Abstract
AIM: To determine the accuracy of 1.5-T magnetic resonance imaging (MRI) in the evaluation of gastric wall invasion and perigastric lymph node metastasis in gastric adenocarcinoma.
METHODS: Twenty resected gastric specimens containing 20 tumors were studied with a 1.5-T MR system using a commercial head surface coil. MR scanning was performed with a T1 weighted image (TR/TE = 500/20), and a T2 weighted image (TR/TE = 2500/90). MR findings were compared with pathologic findings.
RESULTS: A T1-weighted image demonstrated three layers in the normal gastric wall. All of the gastric tumors were well demonstrated by lesions and location. In a MRI findings of gastric wall invasion, there was 1 case of T1, 7 of T2, 11 of T3. Pathologic results of resected specimens included 3 cases of pT1, 4 of pT2, and 12 of pT3. The accuracy of T staging with MRI was 74% (14 of 19). MRI findings of lymph node metastasis included 6 cases of N0, 13 cases of N1. The accuracy of the N staging with MRI was 47% (9 of 19).
CONCLUSION: MRI has a high diagnostic accuracy in the evaluation of the T staging of gastric cancer in vitro and thus potentially enables preoperative histopathologic staging.
Keywords: Gastric cancer, Magnetic resonance imaging, Neoplasm, Staging, Stomach, Depiction of wall layer
INTRODUCTION
The preoperative staging workup of gastric carcinoma is performed mainly with computed tomography (CT). CT has been a favored method for preoperative evaluation and staging in patients with gastric carcinoma[1–3]. Parallel advances in CT equipment and scanning techniques have reduced scanning time and decreased motion artifacts. Simultaneously, rapid IV contrast administration with an automatic power injector has improved contrast enhancement of the gastric wall and gastric cancer. Helical CT has advantages over conventional CT, including faster scanning time and fewer respiratory misregistration artifacts in a single breath-hold[4]. However, CT is limited, particularly in the diagnosis of lymph node metastasis, peritoneal metastasis, and small hematogenous metastasis[5,6].
Endoscopic sonography has been reported to be the most accurate technique for the T staging of gastric carcinoma because it can define five layers of the gastric wall. But this technique cannot evaluate other factors such as liver metastasis and peritoneal seeding[7]. In addition, endoscopic sonography is an invasive technique dependent on the operator.
Magnetic resonance imaging (MRI) has not become popular for staging because of a number of limitations, including motion artifacts, lack of a stable contrast medium, and the high cost[8,9]. However, continuous technical improvements have been made in MRI of the abdomen, thereby reducing motion artifacts and improving image quality. These improvements include breath-hold fast imaging techniques, placement of abdominal binders, administration of antiperistaltic agents, and the use of phased array coils[10]. In vitro studies using 1-4.7- T MR systems have shown that MRI allows the depiction of gastric wall layers and therefore, technically permits the evaluation of the local tumor stage of gastric carcinomas[11–13].
The purpose of this study was to assess the accuracy of the evaluation of gastric carcinomas and lymph node metastasis in vitro by using gastrectomy specimens that were studied with 1.5-T MRI.
MATERIALS AND METHODS
Subjects
Over a period of 13 mo, a total number of 20 consecutive patients with histopathologically proven gastric carcinoma underwent subtotal or total gastrectomy. There were twenty resected gastric specimens that were obtained from the patients who were diagnosed with gastric carcinoma histologically by fiberoptic biopsy. The patients underwent subtotal or total gastrectomy. They consisted of fourteen men and six women [mean age, 53 years: 34-77 years (14 men, 6 women)]. Nineteen subtotal gastrectomy specimens and one total gastrectomy specimen was obtained and used in this study.
Specimen preparation
We needed to distend the gastric specimens for MRI. During their operations, all the stomachs were not opened for the purpose of this study. To distend the stomach of the specimens, the duodenal resection border was sealed with a continuous suture before the specimen was filled with saline solution. The specimens were then placed in a plastic box that had been filled with 5-6 L of saline solution.
The proximal portion of the gastrectomy specimen was hanged at the cap by strings. The box was capped and placed in the head coil of a 1.5-T MRI (SMT 1.5 scanner, Shimazu Co., Tokyo, Japan). Then the MR examination was performed. The study protocol was approved by the Institutional Review Board, and agreement was obtained from each patient before surgery.
MRI protocol
The MRI obtained in this study was based on the following multisection spin echo sequences for T1-weighted images, repetition time (TR) ms/echo time (TE) ms = 500/20, and for T2-weighted images, 2500/90. Two numbers of excitation were applied in this scanning. The matrix size was 256 × 256. Slice thickness was 5 mm and the intersection gap was 1 mm. Field of view was 20 cm. MR scans of the gastrectomy specimen were taken along the axial and sagittal planes. A head coil was used for scanning.
Image analysis
The MR images of 20 resected gastric specimens were analyzed by two radiologists (H.S and S.K with 3 and 10 years of abdominal CT and MRI experience, respectively) in consensus before the results from the pathologic examination were available. The number of visible wall layers and their specific signal intensity (SI) characteristics were examined. Wall-layer correlation was made on the basis of the layer thickness of the visible layers in MRI compared with the ones visible in histology. The presence of a tumor, defined as destruction of the normal gastric wall layers, was noted. The tumors were examined for variations in SI. The depth of infiltration was evaluated according to earlier publications[12,13]. A normal gastric wall was identified as having 3 layers. In terms of scanning direction and degree of distention of the wall, a gastric wall that was more than 1 cm thick or that showed an abrupt change of pattern from normal to pathologic was considered abnormal. The location, gross appearance, size and degree of serosal invasion of tumors were evaluated. Location was classified according to four areas: antrum, body, body and antrum, and fundus. Gross appearance was classified into four categories by Bormann’s classification for advanced gastric carcinoma[14]. T and N staging were based on the TNM system developed by the American Joint Committee on Cancer (AJCC)[15]. Early gastric cancer was evaluated according to the Japanese Research Society for Gastric Cancer[16]. The degree of tumor invasion in the gastric wall according to the T stage was measured as follows: T1 meant that MR showed obliteration of SI within the thickened mucosal layer and second submucosal layer, T2 meant that thickening of the gastric wall and obliteration of the third layer of muscularis propria, and T3 meant irregular SI in the outer margin of the third layer.
We counted the total number of lymph nodes which were located in the perigastric area. A lymph node of > 8 mm at the short axis was considered to be pathologic[17]. N staging of lymph nodes was performed. N0 is defined as no regional lymph node metastasis, N1 as metastasis in one to six regional lymph nodes, N2 as metastasis in seven to 15 regional lymph nodes, N3 as metastasis in more than 15 regional lymph nodes. Results of MR images were compared with findings in pathologic specimens and a report made by pathologists.
Histologic preparation
Immediately after the MR examination of resected gastric specimens, the specimens were transferred to the department of pathology. The time interval between resection and fixation of the specimens was 2-3.5 h. The pathologist was not informed of the findings of the MRI. The gastrectomy specimens were cut in planes corresponding to MRI imaging planes. The location, gross appearance, tumor size, and the histologic depth of invasion were determined for each specimen. The gastric carcinomas were staged according to the American Joint Committee on Cancer[15]. The diagnosed early gastric carcinomas (EGCs) were evaluated[16]. Finally, the histopathological staging of the specimens were compared with the staging by MRI. The depth of tumor invasion was decided according to the T factor of the TNM classification. Invasion of the mucosa or submucosa is classified as pT1, invasion of the muscularis propria as pT2, and tumor penetration of the serosa as pT3.
All the lymph nodes in the perigastric area of the specimens were counted and examined. Lymph nodes were stained with hematoxylin-eosin and examined by a light microscope for metastasis. Correlation between MR staging of lymph node metastasis and pathologic staging were performed by AJCC protocol[15].
RESULTS
SI characteristics of normal gastric wall
On MRI, two to three layers with different SI in the normal gastric wall can be depicted. However, there was a mainly three-layered structure (multilayered pattern) of the gastric wall by MRI. The inner layer showed an increase of SI and was 1-3 mm thick on the T1-weighted images. The second had a lower SI with thickness that varied at different sites in the same individual. The outer layer showed a slightly higher SI.
On T2-weighted images, the inner and outer layers regularly had a low SI, and the middle layer a high SI. On the basis of the comparison, the inner layer corresponds to the mucosa, the middle to the submucosa and the outer to the muscularis propria and serosal layers (Figure 1).
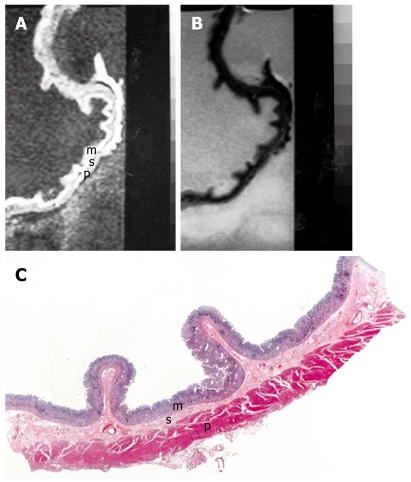
Figure 1.
MRI and histology of normal gastric wall. A: T1-weighted (500/20) sagittal image of resected gastric wall showed three layers. The inner layer corresponds to the mucosa (m) and the middle layer to the submucosa (s). The outer layer basically consists of the muscularis propria (p) from which the serosa cannot be differentiated; B: T2-weighted (2500/90) MRI showed low SI on mucosa and muscularis propria and relatively high SI on submucosa; C: Light microscopic section of normal gastric wall obtained from the greater curvature site of stomach body showed three layers which are compatible with inner mucosal layer (m), middle submucosa layer (s) and outer muscularis propria and serosal layer (p) (HE stain; original magnification, × 1).
Detection of primary tumor
MRI of gastric carcinoma on resected specimens showed as follows: two cases of Bormann’s type 1 carcinoma (polypoid type), seven cases of Bormann’s type 2 (ulcerative type), six cases of Bormann’s type 3 (ulcerative type with infiltration), and four cases of Bormann’s type 4 (infiltrating type). One case of early gastric carcinoma with type IIc was observed, whose lesion was seen as a depression of the mucosa with thinning of the gastric wall on axial and sagittal scannings (Figure 2). Gross pathologic findings showed tumor lesions as follows; two cases of Bormann’s type 1, four of Bormann’s type 2, nine of Bormann’s type 3, four of Bormann’s type 4. One case of early gastric carcinoma with type IIc was proved upon histologic examination.
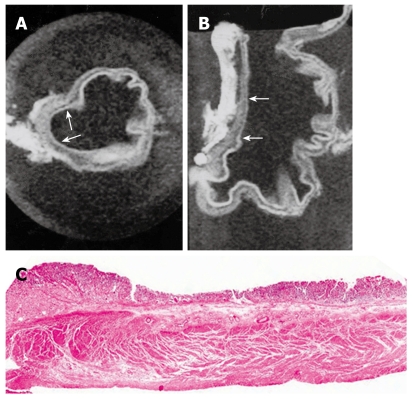
Figure 2.
MRI and histology of early gastric cancer. A: T1-weighted (500/20) axial image showed depression of gastric wall and obliteration of submucosal low SI (arrows); B: T1-weighted sagittal MRI showed depressed mucosa with tumor invasion to submucosa layer (arrows); C: Light microscopic section showed depressed mucosa with tumor invasion to submucosa (HE stain; original magnification, × 1).
In terms of the classification of gross appearance in the nineteen lesions detected as advanced gastric carcinoma, the accuracy of MRI in the Bormann’s type classification was 89% (16 of 19). Differentiation between Bormann’s type 2 and type 3 lesions was erroneous in three lesions.
The location of gastric carcinoma was also identified on the MR images. There were nine cases of gastric carcinoma involvement in the gastric antrum, three cases in the stomach body, seven cases in the antrum and the body, one case involving the entire stomach. Upon gross specimen examination, there was no difference between them and the MRIs.
Depth of tumor invasion
Degree of invasion was evaluated in the nineteen cases of advanced gastric carcinoma (Table 1). MRIs of gastric carcinoma in resected specimens showed various findings, including thickening of the gastric wall with irregularity in the mucosal SI obliteration, thickening of the gastric wall with first and second layer SI obliteration, diffuse thickening of the gastric wall with third layer SI obliteration and irregularity with ulceration as well.
Table 1.
Magnetic resonance imaging (MRI) and pathologic correlation of tumor invasion in gastric wall
|
Diagnosis at histologic examination | |||||
| Diagnosis’ at MR | pT0 | pT1 | pT2 | pT3 | Total |
| T0 | |||||
| T1 | 1 | 1 | |||
| T2 | 2 | 3 | 2 | 7 | |
| T3 | 1 | 10 | 11 | ||
| 3 | 4 | 12 | 19 | ||
T1-weighted images showed intermediate SI in regions affected by gastric carcinoma compared to the surrounding normal mucosa and muscularis propria SI. T2-weighted images showed low SI in the gastric carcinoma. Most tumors had a homogenous SI. However, in some cases necrosis and calcification caused an inhomogeneous SI. It is not possible to differentiate between the muscularis propria, subserosa, and serosa. The reason for this inability was that we considered the subserosa and serosa as being located on the outer border of the joint layer representing the muscularis propria, subserosa, and serosa. If an infiltration was visible, the tumor was classified as T2 as long as it did not reach the outer border. Penetration of the external margin meant at once infiltration of the serosa, and the tumor was staged as a T3 carcinoma. A tumor infiltrating the subserosa without penetrating the serosa was still considered T2, according to the AJCC[5]. The MRI findings of gastric wall invasion included 1 case of T1, 7 of T2 (Figure 3), and 11 of T3 (Figure 4). Pathologic results of resected specimens included 3 cases of pT1, 4 of pT2, and 12 of pT3. Differentiation between T1 and T2 classifications was not difficult in cases displaying a distinction between three layers. However, two cases of pT1 were over staged as T2. One case of pT2 was over staged as T3. Two cases of pT3 were understaged as T2. Differentiation between T2 and T3 lesions was difficult due to the outer muscularis propria and serosal layer’s thinness and could not always be demonstrated by MRIs. The level of accuracy in determining the T factor according to the TNM classification was 74% (14 of 19 lesions).
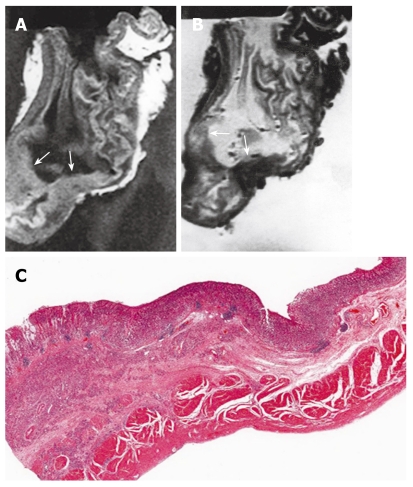
Figure 3.
MRI and histology of T2 gastric cancer. A: T1-weighted (500/20) sagittal image showed diffuse thickening of gastric wall with obliteration of mucosa, submucosa and muscularis propria SI in antrum and lower body, while preserved outer marginal SI (arrows); B: T2-weighted (2000/90) sagittal MRI showed ill defined lesion with minimal increased and same SI compared to surrounding normal gastric wall (arrows); C: Light microscopic section demonstrate proper muscle invasion of gastric cancer (HE stain; original magnification, × 1).
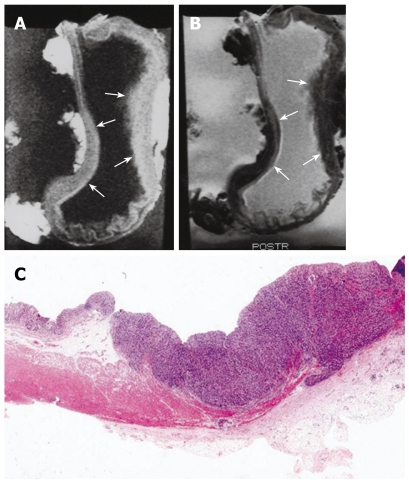
Figure 4.
MRI and histology of T3 gastric adenocarcinoma. A: T1-weighted (500/20) sagittal image showed thickening of gastric wall with all three layer SI change in lesser and greater curvature site of stomach body (arrows); B: T2-weighted (2000/90) sagittal MRI showed minimal increase of SI on lesion site and poor delineation of gastric wall SI at out layer margin compared to normal gastric wall (arrows); C: Light microscopic section showed extension of tumor invasion to serosal layer (HE stain; original magnification, × 1).
Regional lymph node metastasis
The lymph nodes presented with intermediate SI on T1-weighted images, intermediate SI on T2-weighted images. The sizes were measured as being from 0.35 cm to 3.5 cm. We counted 34 lymph nodes in the MRIs. Only 1 lymph node was measured as less than 0.8 cm on its short axis and the other 33 lymph nodes were measured as more than 0.8 cm on their short axis.
One hundred forty lymph nodes were removed from 19 cases of resected gastric specimens. The short axis of resected nodes proved malignant upon pathologic examination ranging from 0.3 to 3.5 cm. Overall metastasis was found in 60 lymph nodes. Degree of lymph node metastasis was also evaluated in 19 cases of gastric carcinoma (Table 2). MRI findings of lymph node metastasis included 6 cases of N0, and 13 of N1 (Figure 5). Pathologic findings of lymph node metastasis included 6 cases of pN0, 10 of pN1 and 3 of pN2 (Figure 6). Four cases of pN0 were over staged as N1 on the MR images. Three cases of pN1 were understaged as N0. Three cases of pN2 were understaged as N1 and N0. The accuracy of N staging by MRI was 47% (9 of 19).
Table 2.
MRI and pathologic correlation of lymph node metastasis
|
Diagnosis at histologic examination | ||||
| Diagnosis at MR | pN0 | pN1 | pN2 | Total |
| N0 | 2 | 3 | 1 | 6 |
| N1 | 4 | 7 | 2 | 13 |
| N2 | ||||
| 6 | 10 | 3 | 19 | |
Figure 5.
MRI of N1 gastric adenocarcinoma. A: T1-weighted (500/20) MR image showed single lymph node on lesser curvature site of stomach body (arrow); B: T2-weighted (2000/90) MRI showed intermediated signal SI of lymph node (arrow).
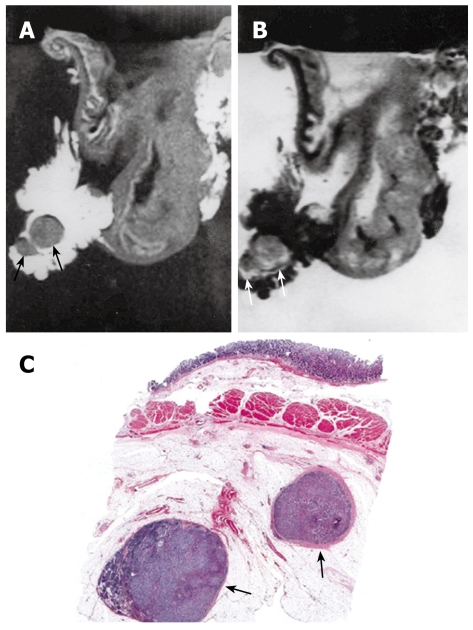
Figure 6.
MRI and histology of N2 gastric adenocarcinoma. A: T1-weighted (500/20) MRI showed two lymph nodes in lesser curvature site of stomach antrum (arrows). Eight lymph nodes are detected in total in perigastric area (not shown); B: T2-weighted (2000/90) MRI showed intermediate SI in the lymph nodes (arrows); C: Light microscopic section showed two lymph nodes in lesser curvature site of gastric antrum (arrows) (HE stain; original magnification, × 1).
DISCUSSION
Imaging modalities, such as CT and endoscopic sonography are performed for the staging of gastric carcinoma. However, the accuracy of staging gastric carcinoma is still controversial in any diagnostic modality and a definitive diagnostic modality has not been established yet. Preoperative staging of gastric carcinoma is limited by the fact that available imaging modalities do not enable accurate evaluation of depth of infiltration of the gastric wall[18–21]. CT imaging is still evaluated for gastric wall invasion and staging of gastric carcinoma. However, the results of CT in local tumor staging are also insufficient, particularly because no reliable anatomic layer differentiation of the gastric wall can be achieved[20]. Without depiction of the wall layers, a secure distinction between T1, T2 and T3 tumors cannot be achieved. Results of early studies concerning CT diagnosis of gastric carcinoma were encouraging; however, findings in later articles were pessimistic about the ability of CT to enable staging of gastric carcinoma[7,9]. Recently, spiral CT has been founded to be more accurate than previous CT studies[22,23].
Endoscopic sonography is effective for detection of lymph node involvement in the perigastric area[24]. Moreover, Botet et al[7] reported the accuracy of the N factor and overall staging with endoscopic sonography to be 78% (39 of 50), which is significantly higher to that examined with the conventional dynamic CT technique. However, there are limitations to endoscopic sonography in the evaluation of distant perigastric lymph node metastasis. Another problem is that endoscopic sonography is invasive and the results are highly operator-dependent.
Interest in the use of MRI for the staging of gastric carcinoma is increasing, but most clinical studies stage the local tumor situation without the differentiation of gastric wall layers[4,8–10]. Studies that use depiction of gastric wall layers as a basis for local tumor staging and lymph node metastasis are rare[13,25].
The high quality of soft-tissue imaging of MR systems enables the depiction of anatomic wall layers. Auh et al[11] studied the gastric wall using an experimental 4.7-T system whereas Lubienski et al[12] used an experimental 2.4-T system. Both groups proved that the depiction of gastric-wall layers is technically possible. Auh et al[11] depicted 3 layers whereas Lubienski et al[12] was able to differentiate 4 layers and correlated them to the mucosa, lamina muscularis mucosa, submucosa and muscularis propria. Typically 3 gastric wall layers are visible. The inner layer corresponds to the mucosa and lamina muscularis mucosa and the middle layer to the submucosa. The outer layer showed the same SI as the muscularis propria in the study of Lubinski’s et al[12] study and therefore mainly consisted of muscle tissue and serosal layers. Palmowski et al[13] demonstrated that a reliable depiction of gastric-wall layers can be achieved by a conventional 1-T MRI. As no subserosa and serosa could be depicted, it must be presumed that they were located on the outer side of the third layer. So the third layer represented the muscularis propria, subserosa, and serosa together[13]. We could demonstrate that the inner and outer layers as hyperintense and the middle layer as hypointense at 1.5-MRI. When the three layers were depicted in the gastric wall, the mucosa and the muscularis propria were clearly different from the intervening submucosal layer on T1-weighted images. The distinction among the layers is based mainly on the lower SI of the submucosa compared with that of the mucosa or muscularis propria. The difference between the three layers was also depicted in the T2-weighted images.
In this study, gastric carcinomas appeared as masses with destruction of the normal structure of the gastric wall or diffuse thickening of the gastric wall and showed intermediate SI compared to surrounding normal gastric walls on T1-weighted images and low SI on T2-weighted images. Both sequences were useful for tumor localization and complement each other because some carcinomas in the study could only be recognized by deviating signal behavior in one of the 2 sequences. In our study, signal characteristics of the carcinoma depending on the MR sequence were not analyzed. Palmowski et al[13] reported that carcinomas show an intermediate SI on T1-weighted images, a low SI on T2-weighted images and a high SI on opposed phase images. Opposed phase images were not obtained in our study, but Dux et al[25] demonstrated that opposed phase images show a very high SI in gastric tumors and insisted that this was useful for the staging of gastric carcinoma. In this study, the infiltration of gastric carcinoma was correctly defined in 74% of the cases. This was not different from that of CT images that had an accuracy rate of 50%-85% and that of MR images that had an accuracy rate of 73%[4,7,26]. Yamada et al[27] reported that gastric specimens that were imaged after fixation in formalin and then MR imaged could also depict early gastric carcinoma. In this study, one case of early gastric carcinoma was depicted on MRI with a shallow depressed wall. This was made possible by adequate distention of the resected stomach with saline. To our knowledge, this is the first MRI depiction of early gastric carcinoma using gastric specimens without fixation in formalin. In this study, unfortunately, cases with pathologic T4 were not included because most patients who were diagnosed as T4 on preoperative imaging studies did not undergo surgery.
The evaluation of lymph node metastasis on MRIs had some limitation in this study, since the size criteria was used only on MRIs and there was no trial of contrast enhancement because of in vitro study of gastric carcinoma. Lymph node borders and signal intensity were not also evaluated for diagnosis of lymph node metastasis. But some cases of lymph nodes showed intermediate SI on T1 and T2-weighted images in the tumor infiltration region and this was correctly correlated with the histology. One-to-one pathologic-to-radiologic correlation on each lymph node was not performed in our study. According to Dux et al[25] study, lymph nodes showed a high SI on opposed phased images. MRI had low rate in depicting lymph node metastasis, with an accuracy of 47%. However, the result was similar to the other reports[25,27]. Further study is needed to increase accuracy in the finding of lymph node metastasis in gastric carcinoma.
In conclusion, the present study demonstrated that MRI can reliably depict several anatomical layers of the gastric wall and also MRI of gastric carcinoma could enable accurate diagnosis of location, gross appearance, degree of gastric wall invasion of the tumors and delineation of regional lymph node metastasis. A clear image of the tumor can be achieved. Therefore, an evaluation of the local tumor stage of gastric carcinoma and perigastric lymph node metastasis based on morphologic criteria is technically possible. This study, using a conventional 1.5-T MRI in combination with standard sequences, demonstrated the potential of MRI in the staging of gastric carcinomas. Although the result obtained in N-staging was not acceptable, it should be explored further. However, we were able to show not only MR findings of gastric wall invasion but also perigastric lymph node involvement in the gastric carcinoma. The results of our study cannot, at this time, be transferred to clinical practice, because conventional imaging acquisition techniques do not provide an image quality comparable to that of those taken of in vitro specimens. Advances in the variation of sequence techniques, as well as application of ultrafast imaging techniques, may in the future allow preoperative staging of gastric carcinomas by MRI.
COMMENTS
Background
The preoperative staging workup of gastric cancer is performed mainly with computed tomography (CT). Magnetic resonance imaging (MRI) has not become popular for staging because of a number of limitations, including motion artifacts, lack of a suitable oral contrast medium, and the high cost. However, continuous technical improvements have been made in MRI of the abdomen, thereby reducing motion artifacts and improving image quality.
Research frontiers
MRI has not yet reached clinical importance because of some limitations. However, in vitro studies using experimental 2.4-4.7-T MR systems have shown that MRI allows the depiction of gastric wall layers and therefore technically permits the evaluation of the local tumor stage of gastric cancer.
Innovations and breakthroughs
MRI in the staging of gastric cancer is not usually applied in clinical practice. Its results are the same or inferior to CT in accuracy. There are many MRI in vitro and clinical imaging gastric cancer studies. In vitro MRI systems demonstrate well gastric wall layers and tumor invasion well and MRI has shown tumor invasion and lymph node metastasis in some clinical studies as well. However, normal gastric wall depiction and tumor invasion of the gastric wall are important to tumor staging, as are demonstrations of perigastric lymph node metastasis. The authors studied resected gastric specimens for the accurate depiction of normal gastric walls, tumor invasion and lymph node metastasis using 1.5-T MRI.
Applications
The study results suggest that MRI could be useful in gastric wall invasion in the staging of gastric cancer. However, further study in the staging of lymph node metastasis is still needed.
Peer review
The authors successfully demonstrated normal gastric wall layers and tumor invasion in their in vitro study using 1.5-T MRI. The results suggest that MRI is a potential diagnostic tool in the staging of gastric cancer.
Peer reviewer: Kiichi Tamada, MD, Department of Gastroenterology, Jichi Medical Shool, 3311-1 Yakushiji, Minamikawachi, Kawachigun, Tochigi 329-0498, Japan
S- Editor Tian L L- Editor Alpini GD E- Editor Ma WH
References
- 1.Sussman SK, Halvorsen RA Jr, Illescas FF, Cohan RH, Saeed M, Silverman PM, Thompson WM, Meyers WC. Gastric adenocarcinoma: CT versus surgical staging. Radiology. 1988;167:335–340. doi: 10.1148/radiology.167.2.3357941. [DOI] [PubMed] [Google Scholar]
- 2.Komaki S, Toyoshima S. CT's capability in detecting advanced gastric cancer. Gastrointest Radiol. 1983;8:307–313. doi: 10.1007/BF01948140. [DOI] [PubMed] [Google Scholar]
- 3.Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, Kim TS, Lee JD, Noh SH, Kim KW. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143–156. doi: 10.1148/rg.261055078. [DOI] [PubMed] [Google Scholar]
- 4.Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol. 2000;174:1551–1557. doi: 10.2214/ajr.174.6.1741551. [DOI] [PubMed] [Google Scholar]
- 5.Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993;72:1631–1636. doi: 10.1002/1097-0142(19930901)72:5<1631::aid-cncr2820720523>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Miller FH, Kochman ML, Talamonti MS, Ghahremani GG, Gore RM. Gastric cancer. Radiologic staging. Radiol Clin North Am. 1997;35:331–349. [PubMed] [Google Scholar]
- 7.Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:419–425. doi: 10.1148/radiology.181.2.1924783. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg HI, Thoeni RF. MRI of the gastrointestinal tract. Radiol Clin North Am. 1989;27:805–812. [PubMed] [Google Scholar]
- 9.Halvorsen RA Jr, Thompson WM. Primary neoplasms of the hollow organs of the gastrointestinal tract. Staging and follow-up. Cancer. 1991;67:1181–1188. doi: 10.1002/1097-0142(19910215)67:4+<1181::aid-cncr2820671513>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Campeau NG, Johnson CD, Felmlee JP, Rydberg JN, Butts RK, Ehman RL, Riederer SJ. MR imaging of the abdomen with a phased-array multicoil: prospective clinical evaluation. Radiology. 1995;195:769–776. doi: 10.1148/radiology.195.3.7754009. [DOI] [PubMed] [Google Scholar]
- 11.Auh YH, Lim TH, Lee DH, Kim YH, Lee MG, Cho KS, Mun CW, Lee I. In vitro MR imaging of the resected stomach with a 4.7-T superconducting magnet. Radiology. 1994;191:129–134. doi: 10.1148/radiology.191.1.8134558. [DOI] [PubMed] [Google Scholar]
- 12.Lubienski A, Grenacher L, Reith W, Schipp A, Mechtersheimer G, Dux M. [MR imaging of gastric wall layers in vitro: correlation to thehistologic wall structure] Rofo. 2002;174:490–494. doi: 10.1055/s-2002-25116. [DOI] [PubMed] [Google Scholar]
- 13.Palmowski M, Grenacher L, Kuntz C, Heye T, Dux M. Magnetic resonance imaging for local staging of gastric carcinoma: results of an in vitro study. J Comput Assist Tomogr. 2006;30:896–902. doi: 10.1097/01.rct.0000224624.17574.27. [DOI] [PubMed] [Google Scholar]
- 14.Douglass HO Jr, Nava HR. Gastric adenocarcinoma--management of the primary disease. Semin Oncol. 1985;12:32–45. [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer. Cancer staging handbook. Philadelphia. Pa: Springer-Verlag; 2002. Available from: URL: www.cancerstaging.net. [Google Scholar]
- 16.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–139. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 17.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 18.Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc. 2000;14:951–954. doi: 10.1007/s004640010040. [DOI] [PubMed] [Google Scholar]
- 19.Bosing N, Schumacher B, Frieling T, Ohmann C, Jungblut R, Lubke H, Bohner H, Verreet P, Roher HD. [Endoscopic ultrasound in routine clinical practice for staging adenocarcinomas of the stomach and distal esophagus] Chirurg. 2003;74:214–221; discussion 222-223. doi: 10.1007/s00104-002-0521-6. [DOI] [PubMed] [Google Scholar]
- 20.Dux M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW. Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr. 1999;23:913–922. doi: 10.1097/00004728-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Yanai H, Noguchi T, Mizumachi S, Tokiyama H, Nakamura H, Tada M, Okita K. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361–365. doi: 10.1136/gut.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, Lee MS, Kim BS. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004;59:619–626. doi: 10.1016/s0016-5107(04)00169-5. [DOI] [PubMed] [Google Scholar]
- 23.Habermann CR, Weiss F, Riecken R, Honarpisheh H, Bohnacker S, Staedtler C, Dieckmann C, Schoder V, Adam G. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–471. doi: 10.1148/radiol.2302020828. [DOI] [PubMed] [Google Scholar]
- 24.Akahoshi K, Misawa T, Fujishima H, Chijiiwa Y, Nawata H. Regional lymph node metastasis in gastric cancer: evaluation with endoscopic US. Radiology. 1992;182:559–564. doi: 10.1148/radiology.182.2.1732981. [DOI] [PubMed] [Google Scholar]
- 25.Dux M, Roeren T, Kuntz C, Schipp A, Scheller D, Mechtersheimer G, Kauffmann GW. MRI for staging of gastric carcinoma: first results of an experimental prospective study. J Comput Assist Tomogr. 1997;21:66–72. doi: 10.1097/00004728-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kim AY, Han JK, Seong CK, Kim TK, Choi BI. MRI in staging advanced gastric cancer: is it useful compared with spiral CT? J Comput Assist Tomogr. 2000;24:389–394. doi: 10.1097/00004728-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Yamada I, Saito N, Takeshita K, Yoshino N, Tetsumura A, Kumagai J, Shibuya H. Early gastric carcinoma: evaluation with high-spatial-resolution MR imaging in vitro. Radiology. 2001;220:115–121. doi: 10.1148/radiology.220.1.r01jl10115. [DOI] [PubMed] [Google Scholar]