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkin TJ. Testing the Accelerator Hypothesis: Body Size, {beta}-Cell Function, and Age at Onset of Type 1 (Autoimmune) Diabetes: Response to Dabelea et al. Diabetes Care. 2006;29(6):1462–1463. doi: 10.2337/dc06-0345. [DOI] [PubMed] [Google Scholar]
- 25.Kibirige M, Metcalf, Renuka R, Wilkin TJ. Testing the Accelerator Hypothesis (1): the relationship between body mass and age at onset of type 1 diabetes. Diabetes Care. 2003;26:2865–2870. doi: 10.2337/diacare.26.10.2865. [DOI] [PubMed] [Google Scholar]
- 26.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, et al. Diabetes Autoimmune Study in The Young (DAISY). Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004;89:3896–3902. doi: 10.1210/jc.2003-031887. [DOI] [PubMed] [Google Scholar]
- 27.Kordonouri O, Hartmann R. Higher body weight is associated with earlier onset of Type 1 diabetes in children: confirming the ‘Accelerator Hypothesis’. Diabet Med. 2005;22(12):1783–4. doi: 10.1111/j.1464-5491.2005.01792.x. [DOI] [PubMed] [Google Scholar]
- 28.Knerr I, Wolf J, Reinehr T, Stachow R, Grabert M, et al. DPV Scientific Initiative of Germany and Austria. The ‘accelerator hypothesis’: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia. 2005;48(12):2501–4. doi: 10.1007/s00125-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 29.Dabelea D, D'Agostino RB, Jr, Mayer-Davis EJ, Pettitt DJ, Imperatore G, et al. SEARCH for Diabetes in Youth Study Group. Testing the accelerator hypothesis: body size, beta-cell function, and age at onset of type 1 (autoimmune) diabetes. Diabetes Care. 2006;29(2):290–4. doi: 10.2337/diacare.29.02.06.dc05-1339. [DOI] [PubMed] [Google Scholar]
- 30.Gimenez M, Aguilera E, Castell C, De Lara N, Nicolau J, et al. Relationship Between BMI and Age at Diagnosis of Type 1 Diabetes in a Mediterranean Area in the Period of 1990–2004. Diabetes Care. 30(6):1593–1595. doi: 10.2337/dc06-2578. [DOI] [PubMed] [Google Scholar]
- 31.Libman IM, Pietropaolo M, Arsalinia SA, LaPorte RE, Becker DJ. Changing Prevalence of Overweight Children and Adolescents at Onset of Insulin-Treated Diabetes. Diabetes Care. 26:2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 32.Wisconsin Hospital Association. Hosptial Utilization Report www.wha.org.
- 33.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Center for Disease Control and Prevention. Epi Info Software. www.cdc.gov.
- 35.Wisconsin Department of Health and Family Services. Services Geographical Regions http://dhfs.wisconsin.gov/aboutdhfs/regions.htm.
- 36.United States Census Bureau. County Census Data http://factfinder.census.gov:
- 37.Dawson B, Trapp R. Basic & Clinical Biostatistics. NCSS CD-ROM. Springfield, IL: McGrew-Hill 2001 [Google Scholar]
- 38.Wisconsin Department of Health and Family Services. The Obesity Epidemic and Wisconsin Students http://www.cdc.gov/HealthyYouth/overweight/pdf/Wisconsin.pdf. [Google Scholar]
- 39.Bjork E, Kampe O, Karlsson FA, Pipeleers DG, Andersson A, et al. Glucose regulation of the autoantigen GAD65 in human pancreatic islets. J Clin Endocrinol Metab. 1992;75:1574–1576. doi: 10.1210/jcem.75.6.1464667. [DOI] [PubMed] [Google Scholar]