Abstract
Allogeneic blood transfusion during liver resection for malignancies has been associated with an increased incidence of different types of complications: infectious complications, tumor recurrence, decreased survival. Even if there is clear evidence of transfusion-induced immunosuppression, it is difficult to demonstrate that transfusion is the only determinant factor that decisively affects the outcome. In any case there are several motivations to reduce the practice of blood transfusion. The advantages and drawbacks of different transfusion alternatives are reviewed here, emphasizing that surgeons and anesthetists who practice in centers with a high volume of liver resections, should be familiar with all the possible alternatives.
Keywords: Blood transfusion, Blood products, Allogeneic blood transfusion, Intraoperative autotransfusion, Preoperative autologous blood donation, Intraoperative isovolemic hemodilution, Infectious complications, Liver resection, Hepatocellular carcinoma
INTRODUCTION
Improvements in surgical techniques, in pre- and post-operative care, and increased experience have improved the safety of liver resections for hepatocellular carcinoma (HCC), and these procedures frequently can be carried out without blood transfusions[1–5]. By contrast, riskier hepatectomies, including posterior resections with reconstruction of the vena cava or resection of the caudate lobe, represent complex procedures which could require perioperative blood transfusion. Transfusion of allogenic blood has been reported to be associated with potentially devastating complications such as transmission of human immunodeficiency virus and hepatitis, transfusion reactions, increased postoperative infection rate, and increased incidence of recurrences for certain cancers[6]. Moreover, pulmonary oedemas occurring during or after a blood transfusion appear as the most frequent serious immediate incidents: they include transfusion-associated circulatory overload and transfusion-related acute lung injury (TRALI)[6]. Transfusion of allogenic whole blood products has been shown to induce variations in certain immune functions[7,8], such as reduced NK cell activity, T lymphocyte blastogenesis, and increased suppressor T lymphocyte activity, which may be of great relevance for host resistance to infection and the spread of neoplastic cells. But, the adverse effects of allogeneic whole blood transfusion on cancer recurrence and survival rates[9–12], regardless of innumerable published studies, continue to be debatable, since as many studies can be found that invalidate[13–18] as those that substantiate[19–27] this hypothesis.
Recent advances in surgical techniques to control blood loss and transfusion need[28–32], and the growing vast experience with hepatic resections, have been responsible for a remarkable reduction in the use of blood and blood products during surgery. Despite these efforts, allogeneic blood transfusion rates during hepatic resections have been reported at 40% to 80% depending upon the magnitude of the resection[3]. Furthermore, even though the introduction of the hepatic inflow occlusion technique introduced by Pringle[33] and selective and/or intermittent inflow occlusion have been very effective at reducing blood loss during hepatic resection, back bleeding from the hepatic veins and their tributaries during the Pringle manoeuvre can still be unpredictable, severe, and unexpected[34].
This paper outlines the current perspectives on blood transfusion in hepatic resection, focusing on allogeneic blood transfusion, intraoperative autotransfusion, preoperative autologous blood donation, and intraoperative isovolemic hemodilution.
ALLOGENEIC BLOOD TRANSFUSION
New measures to reduce transfusion errors have recently been defined by Regan et al[35]. The incidence of allogeneic blood transfusion is high in patients with cirrhotic livers undergoing liver resections for HCC, and for that reason it is vital to determine whether these transfusions stimulate tumor recurrence. The postoperative recurrence of HCC associated with perioperative blood transfusion has been supported[36] and disputed[37]. Furthermore the relationship between perioperative allogeneic blood transfusions, recurrence free survival, and the immunologic profiles of patients with HCC who have undergone curative liver resections has been investigated[38]. These studies have shown that in transfused patients, the CD4 levels are decreased by 90 postoperative days, whereas the CD8 levels are elevated during 14-90 d after surgery, as compared with nontransfused patients. Postoperative levels of the CD57+NK-cell subset and PHA responses in the transfused group are elevated as compared with the nontransfused group, and the PHA response of the transfused patients is significantly increased at seven postoperative days. Recurrence free survival seems not to be affected by perioperative blood transfusions.
All these studies suggest the significance of perioperative blood transfusion as an independent prognostic variable in terms of recurrence, survival, complications, and death. Patients who need preoperative, intraoperative, or postoperative transfusions are generally those with large lesions that either require a tri-segmentectomy, or are too close to the vena cava. On the other side, patients who do not need blood transfusions tend to have smaller, more peripheral lesions that can be resected under close hemostatic control. This suggests that patients with large HCC (with poor prognosis) are more likely to receive blood, and possible other factors should be taken into consideration for a more accurate evaluation. In regard to survival, for instance, the margin of resection, evidence of metastatic disease, liver failure or other perioperative complications should always be reviewed.
INTRAOPERATIVE AUTOTRANSFUSION
Intraoperative autotransfusion [also known as autologous blood salvage or intraoperative blood salvage (IBS)] is a medical procedure involving recovering blood lost during surgery and re-infusing it into the patient. Different medical devices have been developed to assist in salvaging the patient’s own blood in the perioperative setting. IBS is widely used in a variety of surgical procedures, including cardiovascular, orthopedic, and gynecologic procedures, and emergency medical situations[39–41], but IBS in oncologic patients has not been widely studied. IBS has been cited as a contraindication[42] because of the potential risk of disseminating metastasis. This concept was introduced firstly by Yaw et al[43] who demonstrated tumor cells in processed blood that passed through filters in the Bentley autotransfusion device. Other studies support that IBS can be safely used in patients with cancer[44–46]. Because the Haemonetics cell saver processes blood by centrifuge-based washing after filtration, the risk of reinfusion of malignant cells seems to be lower than by the Bentley system. Clinical evidence of dissemination of cancer cells caused by IBS has not been reported, and several studies show no correlation between the presence of malignant cells and their subsequent dissemination[47,48]. The haemonetics cell saver was employed by Fujimoto et al[49] as an intraoperative scavenger of blood in patients undergoing hepatectomy for HCC. In this study autotransfusion was shown to be safe and effective, and the pattern and frequency of recurrence suggest that autotransfusion is not responsible for recurrence or metastasis. Hashimoto et al[50] showed that IBS in living liver donors undergoing liver resection for graft procurement offered the advantage of reduced blood loss during parenchymal transection.
At the present time, the processes used to assist in salvaging the patient’s own whole blood in the perioperative setting can be categorized into three general types: (1) Cell processors and salvage devices that wash and save red blood cells (RBCs), i.e. “cell washers” or RBC-savers; (2) Direct transfusion; (3) Ultrafiltration of whole blood. Cell processors are red cell washing devices that collect anticoagulated shed or recovered blood, wash and separate the RBCs by centrifugation, and reinfuse the RBCs. RBC washing devices can help remove byproducts in salvaged blood such as activated cytokines, anaphylatoxins, and other waste substances that may have been collected in the reservoir suctioned from the surgical field. However, they also remove viable platelets, clotting factors, and other plasma proteins essential for homeostasis. Direct transfusion is a blood salvaging method associated with cardiopulmonary bypass circuits or other extracorporeal circuits that are used in surgery such as coronary artery bypass grafts, valve replacement, or surgical repair of the great vessels. Hemofiltration or ultrafiltration devices constitute the third major type of blood salvage appearing in operating rooms. In general, ultrafiltration devices filter the patient’s anticoagulated whole blood. The filtration process removes unwanted, excess non-cellular plasma water, low molecular weight solutes, platelet inhibitors and some particulate matter through hemoconcentration, including activated cytokines, anaphylatoxins, and other waste substances making concentrated whole blood available for reinfusion. Hemofiltration devices return the patient’s whole blood with all the blood elements and fractions including platelets, clotting factors, and plasma proteins with a substantial Hb level. Presently, the only whole blood ultrafiltration device in clinical use is the Hemobag.
Concerns about possible contamination of autologous RBC with cancer cells responsible for metastasis still continues to limit the use of IBS in cancer patients. This is despite the fact that no evidence has been reported showing an increase in metastasis or a decrease in patient survival, regardless of the obvious demonstration that salvaged blood is contaminated with viable tumor cells which are not washed out of the RBC layer during IBS. Total elimination of the risk of reinfusion of cancer cells by irradiation has been proposed by Hansen[51], who has been able to show that IBS with blood irradiation is safe as it provides efficient elimination of contaminating cancer cells, does not compromise the quality of RBC, and is very effective in saving blood resources. The effectiveness of this procedure has been shown on a large number of oncologic patients[52].
PREOPERATIVE AUTOLOGOUS BLOOD DONATION
Evidence that allogeneic transfusion may lead to a potential risk of postoperative infections, and the increased demand for blood with a declining population of qualified, willing, and healthy donors, give reason for the current support for preoperative autologous transfusion (PAD)[53,54]. The overall benefits of PAD have been assessed in both randomized trials and cohort studies[55]. Assuming that the donor is not bacteremic at the time of donation and/or there are no clerical errors resulting in the accidental transfusion of the wrong unit of blood, the patient is also protected against hemolytic, febrile or allergic transfusion reactions; alloimmunization to erythrocyte, leukocyte, platelet or protein antigens; and graft-versus-host disease (GVHD). An additional benefit is that erythropoiesis may be stimulated by repeated phlebotomies, thereby enabling the patient to regenerate hemoglobin at an accelerated rate after surgery.
PAD programs are not without some disadvantages. Perhaps the most important is that autologous blood is considerably more expensive than allogeneic blood. This problem is compounded by the fact that current reimbursement programs of most of the National Health systems around the world either deny the medical necessity of PAD or ignore the well-documented increase in cost[56]. Moreover, the blood that is not transfused to the intended recipient (approximately 50% of donated blood) is generally wasted rather than being transfused to other patients[57]. This wastage of blood and the costs of administering autologous programmes result in collection expenses that are higher than those for allogeneic transfusion.
Patients undergoing PAD may donate a unit (450 ± 45 mL) of blood as often as twice weekly, until 72 h before surgery. Under normal conditions, patients conventionally donate once weekly. Oral iron supplements are routinely prescribed. This iatrogenic blood loss is accompanied by a response in endogenous erythropoietin (EPO) levels that, although increased significantly over basal levels, remain within the normal range. The erythropoietic response that occurs under these conditions is therefore modest[58]. With routine PAD, erythropoiesis of 220-351 mL (11%-19% RBC expansion)[59,60] or the equivalent of 1-1.75 blood units, occurs in excess of basal erythropoiesis, which indicates the efficacy of this blood conservation practice.
The use of autologous blood deposits for cancer patients undergoing elective surgical procedures has been studied by Lichtiger[61], who was able to show that the majority (132/182) of his patients (with head and neck, neurosurgical, gastrointestinal and colorectal, adrenal, gynecologic, soft tissue and bone, breast, and genitourinary tumors) underwent surgery using only autologous transfusions. Kajikawa et al[62] evaluated the benefit of autologous blood transfusion and the effect of recombinant human erythropoietin (rh-EPO) on preoperative autologous blood donation for hepatectomy in patients with cirrhosis. Their study shows that autologous blood transfusion yields clinically superior results for hepatectomy in patients with cirrhosis when compared with homologous transfusion. In addition preoperative rh-EPO administration minimizes presurgical decreases in hematocrit (HCT) caused by autologous blood donation[62]. Likewise preoperative autologous blood donation in combination with rh-EPO therapy markedly reduces the requirements for homologous blood transfusion during hepatic resections[63].
Other studies on patients undergoing hepatic resection have shown that the predeposition of autologous blood decreased the need for homologous transfusions from 56% to 38%. A further reduction in the transfusion rate of 25% could have been possible if all patients had donated 2 U of autologous blood[64].
To determine if predonation of autologous blood impacts upon transfusion practice and clinical outcome following liver resection, clinical records of 379 consecutive patients undergoing hepatic resection for metastases of colorectal cancer were identified from the prospective hepatobiliary database and reviewed by Chan et al[65]. No conclusion could be drawn from their data concerning the influence of allogeneic transfusion on tumor recurrence, since their study was not a randomized trial comparing allogeneic blood transfusion with autologous transfusion. Data from their study however demonstrated that PAD alone is insufficient to alter the rate of tumor recurrence or disease-specific survival. Furthermore major hepatic resections using current surgical techniques can be performed safely with low blood loss so that transfusion is required for only a minority of patients. PAD may further reduce the need for allogeneic blood. Autologous blood transfusion is safe after storage and it has advantages if compared with homologous blood transfusion with regard to postoperative liver function and survival rate after hepatectomy for HCC[66].
In a recent study, Hirano et al[67] have shown that their autologous blood program, with IBS and preoperative blood donation, reduces the volume of banked blood needed and improves the prognosis of patients undergoing hepatectomy for HCC.
INTRAOPERATIVE ISOVOLEMIC HEMODILUTION
Acute isovolemic hemodilution (ANH) is another possible alternative to allogeneic blood transfusions, which was introduced in the early 1970s[68]. The procedure implies the removal of blood from the patient immediately before operation and the simultaneous replacement with appropriate volume of crystalloid or colloid fluids. ANH will reduce the HCT so that blood shed during the operative procedure will result in less RBC mass loss. The amount of blood removed varies between one and three units (450-500 mL constitutes 1 U), although larger volumes may be withdrawn safely in certain circumstances. The removed blood is then reinfused as autologous whole blood after the major blood loss portion of the procedure is completed. The blood withdrawn is anticoagulated and maintained at room temperature, in the operating room, for up to 8 h. It is reinfused into the patient as needed during, or after, the surgical procedure. ANH can be used as the only blood preservation technique, or it can be combined with preoperative autologous donation, blood salvage, or both.
Hemodilution could be classified according to the target HCT as mild (HCT ≥ 30%), moderate (30% < HCT ≥ 20%), or severe (HCT < 20%)[16]. The target HCT with ANH is variable but is often around 25%-30%. Severe hemodilution (e.g. 20%) is likely to be more efficacious with regards to blood conservation, but the risks are greater, particularly for patients with preexisting medical conditions such as coronary heart disease[69].
ANH should be taken into consideration for patients with good initial HCTs who are assumed to be deprived of more than two units of blood (900-1000 mL) during surgery. This technique works better in healthy, young adults, but it has been successfully employed in children and elderly patients. ANH has been used in vascular, orthopedic, and in some general surgical procedures. In addition, Jehovah’s Witnesses patients accept this technique with the modification that we keep the blood moving and in direct contact with the patient’s vascular system. Some Jehovah’s Witnesses will agree to ANH if the blood is maintained in a closed circuit continuous flow system[70].
ANH is contraindicated in cardiac disease, since the main compensatory mechanism for the induced anemia is an increase in the cardiac output, when renal function is impaired, since large amounts of infused fluids need to be excreted, and when baseline hemoglobin is below 110 mg/L (11 g/dL). Furthermore low concentrations of coagulation proteins, inadequate vascular access, and the absence of appropriate monitoring capability indicate that ANH should not be used[71].
In the last 20 years several groups reported the use of ANH during major hepatic resections[72–76], and the overall conclusion is that ANH, in selected patients, is a safe and effective technique that appears to reduce the number of patients requiring homologous blood transfusion as well as the number of units transfused per patient. Furthermore, Jehova’s Witnesses with hepatic tumors represent a major problem for liver surgeons to achieve good outcome, in fact these patients, because of their religious beliefs, refuse transfusion of blood and blood products. In order to avoid transfusion Barakat et al[75] have recently described the use of ANH in a Jehova’s Witness who underwent a combined left trisegmentectomy and caudate lobectomy to treat a large intrahepatic cholangiocarcinoma.
ANH is considered a simple and inexpensive procedure, and has the advantage that fresh autologous blood is readily available. Numerous studies of its efficacy, however, have produced conflicting results, perhaps because of the heterogeneity of the surgeries in which it was used, differences in study protocol, and differences in the definition of outcome variables[77,78].
DISCUSSION
Liver resection is still the mainstay of treatment for patient with HCC. Even though improved surgical techniques and anesthesia have remarkably decreased the mortality rates of liver resections, morbidity rates, remain high. One of the major risks of hepatectomy is large-volume blood loss, which necessitates perioperative blood transfusion. The possible consequences of homologous blood transfusion are well known and include noninfectious risks such as transfusion reactions, transient immunodeficiency, transfusion-associated GVHD, and TRALI[79–84]. Thus there are conclusive motivations to reduce blood loss during surgery and, as a consequence to lessen blood transfusion. It has been clearly shown that transfusion has a significant negative effect on perioperative mortality, complications, and length of hospital stay, even if it is difficult to demonstrate that transfusion is the only factor that decisively affects the outcome. The magnitude of the surgical procedure has always to be considered the most critical factor. It is intuitive that anterior, small, marginal atypical resections are quite different to complicated posterior large resections which include reconstruction of resected vena cava.
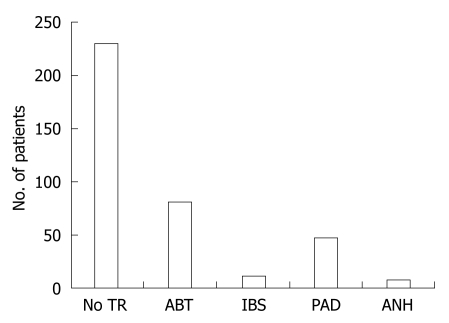
An association between transfusion and postoperative complications has been shown in preclinical models[85,86] and in clinical studies[87–91]. The review of 378 consecutive elective liver resections performed in our institution shows that 62% of the patients were not transfused, and the remaining 38% received blood products delivered with different procedures (Figure 1).
Figure 1.
Transfusion procedures in 378 patients undergoing liver resection. No TR: Not transfused (62%); ABT: Autologous blood transfusion (21%); IBS: Intraoperative blood salvage (3%); PAD: Preoperative autologous blood donation (12%); ANH: Acute normovolemic hemodilution (2%, seven of the eight pts were Jehowa’s Witnesses). Data from the Department of Surgical Sciences, University of Insubria, Varese, Italy.
Infectious complications (wound infections, pneumonia, urinary tract infections, central venous catheter infections, abscesses, and undiagnosed postoperative fever) have been more frequent in the transfused group of patients (33 vs 7). Most of the infections complications (18) have been recorded in the patients receiving autologous blood transfusions, the most frequent being wound infections (7) and pneumonia (5). Our results confirm the observation of Alfieri et al[92] who in a series of 254 liver resections found a significant association between blood transfusions and development of complications. More recently, Kooby et al[93] have been able to show that perioperative blood transfusion is a prognostic factor for the development of complications in univariate and multivariate analysis. Transfusion predicted development of both minor and major complications. Transfused patients had twice as high a chance of developing major complications and four times the risk of perioperative death. Transfused patients also had a higher incidence of infectious complications (17% vs 13%, P = 0.03)[93].
Despite these results and studies, it is still debatable whether transfusion is the only and independent factor related to short term outcome, and specifically the only determinant of postoperative infectious complications. Is the transfusion itself and not the reason for the transfusion the cause of postoperative morbidity? Intraoperative hypotension, complexity of operation (extended hepatectomies vs lesser resections), duration of anesthesia, age, stage of the neoplastic lesion, degree of liver dysfunction, nutritional status, and possible neoadjuvant treatment, are all factors which could interfere with some aspects of the complex immunologic response. Furthermore, timing of the transfusion and the circumstances necessitating transfusions have been proposed as the real determinants of prognosis[94]. Today we are not able to conclude that transfusion is the factor producing the infectious complication, and the correlation we found of transfusion with complications should not be interpreted as a direct cause and effect relationship. The infectious complications are different in the transfused and non-transfused patients, but we cannot say for sure that immunologic irregularities are what produces the difference.
In recent years, we have had the occasion to carry out seven major liver resections on Jehova’s Witnesses with large tumors. The management of Jehova’s Witnesses with HCC, or any other type of liver tumor, entails a multidisciplinary, adapted plan in harmony with their religious beliefs to achieve good outcome[95]. This approach enabled us to perform the surgical procedure respecting their religious conviction, and authorized us to anticipate that ANH could be considered a safe alternative for use in selected cases in which allogeneic blood transfusion is considered of high risk. This approach, in our series, has been associated with a relative high incidence of infectious complications, if compared with other autologous blood transfusion procedures (Figure 2).
Figure 2.
Details of postoperative infectious complications (44 pts, 11.6%) occurred in 378 patients undergoing liver resections and correlated to transfusion procedures. UTV: Urinary tract infection; CVC: Central venous catheter; UPF: Undiagnosed post-operative fever. Data from the Department of Surgical Sciences, University of Insubria, Varese, Italy.
CONCLUSION
A substantial discrepancy is apparent in transfusion practice for elective surgery, and even more so for liver resections[96]. Reducing unneeded exposure to blood components by blood saving measures is essential in patients undergoing elective surgery. A publication for anesthesists reviews good transfusion practices in surgical patients[97].
Perioperative blood transfusion has been described as one of the risk factors for poor outcome after liver resection. This seems particularly verifiable for infectious complications. The postoperative recurrence of HCC associated with perioperative blood transfusion has been the subject of controversy due to conflicting results. Although allogeneic blood transfusion may have immunosuppressive effects, perioperative blood transfusions seem not to influence the cancer free survival rate in patients with HCC. Even if there is no evidence of one transfusion procedure which prevails over the others, surgeons who practice in Centers with high volume of liver resections should be familiar with all the possible alternatives (ABT, IBS, PAD, ANH), since each of them, when blood products are needed, have a place depending upon the different clinical pattern.
Finally, maintaining a low central venous pressure has been shown recently to be effective in reducing blood loss during partial liver resections. Moreover antifibrinolytic drugs have proved to be effective in reducing blood loss during liver transplantation[98].
Peer reviewer: Ton Lisman, PhD, Thrombosis and Haemostasis Laboratory, Department of Haematology G.03.550, University Medical Centre, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands
S- Editor Tian L L- Editor Lalor PF E- Editor Zheng XM
References
- 1.Tsao JI, Loftus JP, Nagorney DM, Adson MA, Ilstrup DM. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Ann Surg. 1994;220:199–205. doi: 10.1097/00000658-199408000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–1529. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 3.Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, Frena A, Morganti M, Ercolani G, Masetti M. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–1110. doi: 10.1002/bjs.1800820833. [DOI] [PubMed] [Google Scholar]
- 4.Torzilli G, Gambetti A, Del Fabbro D, Leoni P, Olivari N, Donadon M, Montorsi M, Makuuchi M. Techniques for hepatectomies without blood transfusion, focusing on interpretation of postoperative anemia. Arch Surg. 2004;139:1061–1065. doi: 10.1001/archsurg.139.10.1061. [DOI] [PubMed] [Google Scholar]
- 5.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen HJ. Detrimental effects of perioperative blood transfusion. Br J Surg. 1995;82:582–587. doi: 10.1002/bjs.1800820505. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–310. [PubMed] [Google Scholar]
- 8.Gafter U, Kalechman Y, Sredni B. Induction of a subpopulation of suppressor cells by a single blood transfusion. Kidney Int. 1992;41:143–148. doi: 10.1038/ki.1992.19. [DOI] [PubMed] [Google Scholar]
- 9.Chung M, Steinmetz OK, Gordon PH. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg. 1993;80:427–432. doi: 10.1002/bjs.1800800407. [DOI] [PubMed] [Google Scholar]
- 10.Tartter PI. The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg. 1992;216:633–638. doi: 10.1097/00000658-199212000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, Adson MA. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504; discussion 504-505. doi: 10.1097/00000658-199210000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang R, Wang JY, Chien CR, Chen JS, Lin SE, Fan HA. The association between perioperative blood transfusion and survival of patients with colorectal cancer. Cancer. 1993;72:341–348. doi: 10.1002/1097-0142(19930715)72:2<341::aid-cncr2820720206>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Weiden PL, Bean MA, Schultz P. Perioperative blood transfusion does not increase the risk of colorectal cancer recurrence. Cancer. 1987;60:870–874. doi: 10.1002/1097-0142(19870815)60:4<870::aid-cncr2820600425>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Keller SM, Groshen S, Martini N, Kaiser LR. Blood transfusion and lung cancer recurrence. Cancer. 1988;62:606–610. doi: 10.1002/1097-0142(19880801)62:3<606::aid-cncr2820620327>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Foster RS Jr, Foster JC, Costanza MC. Blood transfusions and survival after surgery for breast cancer. Arch Surg. 1984;119:1138–1140. doi: 10.1001/archsurg.1984.01390220024005. [DOI] [PubMed] [Google Scholar]
- 16.Voogt PJ, van de Velde CJ, Brand A, Hermans J, Stijnen T, Bloem R, Leer JW, Zwaveling A, van Rood JJ. Perioperative blood transfusion and cancer prognosis. Different effects of blood transfusion on prognosis of colon and breast cancer patients. Cancer. 1987;59:836–843. doi: 10.1002/1097-0142(19870215)59:4<836::aid-cncr2820590430>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989;13:637–643. doi: 10.1007/BF01658891. [DOI] [PubMed] [Google Scholar]
- 18.Moriguchi S, Maehara Y, Akazawa K, Sugimachi K, Nose Y. Lack of relationship between perioperative blood transfusion and survival time after curative resection for gastric cancer. Cancer. 1990;66:2331–2335. doi: 10.1002/1097-0142(19901201)66:11<2331::aid-cncr2820661113>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Foster RS Jr, Costanza MC, Foster JC, Wanner MC, Foster CB. Adverse relationship between blood transfusions and survival after colectomy for colon cancer. Cancer. 1985;55:1195–1201. doi: 10.1002/1097-0142(19850315)55:6<1195::aid-cncr2820550610>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Parrott NR, Lennard TW, Taylor RM, Proud G, Shenton BK, Johnston ID. Effect of perioperative blood transfusion on recurrence of colorectal cancer. Br J Surg. 1986;73:970–973. doi: 10.1002/bjs.1800731208. [DOI] [PubMed] [Google Scholar]
- 21.Lange MM, van Hilten JA, van de Watering LM, Bijnen BA, Roumen RM, Putter H, Brand A, van de Velde CJ. Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg. 2009;96:734–740. doi: 10.1002/bjs.6636. [DOI] [PubMed] [Google Scholar]
- 22.Hyman NH, Foster RS Jr, DeMeules JE, Costanza MC. Blood transfusions and survival after lung cancer resection. Am J Surg. 1985;149:502–507. doi: 10.1016/s0002-9610(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 23.Moores DW, Piantadosi S, McKneally MF. Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg. 1989;47:346–351. doi: 10.1016/0003-4975(89)90371-8. [DOI] [PubMed] [Google Scholar]
- 24.Little AG, Wu HS, Ferguson MK, Ho CH, Bowers VD, Segalin A, Staszek VM. Perioperative blood transfusion adversely affects prognosis of patients with stage I non-small-cell lung cancer. Am J Surg. 1990;160:630–632; discussion 633. doi: 10.1016/s0002-9610(05)80762-7. [DOI] [PubMed] [Google Scholar]
- 25.Nowak MM, Ponsky JL. Blood transfusion and disease-free survival in carcinoma of the breast. J Surg Oncol. 1984;27:124–130. doi: 10.1002/jso.2930270214. [DOI] [PubMed] [Google Scholar]
- 26.Manyonda IT, Shaw DE, Foulkes A, Osborn DE. Renal cell carcinoma: blood transfusion and survival. Br Med J (Clin Res Ed) 1986;293:537–538. doi: 10.1136/bmj.293.6546.537-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Seipp CA, White DE, Wesley R. Perioperative blood transfusions are associated with increased rates of recurrence and decreased survival in patients with high-grade soft-tissue sarcomas of the extremities. J Clin Oncol. 1985;3:698–709. doi: 10.1200/JCO.1985.3.5.698. [DOI] [PubMed] [Google Scholar]
- 28.Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155–161. doi: 10.1097/00000658-199608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711; discussion 711-713. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 31.Konopke R, Kersting S, Bunk A, Dietrich J, Denz A, Gastmeier J, Saeger HD. Colorectal liver metastasis surgery: analysis of risk factors predicting postoperative complications in relation to the extent of resection. Int J Colorectal Dis. 2009;24:687–697. doi: 10.1007/s00384-009-0669-3. [DOI] [PubMed] [Google Scholar]
- 32.Johnson LB, Plotkin JS, Kuo PC. Reduced transfusion requirements during major hepatic resection with use of intraoperative isovolemic hemodilution. Am J Surg. 1998;176:608–611. doi: 10.1016/s0002-9610(98)00284-0. [DOI] [PubMed] [Google Scholar]
- 33.Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541–549. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong KH, Hamady ZZ, Malik HZ, Prasad R, Lodge JP, Toogood GJ. Intermittent Pringle manoeuvre is not associated with adverse long-term prognosis after resection for colorectal liver metastases. Br J Surg. 2008;95:985–989. doi: 10.1002/bjs.6129. [DOI] [PubMed] [Google Scholar]
- 35.Regan F, Taylor C. Blood transfusion medicine. BMJ. 2002;325:143–147. doi: 10.1136/bmj.325.7356.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Mizuno S, Makuuchi M. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 37.Matsumata T, Ikeda Y, Hayashi H, Kamakura T, Taketomi A, Sugimachi K. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72:1866–1871. doi: 10.1002/1097-0142(19930915)72:6<1866::aid-cncr2820720613>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 38.Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771–778. [PubMed] [Google Scholar]
- 39.Hallett JW Jr, Popovsky M, Ilstrup D. Minimizing blood transfusions during abdominal aortic surgery: recent advances in rapid autotransfusion. J Vasc Surg. 1987;5:601–606. [PubMed] [Google Scholar]
- 40.Dzik WH, Jenkins R. Use of intraoperative blood salvage during orthotopic liver transplantation. Arch Surg. 1985;120:946–948. doi: 10.1001/archsurg.1985.01390320070014. [DOI] [PubMed] [Google Scholar]
- 41.Williamson KR, Taswell HF. Intraoperative blood salvage: a review. Transfusion. 1991;31:662–675. doi: 10.1046/j.1537-2995.1991.31791368347.x. [DOI] [PubMed] [Google Scholar]
- 42.Autologous blood transfusions. Council on Scientific Affairs. JAMA. 1986;256:2378–2380. [PubMed] [Google Scholar]
- 43.Yaw PB, Sentany M, Link WJ, Wahle WM, GGlover JL. Tumor cells carried through autotransfusion. Contraindication to intraoperative blood recovery? JAMA. 1975;231:490–491. [PubMed] [Google Scholar]
- 44.Klimberg I, Sirois R, Wajsman Z, Baker J. Intraoperative autotransfusion in urologic oncology. Arch Surg. 1986;121:1326–1329. doi: 10.1001/archsurg.121.11.1326. [DOI] [PubMed] [Google Scholar]
- 45.Muscari F, Suc B, Vigouroux D, Duffas JP, Migueres I, Mathieu A, Lavayssiere L, Rostaing L, Fourtanier G. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int. 2005;18:1236–1239. doi: 10.1111/j.1432-2277.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 46.Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia. 2008;63:1332–1338. doi: 10.1111/j.1365-2044.2008.05637.x. [DOI] [PubMed] [Google Scholar]
- 47.Salsbury AJ. The significance of the circulating cancer cell. Cancer Treat Rev. 1975;2:55–72. doi: 10.1016/s0305-7372(75)80015-6. [DOI] [PubMed] [Google Scholar]
- 48.Griffiths JD, McKinna JA, Rowbotham HD, Tsolakidis P, Salsbury AJ. Carcinoma of the colon and rectum: circulating malignant cells and five-year survival. Cancer. 1973;31:226–236. doi: 10.1002/1097-0142(197301)31:1<226::aid-cncr2820310130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto J, Okamoto E, Yamanaka N, Oriyama T, Furukawa K, Kawamura E, Tanaka T, Tomoda F. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg. 1993;128:1065–1069. doi: 10.1001/archsurg.1993.01420210129021. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto T, Kokudo N, Orii R, Seyama Y, Sano K, Imamura H, Sugawara Y, Hasegawa K, Makuuchi M. Intraoperative blood salvage during liver resection: a randomized controlled trial. Ann Surg. 2007;245:686–691. doi: 10.1097/01.sla.0000255562.60215.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen E, Bechmann V, Altmeppen J. Intraoperative blood salvage in cancer surgery: safe and effective? Transfus Apher Sci. 2002;27:153–157. doi: 10.1016/s1473-0502(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 52.Valbonesi M, Bruni R, Lercari G, Florio G, Carlier P, Morelli F. Autoapheresis and intraoperative blood salvage in oncologic surgery. Transfus Sci. 1999;21:129–139. doi: 10.1016/s0955-3886(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 53.Toy PT, Strauss RG, Stehling LC, Sears R, Price TH, Rossi EC, Collins ML, Crowley JP, Eisenstaedt RS, Goodnough LT. Predeposited autologous blood for elective surgery. A national multicenter study. N Engl J Med. 1987;316:517–520. doi: 10.1056/NEJM198702263160906. [DOI] [PubMed] [Google Scholar]
- 54.Festa V, Bosio P, Giraudo G, Cavuoti G, Soncini S, Morino M. Possible impact of autologous blood towards elective open and laparoscopic surgery for colorectal carcinoma. Hepatogastroenterology. 2006;53:687–692. [PubMed] [Google Scholar]
- 55.Vanderlinde ES, Heal JM, Blumberg N. Autologous transfusion. BMJ. 2002;324:772–775. doi: 10.1136/bmj.324.7340.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yomtovian R, Kruskall MS, Barber JP. Autologous-blood transfusion: the reimbursement dilemma. J Bone Joint Surg Am. 1992;74:1265–1272. [PubMed] [Google Scholar]
- 57.Fontaine MJ, Winters JL, Moore SB, McGregor CG, Santrach PJ. Frozen preoperative autologous blood donation for heart transplantation at the Mayo Clinic from 1988 to 1999. Transfusion. 2003;43:476–480. doi: 10.1046/j.1537-2995.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 58.Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823–833. [PubMed] [Google Scholar]
- 59.Kasper SM, Gerlich W, Buzello W. Preoperative red cell production in patients undergoing weekly autologous blood donation. Transfusion. 1997;37:1058–1062. doi: 10.1046/j.1537-2995.1997.371098016445.x. [DOI] [PubMed] [Google Scholar]
- 60.Kasper SM, Lazansky H, Stark C, Klimek M, Laubinger R, Börner U. Efficacy of oral iron supplementation is not enhanced by additional intravenous iron during autologous blood donation. Transfusion. 1998;38:764–770. doi: 10.1046/j.1537-2995.1998.38898375516.x. [DOI] [PubMed] [Google Scholar]
- 61.Lichtiger B, Huh YO, Armintor M, Fischer HE. Autologous transfusions for cancer patients undergoing elective ablative surgery. J Surg Oncol. 1990;43:19–23. doi: 10.1002/jso.2930430106. [DOI] [PubMed] [Google Scholar]
- 62.Kajikawa M, Nonami T, Kurokawa T, Hashimoto S, Harada A, Nakao A, Takagi H. Autologous blood transfusion for hepatectomy in patients with cirrhosis and hepatocellular carcinoma: use of recombinant human erythropoietin. Surgery. 1994;115:727–734. [PubMed] [Google Scholar]
- 63.Shinozuka N, Koyama I, Arai T, Numajiri Y, Watanabe T, Nagashima N, Matsumoto T, Ohata M, Anzai H, Omoto R. Autologous blood transfusion in patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2000;179:42–45. doi: 10.1016/s0002-9610(99)00256-1. [DOI] [PubMed] [Google Scholar]
- 64.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–1056. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 65.Chan AC, Blumgart LH, Wuest DL, Melendez JA, Fong Y. Use of preoperative autologous blood donation in liver resections for colorectal metastases. Am J Surg. 1998;175:461–465. doi: 10.1016/s0002-9610(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 66.Kitagawa K, Taniguchi H, Mugitani T, Koh T, Obayashi T, Kunishima S, Yamaguchi A, Yamagishi H. Safety and advantage of perioperative autologous blood transfusion in hepatic resection for hepatocellular carcinoma. Anticancer Res. 2001;21:3663–3667. [PubMed] [Google Scholar]
- 67.Hirano T, Yamanaka J, Iimuro Y, Fujimoto J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg Today. 2005;35:1042–1046. doi: 10.1007/s00595-005-3082-8. [DOI] [PubMed] [Google Scholar]
- 68.Messmer K. Hemodilution. Surg Clin North Am. 1975;55:659–678. doi: 10.1016/s0039-6109(16)40641-9. [DOI] [PubMed] [Google Scholar]
- 69.Napier JA, Bruce M, Chapman J, Duguid JK, Kelsey PR, Knowles SM, Murphy MF, Williamson LM, Wood JK, Lee D, et al. Guidelines for autologous transfusion. II. Perioperative haemodilution and cell salvage. British Committee for Standards in Haematology Blood Transfusion Task Force. Autologous Transfusion Working Party. Br J Anaesth. 1997;78:768–771. doi: 10.1093/bja/78.6.768. [DOI] [PubMed] [Google Scholar]
- 70.Schaller RT Jr, Schaller J, Morgan A, Furman EB. Hemodilution anesthesia: a valuable aid to major cancer surgery in children. Am J Surg. 1983;146:79–84. doi: 10.1016/0002-9610(83)90263-5. [DOI] [PubMed] [Google Scholar]
- 71.Kreimeier U, Messmer K. Hemodilution in clinical surgery: state of the art 1996. World J Surg. 1996;20:1208–1217. doi: 10.1007/s002689900184. [DOI] [PubMed] [Google Scholar]
- 72.Chen H, Sitzmann JV, Marcucci C, Choti MA. Acute isovolemic hemodilution during major hepatic resection--an initial report: does it safely reduce the blood transfusion requirement? J Gastrointest Surg. 1997;1:461–466. doi: 10.1016/s1091-255x(97)80134-5. [DOI] [PubMed] [Google Scholar]
- 73.Imai R, Matsumura H, Uchida R, Watanabe K. Perioperative hemodilutional autologous blood transfusion in burn surgery. Injury. 2008;39:57–60. doi: 10.1016/j.injury.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 74.Rhim CH, Johnson LB, Kitisin K, Lu AD, Fishbein T, Broseker L, Yosaitis J, Manley J, Plotkin JS. Intra-operative acute isovolemic hemodilution is safe and effective in eliminating allogeneic blood transfusions during right hepatic lobectomy: Comparison of living donor versus non-donors. HPB (Oxford) 2005;7:201–203. doi: 10.1080/13651820510016515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adelola OA, Ahmed I, Fenton JE. Management of Jehovah's Witnesses in otolaryngology, head and neck surgery. Am J Otolaryngol. 2008;29:270–278. doi: 10.1016/j.amjoto.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Balci ST, Pirat A, Torgay A, Cinar O, Sevmis S, Arslan G. Effect of restrictive fluid management and acute normovolemic intraoperative hemodilution on transfusion requirements during living donor hepatectomy. Transplant Proc. 2008;40:224–227. doi: 10.1016/j.transproceed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Segal JB, Blasco-Colmenares E, Norris EJ, Guallar E. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004;44:632–644. doi: 10.1111/j.1537-2995.2004.03353.x. [DOI] [PubMed] [Google Scholar]
- 78.Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesth Analg. 1998;86:9–15. doi: 10.1213/00000539-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Doyle DJ. Blood transfusions and the Jehovah's Witness patient. Am J Ther. 2002;9:417–424. doi: 10.1097/00045391-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 80.America's Blood Centers. West Nile virus and the blood supply: 2003. Blood Bulletin. 2003;6:12–14. [Google Scholar]
- 81.Marcucci C, Madjdpour C, Spahn DR. Allogeneic blood transfusions: benefit, risks and clinical indications in countries with a low or high human development index. Br Med Bull. 2004;70:15–28. doi: 10.1093/bmb/ldh023. [DOI] [PubMed] [Google Scholar]
- 82.Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, Herruzo-Avilés A, Camacho-Laraña P, Garnacho-Montero J, Amaya-Villar R. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. 2001;119:1461–1468. doi: 10.1378/chest.119.5.1461. [DOI] [PubMed] [Google Scholar]
- 83.Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–320. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 84.Roth VR, Kuehnert MJ, Haley NR, Gregory KR, Schreiber GB, Arduino MJ, Holt SC, Carson LA, Elder KV, Jarvis WR. Evaluation of a reporting system for bacterial contamination of blood components in the United States. Transfusion. 2001;41:1486–1492. doi: 10.1046/j.1537-2995.2001.41121486.x. [DOI] [PubMed] [Google Scholar]
- 85.Tadros T, Wobbes T, Hendriks T. Blood transfusion impairs the healing of experimental intestinal anastomoses. Ann Surg. 1992;215:276–281. doi: 10.1097/00000658-199203000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tadros T, Wobbes T, Hendriks T. Opposite effects of interleukin-2 on normal and transfusion-suppressed healing of experimental intestinal anastomoses. Ann Surg. 1993;218:800–808. doi: 10.1097/00000658-199312000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van de Watering LM, Hermans J, Houbiers JG, van den Broek PJ, Bouter H, Boer F, Harvey MS, Huysmans HA, Brand A. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–568. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 88.Vamvakas EC, Carven JH. Allogeneic blood transfusion, hospital charges, and length of hospitalization: a study of 487 consecutive patients undergoing colorectal cancer resection. Arch Pathol Lab Med. 1998;122:145–151. [PubMed] [Google Scholar]
- 89.Bellantone R, Sitges-Serra A, Bossola M, Doglietto GB, Malerba M, Franch G, Pacelli F, Crucitti F. Transfusion timing and postoperative septic complications after gastric cancer surgery: a retrospective study of 179 consecutive patients. Arch Surg. 1998;133:988–992. doi: 10.1001/archsurg.133.9.988. [DOI] [PubMed] [Google Scholar]
- 90.Kinoshita Y, Udagawa H, Tsutsumi K, Ueno M, Nakamura T, Akiyama H, Takahashi K, Kajiyama Y, Tsurumaru M. Usefulness of autologous blood transfusion for avoiding allogenic transfusion and infectious complications after esophageal cancer resection. Surgery. 2000;127:185–192. doi: 10.1067/msy.2000.102048. [DOI] [PubMed] [Google Scholar]
- 91.Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg. 2000;87:1553–1562. doi: 10.1046/j.1365-2168.2000.01570.x. [DOI] [PubMed] [Google Scholar]
- 92.Alfieri S, Carriero C, Caprino P, Di Giorgio A, Sgadari A, Crucitti F, Doglietto GB. Avoiding early postoperative complications in liver surgery. A multivariate analysis of 254 patients consecutively observed. Dig Liver Dis. 2001;33:341–346. doi: 10.1016/s1590-8658(01)80089-x. [DOI] [PubMed] [Google Scholar]
- 93.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869-870. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bossola M, Pacelli F, Bellantone R, Doglietto GB. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2005;241:381. doi: 10.1097/01.sla.0000152989.76942.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barakat O, Cooper JR Jr, Riggs SA, Hoef JW, Ozaki CF, Wood RP. Complex liver resection for a large intrahepatic cholangiocarcinoma in a Jehovah's witness: a strategy to avoid transfusion. J Surg Oncol. 2007;96:249–253. doi: 10.1002/jso.20799. [DOI] [PubMed] [Google Scholar]
- 96.Use of blood products for elective surgery in 43 European hospitals. The Sanguis Study Group. Transfus Med. 1994;4:251–268. [PubMed] [Google Scholar]
- 97.Association of Anaesthetists of Great Britain and Ireland Blood Transfusion and the anaesthetist: red cell transfusion. London: AAGBI; 2001. pp. 134–144. [Google Scholar]
- 98.Alkozai EM, Lisman T, Porte RJ. Bleeding in liver surgery: prevention and treatment. Clin Liver Dis. 2009;13:145–154. doi: 10.1016/j.cld.2008.09.012. [DOI] [PubMed] [Google Scholar]