Abstract
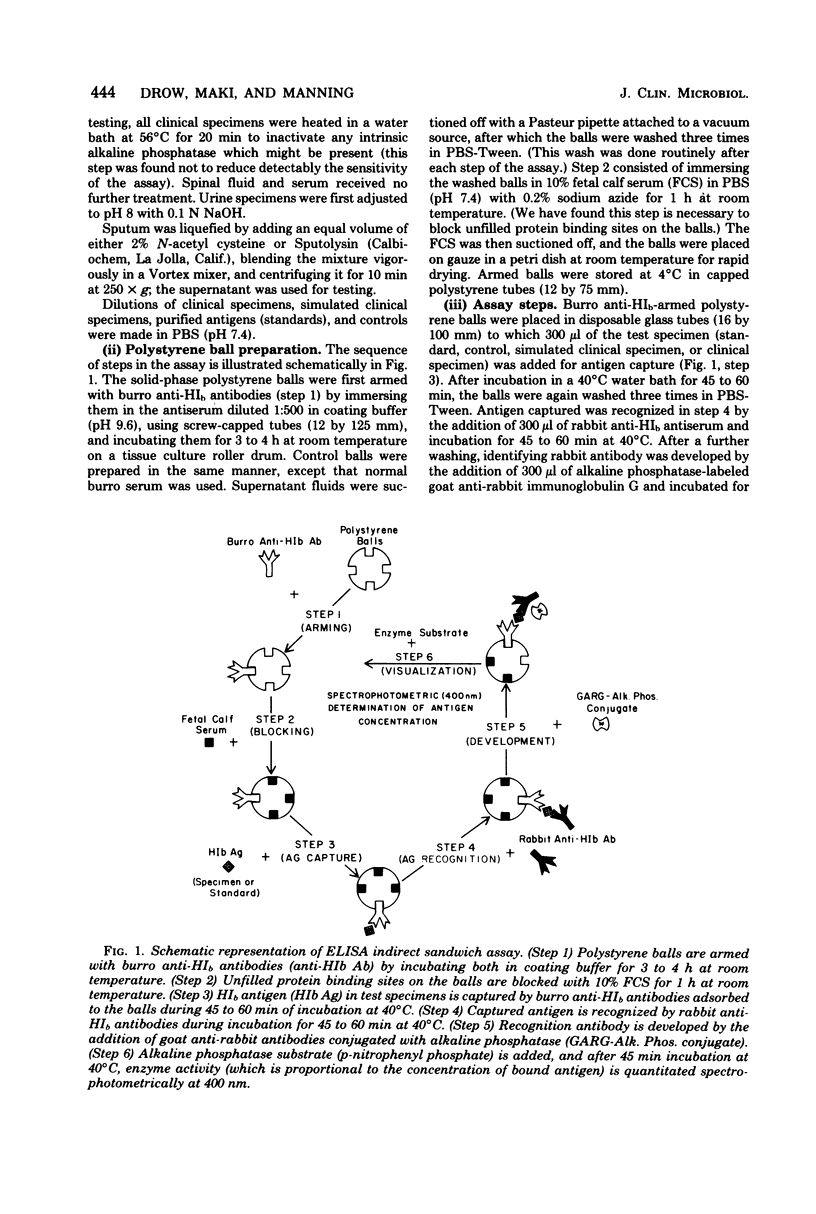
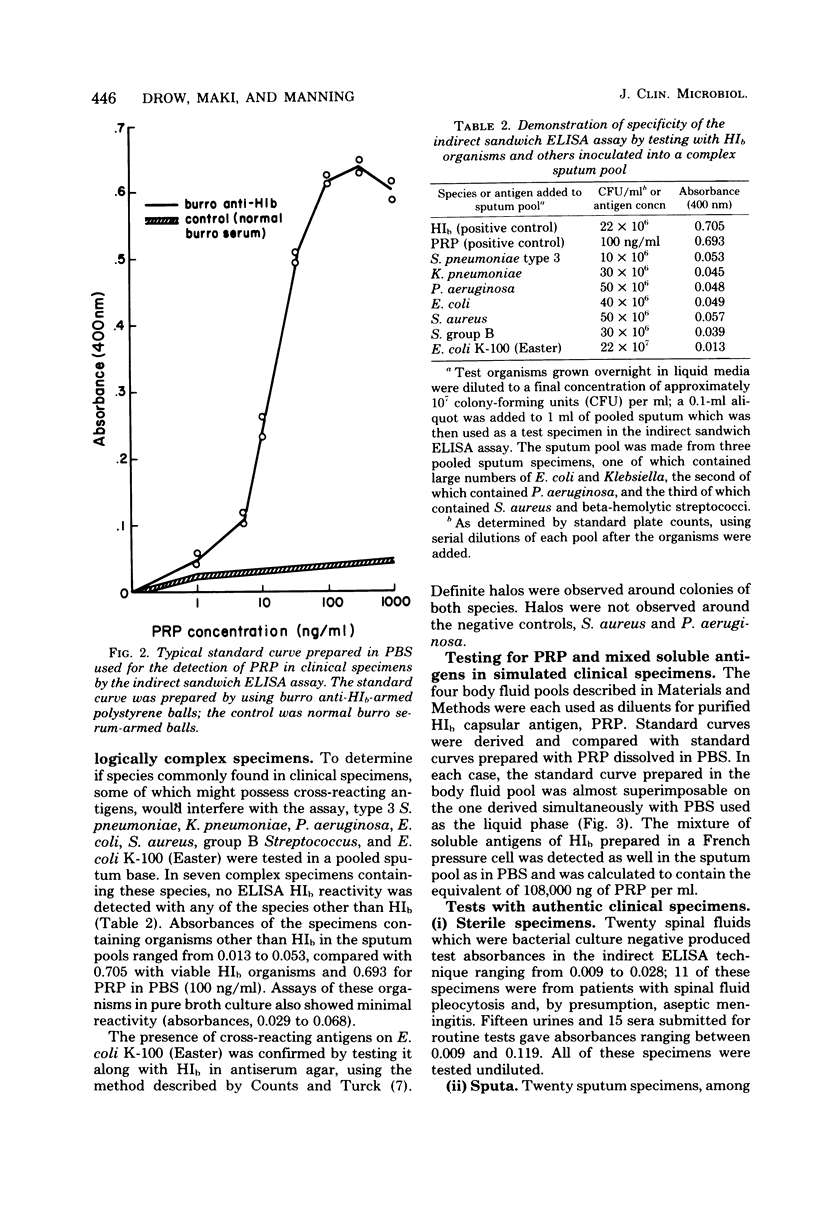
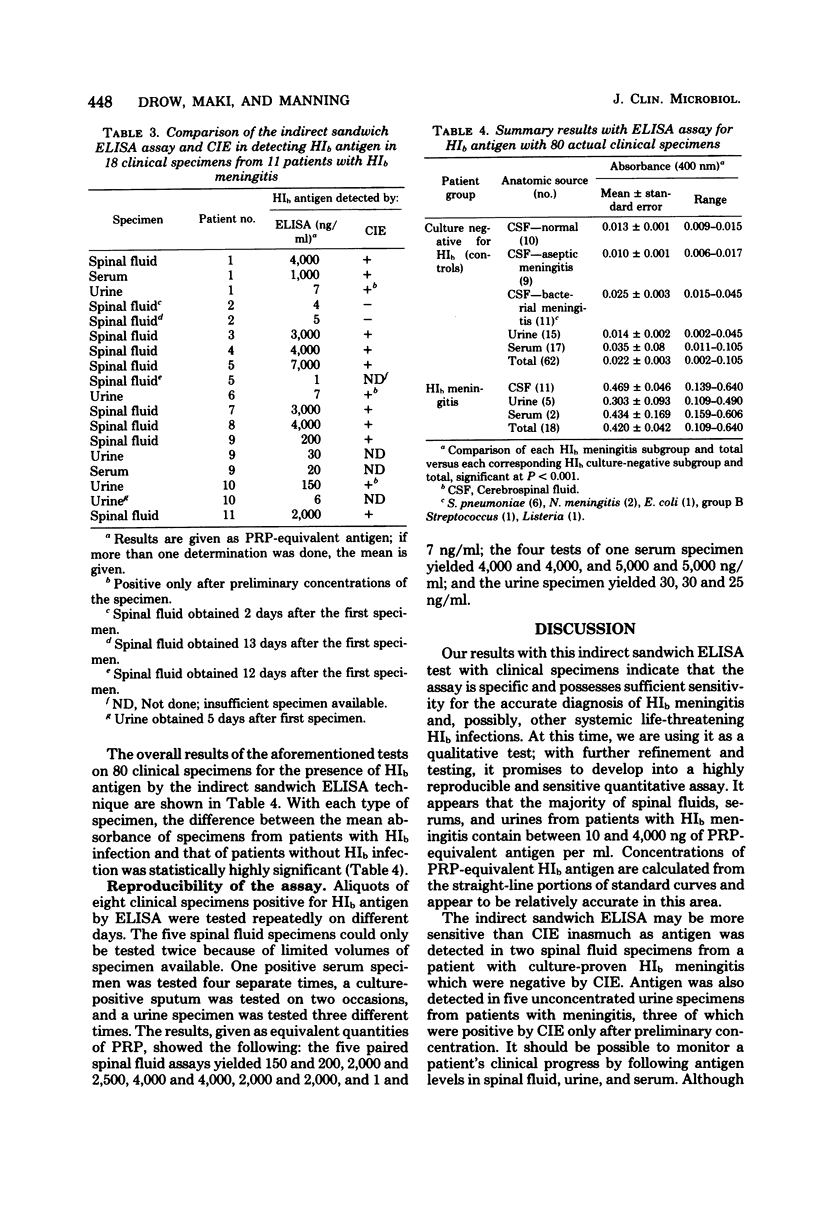
We report the development and testing of an enzyme-linked immunosorbent assay with excellent sensitivity for the detection of Haemophilus influenzae type b (HIb) antigen in clinical specimens from patients with HIb meningitis. The assay, an indirect sandwich technique, uses polystyrene balls as a solid phase and an alkaline phosphatase-labeled goat anti-rabbit globulin conjugate. Specimens are incubated with polystyrene balls armed with burro anti-HIb antiserum, and recognition antibody is visualized by addition of alkaline phosphatase-labeled anti-globulin, together with the enzyme substrate p-nitrophenyl phosphate. Concentrations of antigen are determined from standard curves prepared by using purified HIb capsular antigen polyribophosphate. The assay reproducibly detects polyribophosphate at concentrations between 1 and 5 ng/ml. Cross-reactions have not as yet been encountered in simulated and authentic clinical specimens containing other species including Escherichia coli, Klebsiella pneumoniae, group B Streptococcus, Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus, Neisseria meningitidis, and Listeria monocytogenes. In preliminary tests with 11 spinal fluid specimens, 2 serum specimens, and 5 urine specimens from patients with culture-proved HIb meningitis, antigen was detected in all specimens in concentrations ranging from 1 to 7,000 ng/ml. Antigen was not detected in any of 62 clinical specimens which were culture negative for HIb, including 11 spinal fluid specimens from patients with bacterial meningitis caused by microorganisms other than HIb. The enzyme-linked immunosorbent assay technique described here is considerably simpler than radioimmunoassay and, based on concurrent tests with 14 positive clinical specimens, may be more sensitive than counterimmunoelectrophoresis. It seems, therefore, to hold considerable promise for clinical use in rapid detection of systemic HIb infections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bullock S. L., Walls K. W. Evaluation of some of the parameters of the enzyme-linked immunospecific assay. J Infect Dis. 1977 Oct;136 (Suppl):S279–S285. doi: 10.1093/infdis/136.supplement_2.s279. [DOI] [PubMed] [Google Scholar]
- Burtis C. A., Seibert L. E., Baird M. A., Sampson E. J. Temperature dependence of the absorbance of alkaline solutions of 4-nitrophenyl phosphate--a potential source of error in the measurement of alkaline phosphatase activity. Clin Chem. 1977 Sep;23(9):1541–1547. [PubMed] [Google Scholar]
- Colding H., Lind I. Counterimmunoelectrophoresis in the diagnosis of bacterial meningitis. J Clin Microbiol. 1977 Apr;5(4):405–409. doi: 10.1128/jcm.5.4.405-409.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Rytel M. W. Detection of type-specific pneumococcal antigens by counterimmunoelectrophoresis. I. Methodology and immunologic properties of pneumococcal antigens. J Lab Clin Med. 1973 May;81(5):770–777. [PubMed] [Google Scholar]
- Counts G. W., Turck M. Screening for cross-reacting capsular polysaccharide K antigens of Escherichia coli using antiserum agar. J Clin Microbiol. 1977 Apr;5(4):490–491. doi: 10.1128/jcm.5.4.490-491.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Engvall E. Quantitative enzyme immunoassay (ELISA) in microbiology. Med Biol. 1977 Aug;55(4):193–200. [PubMed] [Google Scholar]
- Käyhty H., Mäkelä P. H., Ruoslahti E. Radioimmunoassay of capsular polysaccharide antigens of groups A and C meningococci and Haemophilus influenzae type b in cerebrospinal fluid. J Clin Pathol. 1977 Sep;30(9):831–833. doi: 10.1136/jcp.30.9.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Schuurs A. H., Van Weemen B. K. Enzyme-immunoassay. Clin Chim Acta. 1977 Nov 15;81(1):1–40. doi: 10.1016/0009-8981(77)90410-7. [DOI] [PubMed] [Google Scholar]
- Van Weemen B. K., Schuurs A. H.W.M. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971 Jun 24;15(3):232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Siber G. R., Scheifele D. W., Smith D. H. Rapid diagnosis of Hemophilus influenzae type b infections by latex particle agglutination and counterimmunoelectrophoresis. J Pediatr. 1978 Jul;93(1):37–42. doi: 10.1016/s0022-3476(78)80596-4. [DOI] [PubMed] [Google Scholar]



