Abstract
AIM: To study the stability of portal hypertension (PHT) caused by partial ligation of the portal vein ligation (PVL) in a rat model.
METHODS: Thirty male adult Wistar rats were divided into two groups: 10 in Group I received a sham operation; and 20 in Group II received partial PVL. Portal vein pressure (PVP) was measured at four time periods: before ligation, 2 wk, 6 wk and 10 wk post-surgery. Portal venography, blood sampling and liver and spleen pathological examinations were conducted at 10 wk after surgery.
RESULTS: The PVP was 9.15 ± 0.58 cmH2O before ligation, and increased to 17.32 ± 0.63 cmH2O 2 wk after PVL. By repeat measurement of the PVP in each rat, it was shown to remain elevated for 10 wk. There were no significant differences in the pressure measurements at 2 wk, 6 wk and 10 wk. Varices were found mainly in the mesenteric vein 2 wk after PVL, which were more obvious later, while these manifestations were similar at week 6 and week 10. Portal venography demonstrated the varices and collaterals. There was no significant change in liver pathology. The volume of the spleen was enlarged 2-fold after ligation, and the sinus of the spleen was enlarged due to congestion. Significant sinus endothelial cell proliferation was observed, but no evidence of hypersplenia was found on hemogram and biochemical examination.
CONCLUSION: These findings suggest that a satisfactory prehepatic PHT rat model can be obtained by partial ligation of the portal vein, and this PHT rat model was stable for at least 10 wk.
Keywords: Ligation, Portal hypertension, Portal vein, Rat
INTRODUCTION
A rat model of prehepatic portal hypertension (PHT) produced by partial portal vein ligation (PVL) is an important tool in studying PHT. The PVL model has been used to study alterations in the splanchnic circulation and in the pathophysiology of the hyperdynamic circulation after ligation[1,2]. However, from the literature almost all of these studies were carried out around 2 wk after PVL; and are rarely reported beyond this time period. It is suspected that elevated portal vein pressure (PVP) can last for a longer period of time. The aim of this study was to determine the stability of artificially elevated PVP using rigidly controlled experiments. Furthermore, we used this model to evaluate the effect of a newly designed spleno-hepatopexy in PHT treatment.
MATERIALS AND METHODS
Thirty male Wistar rats weighing 280-320 g (average 302 g) were kept under temperature controlled conditions and an artificial 12-h light-dark cycle. They were allowed standard chow and water ad libitum. All animals and procedures were approved by the Ethics Committee of the Animal Experiment Center of Capital Medical University.
Animals were randomized into two groups: Ten rats in Group I had a sham operation; and twenty rats in Group II had partial portal-vein ligation. PVP was measured at four time periods: before ligation, 2 wk, 6 wk, and 10 wk after surgery. Portal venography was performed in rats at 10 wk. Other parameters measured included: weight and volume of spleen, hemogram, biochemical parameters and pathologic examination of the liver and spleen.
Model establishment
Animals were anesthetized with intramuscular administration of 2% pentobarbital solution. The model was established according to Harvolsen and Myking[3,4]. A 1.5 cm midline incision was made and the portal vein was exposed. The diameter of the portal vein was measured by Vernier calipers at the point where the ligation would be carried out. A No.4 silk thread was placed around the portal vein together with a pre-placed 21G blunt-tipped needle lying along the portal vein. By tying the ligature snugly beyond the left gastric vein, and then pulling out the needle, a stenosis of the portal vein with a constriction corresponding to the thickness of the pre-placed needle was left behind.
PVP measurement
After anesthesia, a midline incision was made. A segment of the mesenteric branch vein was cannulated with a 24-g cannula needle, and the tip of the cannula was advanced just into the trunk of the superior mesenteric vein. The PVP was recorded, via a pressure transducer, by the BL-420E+ biophysical function experiment system (manufactured by Taimeng Technology Limited Company, Chengdu, China). Pressure measurement lasted for 3 min, and the average value was regarded as the PVP. To maintain stable anesthesia, the measurement was started 20 min after the injection of pentobarbital.
Portal venography
A midline incision was made after anesthesia; the ileocolic vein was cannulated with a 20-g cannula, and the tip of the cannula was advanced into the lower part of the portal vein just above the entrance to the splenic vein. Seventy-six percent meglumine diatrizoate was injected at a speed of 2 mL/5 s, and the intrahepatic and extrahepatic portal vein system could be seen.
Blood samples and liver, spleen pathological examination
At week ten, blood samples from the rats were obtained for hemogram and biochemical examination. Both liver and spleen biopsy were taken for pathological examination, (using Hematoxylin-eosin staining). The rats were sacrificed at the end of the experiment.
Statistical analysis
The data were expressed as mean ± SD. Repeated analysis of variance was used to compare the differences between groups by SPSS 11.5 software. Values of P < 0.05 were considered significant.
RESULTS
Constriction rate of the portal vein
The average diameter of the portal vein before ligation was 2.40 ± 0.18 mm, and the outer diameter of the 21-g needle was 0.80 mm. According to the formula, the constriction rate = (1-лr2/лR2) × 100%[5] = 88.9%.
Survival rate of animals
None of the rats in Group I died, while 4 rats in Group II died after PVL. The survival rate was 80% (16/20). Three rats died within 24 h of ligation, the other rat died between 24 and 48 h after surgery. Autopsy found severe congestion of the mesenteric and splenic veins, as well as marked cyanosis of the gut and spleen. No mortality occurred after this period.
PVP
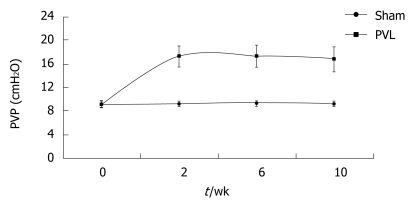
Partial ligation of the portal vein resulted in an immediate increase in PVP up to 25-30 cmH2O. Two weeks after surgery, PVP dropped to about 17.32 cmH2O, which was about twice the value of the control (P < 0.01). The pressure at week 6 and week 10 was maintained at a similar level, with no significant difference compared with the value for week 2. PVP in Group II was significantly higher than that in Group I. (Table 1 and Figure 1).
Table 1.
PVP changes (cmH2O), (mean ± SD)
| Groups | wk-0 | wk-2 | wk-6 | wk-10 |
| G-I (SO, n = 10) | 9.15 ± 0.58 | 9.22 ± 0.49 | 9.27 ± 0.43 | 9.21 ± 0.49 |
| G-II (PVL, n = 16) | 9.21 ± 0.63 | 17.32 ± 1.77a | 17.36 ± 1.93a,b | 16.82 ± 2.20a,b |
Portal vein pressure (PVP) changes at four time periods were compared.
P < 0.001 vs value of G-I;
P < 0.001 vs value of wk-2 in G-II. wk-0: before ligation; wk-2, wk-6, wk-10: 2 weeks, 6 weeks and 10 weeks post-surgery, respectively.
Figure 1.
Pressure tendency of PVP after PVL.
Varices and collateral circulation after PVL
Gross observations: In Group I, in the mesenteric vein, there was no distortion, no varices and no visible collaterals. In Group II, in the mesenteric and gastric veins, varices were found mainly in the mesenteric vein 2 wk after PVL, and collaterals could be seen mainly between the spleen and left kidney. The left adrenal vein was markedly engorged. Collaterals could also be seen between the inferior mesenteric vein and the posterior peritoneum (Figures 2 and 3). Collateral vessels were found in the porta hepatis in some rats. These manifestations were more obvious later, and were similar at week 6 and week 10. The spleen was markedly enlarged 2 wk after surgery with some white fibrin deposits on the splenic capsule.
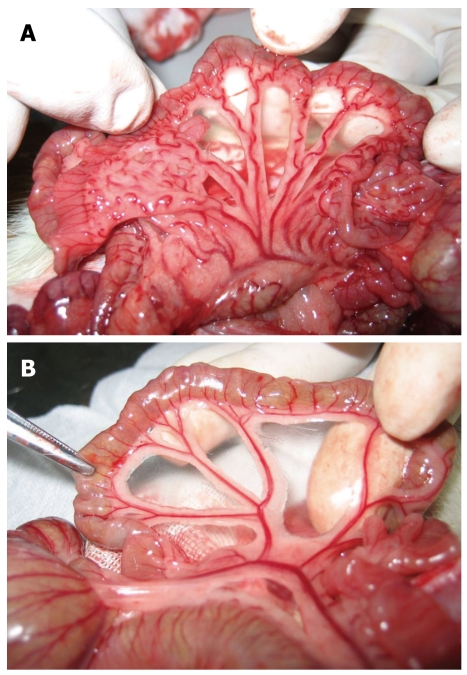
Figure 2.
Changes in the mesenteric vein. A: There was no distortion, no varices, no visible collaterals in the mesenteric vein before portal vein ligation; B: Varices were found mainly in the mesenteric vein 2 wk after portal vein ligation.
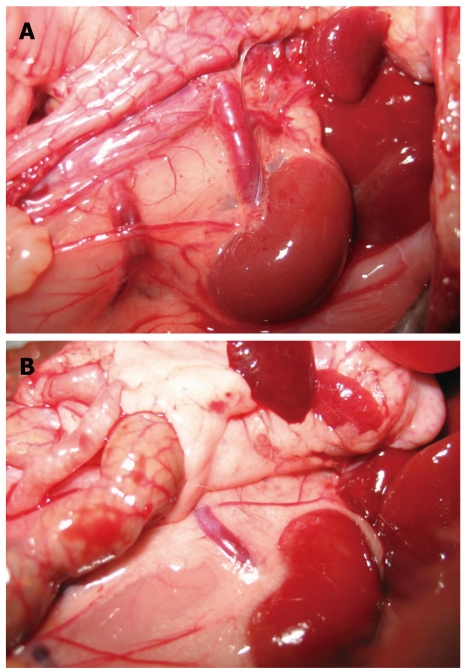
Figure 3.
Collaterals after PVL. A: In the control rat, the left renal vein was thin and of normal size, no collateral vessels could be seen between the spleen and the left kidney; B: Collaterals could be seen mainly between the spleen and the left kidney 10 wk after PVL. The left adrenal vein was markedly engorged. The collaterals could also be seen between the inferior mesenteric vein and the posterior peritoneum.
Portal venography
Group I: An angiographic study revealed normal mesenteric and portal vein image patterns in control rats. The intrahepatic portal vein was shown as tree twig branches. There was only a little contrast medium in the mesenteric vein and the splenic vein. After ligation of the portal vein, images of the superior and inferior mesenteric veins and the splenic vein appeared simultaneously, but no collateral circulation could be seen (Figure 4).
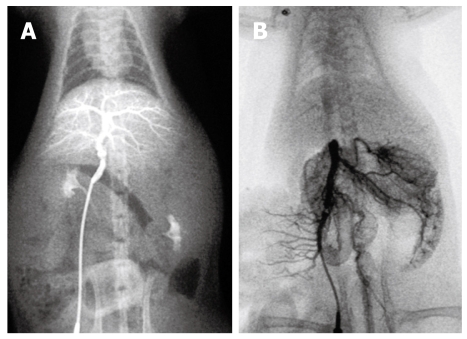
Figure 4.
Portal venography in control rat. A: The intrahepatic portal vein was shown as tree twig branches. There was little contrast medium in the mesenteric vein and the splenic vein; B: After ligation of the portal vein, images of the superior and inferior mesenteric vein and the splenic vein appeared simultaneously, but no collateral circulation could be seen.
Group II: At week 10, portal venography showed the mesenteric vein with varices and collaterals, and a lot of the contrast medium was seen in the vena cava, which indicated the establishment of the collateral circulation. These observations were not seen in the control rats. Following PVL, the left adrenal vein was clearly seen and had an enlarged diameter, while this vein was not seen in the control group. On continuous observation, some contrast medium was seen in the portal vein system which diffused via the left adrenal vein into the left renal vein and the vena cava. Additionally, collateral vessels between the inferior mesenteric vein and the posterior peritoneum were also seen in the angiograph. No obvious esophageal varices were found. Contrast medium in the liver decreased significantly (Figure 5).
Figure 5.
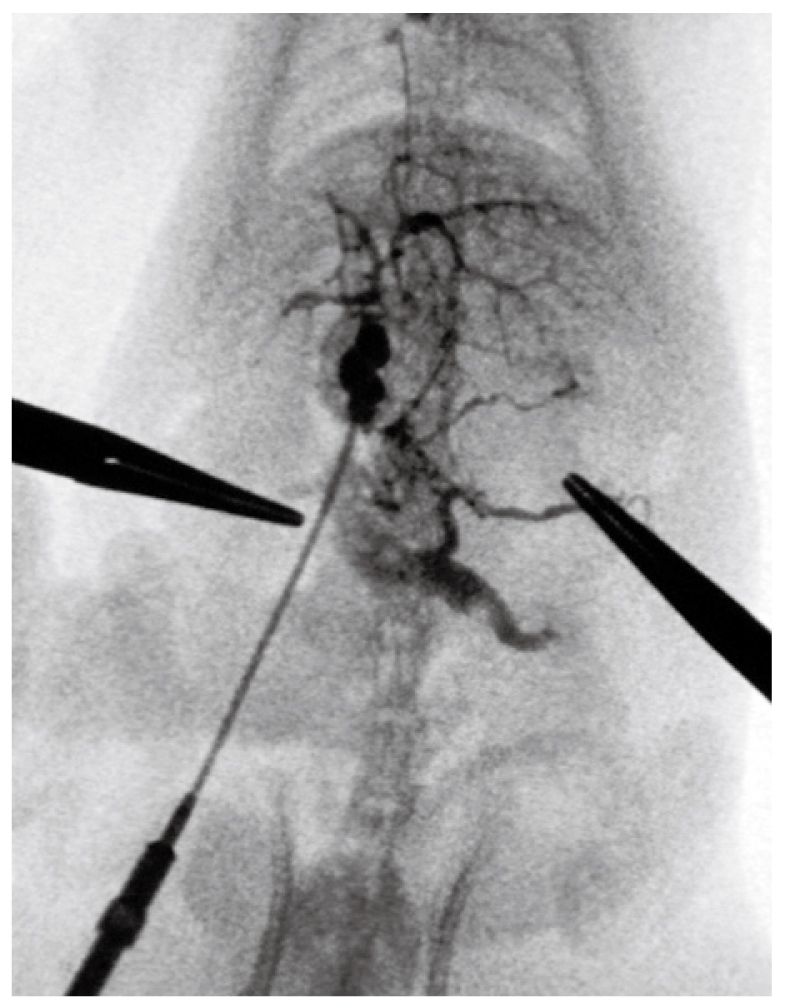
At week 10, portal venography showed the mesenteric vein with varices and collaterals. A lot of contrast medium was seen in the vena cava, which indicated the establishment of the collateral circulation. These observations were not seen in the control rats. The left adrenal vein after PVL was clearly shown with an enlarged diameter, while this vein was not seen in the control group.
Splenic volume
The splenic volume was slightly increased when the weight of the animal increased in Group I, and was almost twice the original size in Group II after PVL. The difference in volume in Group II was significantly different from that in Group I (P < 0.01) (Table 2).
Table 2.
Splenic volume changes (cm3), (mean ± SD)
| Groups | wk-0 | wk-2 | wk-6 | wk-10 |
| G-I (SO, n = 10) | 1.90 ± 0.28 | 1.93 ± 0.24 | 2.03 ± 0.23 | 2.04 ± 0.25 |
| G-II (PVL, n = 16) | 2.07 ± 0.40 | 3.94 ± 1.33a,b | 4.07 ± 1.37a,b | 4.17 ± 1.44a,b |
P < 0.01 vs value of G-I;
P < 0.01 vs value of wk-0 in G-II. wk-0: before ligation; wk-2, wk-6, wk-10: 2 weeks, 6 weeks and 10 weeks post-surgery, respectively.
Hemogram and biochemical parameters
There were no significant differences between Group I and II in hemogram and biochemical parameters. No evidence of hypersplenia was found.
Pathology study
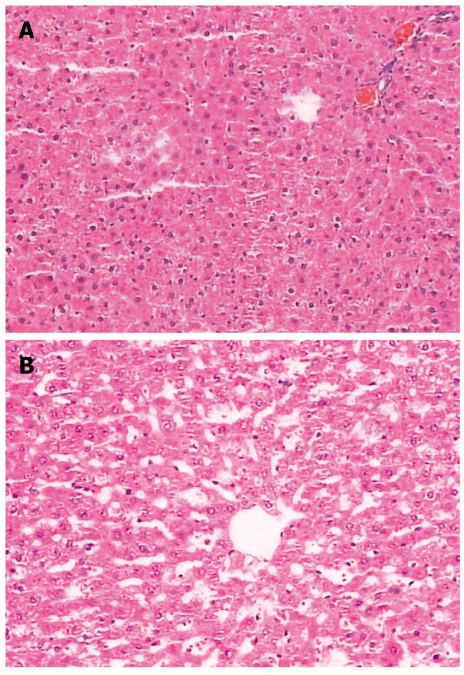
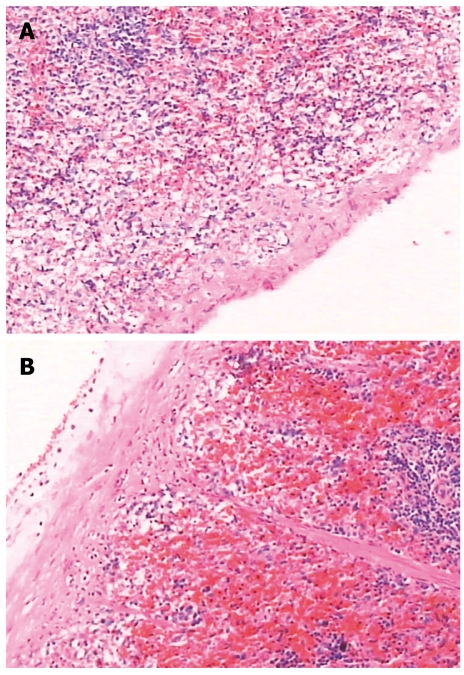
There was no significant change in liver pathology. The spleen capsule was thickened, the sinus of the spleen was enlarged due to congestion, there was significant sinus endothelial cell proliferation, and the spleen trabeculae were widened. The white pulp and germinal center had significant atrophy (Figures 6 and 7).
Figure 6.
There were no significant changes in liver pathology. A: Pathologic image of rat in Group-I; B: Image of Group-II.
Figure 7.
Pathologic image of the spleen. A: Group-I; B: Group-II, the capsule was thickened, the spleen sinus was enlarged due to congestion, there was significant sinus endothelial cell proliferation, and the spleen trabeculae were widened. The white pulp and germinal center had significant atrophy.
DISCUSSION
The rat model of PHT produced by partial PVL is an important tool in studying PHT. It is fast, economic, simple and repeatable. Some authors have applied the model to the study of alterations in splanchnic circulation and the pathophysiology of the hyperdynamic circulation[1]. However, most of these studies were short-term experiments, which were carried out 2-3 wk after ligation. There are few reports of long-term experiments, two months or more after ligation. Some authors even doubt the long-term stability of the model, and are concerned that PVP will drop to the normal level due to establishment of the collateral circulation. For experiments on the surgical treatment of PHT, such as spleno-hepatopexy, a long-term stable model is needed. The aims of the present study were to prove the stability of the PHT rat model under rigidly controlled experimental conditions, and to prove that this model is suitable for evaluating the effect of our newly designed spleno-hepatopexy for PHT treatment. For spleno-hepatopexy, a peripheral cut on the surface of the spleen and the same on the liver are made which adhere together, it is hoped that the high pressure blood in the spleen will diffuse into the liver through the collateral circulation instead of porto-systemic venous shunting to allow detoxification of the liver.
In our experiment, the PVP value before ligation and in control animals was within the same range as that found by other investigators[4,6,7]. The establishment of a partial PVL resulted in instant congestion in the portal vein system, and the PVP immediately increased to 25-30 cmH2O. At week 2 after surgery, following compensation by the animal itself, the pressure dropped to about 17 cmH2O. In subsequent observations from week 2 to week 10, the elevated PVP value was maintained at the increased level of about 17 cmH2O, which was about twice the level before ligation.
Several factors affected the results of these experiments. The portal vein constriction rate is a critical factor in rat survival in the PHT model. After complete portal vein ligation, the portal vein system was suddenly blocked, and this resulted in a serious blood deficiency in the circulation, and the rat died within one hour. For partial ligation, the survival rate following PVL was positively related to the constriction rate[4]. We obtained different constriction rates by using different-sized needles. With a 26-g (diameter = 0.45 mm) or a 23-g needle (diameter = 0.6 mm), all the rats died within 1-2 h after ligation. Using a 22-g needle (diameter = 0.7 mm), mortality was about 80%, which was obviously not acceptable for these experiments. Using a 21-g needle (diameter = 0.8 mm), the constriction rate was 88.9%, and the mortality dropped to 20%-30%, and was acceptable for this model of PTH. By decreasing the constriction rate using a 19-g needle (diameter = 1.0 mm), the survival rate was increased, but the PVP did not reach an ideal level to meet the experimental demand.
Constriction of the portal vein resulted in increased resistance in the portal vein, and therefore increased PVP. The resistance of the portal vein was mainly determined by the constriction rate of the portal vein in the experiment. Other factors such as the thickness of the thread were also important. We tried different sizes of thread in our preliminary experiments, including 3-0, 1-0 and No.1, No.4 and No.7 silk. For thin thread less than No.1, the PVP was unstable, and the pressure was usually low, because the resistance of a tube is positively related to the obstructive length. However, for thread thicker than No.7 silk, it was difficult to make a tight knot on such a small portal vein of nearly 1.0 mm. Eventually, we selected the No.4 thread and obtained satisfactory results. Some authors use triple-ligation on the portal vein to obtain the PVL model. Additionally, right ligation against the portal vein axis is also quite important. The strength used for ligation is important and should be the same in every experiment. Too much strength could damage the portal vein, which may result in thrombosis, while too little strength would be too loose and inaccurate for standard constriction. Cleaning the surrounding tissue next to the portal vein is also useful to reduce errors in constriction diameter.
There have been different reports on the PVP level after PVL. Orda et al[8] induced PVL in a rat model with an elevated pressure for 2-3 wk, which then dropped to normal. This was thought to be due to a large amount of collateral circulation formation. Canty[9] summarized the previous experience and thought that simple ligation or application of a meroid constrictor to the portal vein could only maintain a high pressure for 4-6 wk. He designed a method of using the ameroid constrictor accompanied by portal lymph node ligation, and hence a high portal pressure could be maintained for about 12 months. Myking et al[3] and Halvorsen et al[7] performed serial experiments on the PVL rat model, and successfully elevated PVP for 12 mo after simple ligation, and was considered a stable and repeatable rat model.
The difference in results in the literature might be due to multiple factors, e.g. the animal strain and body weight. In a very low weight rat, a stable elevated pressure is difficult to maintain[3]. With regard to the constriction rate, this might be influenced by the size of the needle, the thickness of the thread, and the design of the experiments.
In the PVL model of Myking and Halvorsen[3,7], only one pressure measurement was carried out for each rat. In our experiment, four measurements were carried out for each rat. By this method, we could observe the PVP changes in every rat, which was much more accurate and reliable than a single measurement. Following surgery, there was little adhesion in the rat abdominal cavity except at the local area of ligation or puncture. Because the mesenteric vein branch can be used repeatedly, multiple measurements were possible for each rat.
The peak PVP level after PVL was usually 24 h post-surgery[10]. Under natural compensation, the collateral circulation is established to release the high pressure. It was proved that the collateral circulation could be observed two days after PVL, and was fully established 3-4 wk post-surgery[10]. The diverted volume of portal vein blood flow was about 95%[3]. In our experiments, we also observed that varices appeared in the mesenteric vein 2 wk after surgery. The collateral vessels between the spleen and left kidney, between the inferior mesenteric vein and the posterior peritoneum, and at the site of porta hepatis were also observed at this time, but were more apparent at 6 wk after ligation. The manifestations at week 10 were similar to those at week 6. Portal venography further proved the establishment of the collateral circulation. The left adrenal vein was significantly enlarged and became an important shunting vessel between the splanchnic circulation and the systemic circulation.
Some researchers[1] have studied the splanchnic and systemic hemodynamics in the PVL rat model. They found that, after 2 wk of PVL, the rats with portal hypertension and greater than 93% portal-systemic shunting had an increase in portal venous inflow of 50%, and a concomitant 40% decrease in splanchnic arteriolar resistance. Cardiac index was elevated by 50%, and total peripheral resistance was decreased by 40%. The resistance to portal blood flow in portal vein-constricted rats was similar to that in control rats, indicating that the hyperdynamic portal venous inflow, and not resistance, was the mainstay of the elevated portal venous pressure. Some researchers[10] have suggested that many vessel-activated substances which accumulate in the systemic system, without inactivation by the liver, were the direct cause of the hyperdynamic circulation. Therefore, it is considered that, in the early period of PVL, portal vein obstruction is the direct cause of elevated PVP, but with the subsequent establishment of the collateral circulation, the hyperdynamic circulation becomes the important factor in maintaining the elevated pressure. Although portal-systemic shunting can reach to more than 90%, the PVP can still maintain an elevated level.
Other features of the PVL portal hypertensive rat model were noted: Hepatic function remained normal, which is similar to the clinical manifestation of PHT. Although the spleen volume increased almost 2-fold due to enlargement and congestion of splenic sinusoids, proliferation of sinus endothelial cells, hypersplenism was not shown by hemogram, which was similar to findings in the dog PHT model[5]. The reason for this may be due to species and anatomy differences to that in humans, or may be due to the short observation period.
In conclusion, portal vein partial ligation increases PVP to around twice the normal value and can be maintained for more than 10 wk. This method can provide a satisfactory model for investigating the surgical treatment of PHT.
COMMENTS
Background
Portal hypertension (PHT) is a serious disease in children. In about half of children with PHT it is prehepatic. However, the results of surgery for PHT are unsatisfactory. To investigate and to improve the results of surgery, a long-term stable animal model of PHT is needed. Partial portal vein ligation is a good method of producing a prehepatic PHT rat model. The technique is fast and economic. However, researchers have had different experiences in the long-term stability of the model. Some authors have suggested that the portal vein pressure (PVP) would drop to normal 2 wk after ligation, but others think that the models would be stable for more than a year.
Research frontiers
Several types of PHT animal models have already been established. But focusing on the long-term stability of these models it is still a frontier of research.
Innovations and breakthroughs
In their experiments, the prehepatic PHT rat model was proved to be stable for more than 10 wk. The anatomic features of this model were described in detail. Moreover, it is interesting that almost no adhesion in the abdominal cavity and no blood thrombosis in the mesenteric vein were found after repeated operations. Thus, it is possible to measure the PVP repeatedly in one rat, providing a better comparison in the same animal. This is superior to the method commonly used, i.e. a single measurement in each experimental rat.
Applications
The prehepatic PHT rat model is quite useful in the study of PHT, as the alterations in the splanchnic circulation and the pathophysiology of the hyperdynamic circulation can be studied. It is a fast and economic model and can be widely used in this research field. In addition, it can also be used in clinical research, e.g. a new design for spleno-hepatopexy, and to explore new surgical techniques for PHT in children.
Terminology
Portal hypertension: A series of syndromes with abnormal circulation and increased blood pressure in the portal system. According to its pathology, PHT can be divided into three types, prehepatic, intrahepatic and posthepatic. Varices: Abnormally dilated/stretched veins, frequently caused by the development of portal collateral vessels as a result of portal hypertension. It often occurs in the portal system, such as the mesenteric vein and the splenic vein. The most clinically important varices in humans are found in the esophagus and stomach-submucosal varices of the lower esophagus or gastric fundus sub-mucosa. Spleno-hepatopexy: Is a newly designed surgical procedure which allows communication of the spleen with the liver to establish a compensatory collateral circulation by-pass which crosses over the blockage of the portal vein. Thus, the blood in the distal portal system may be drawn into the liver, instead of into the systemic circulation allowing liver detoxification. This surgical technique would be beneficial for patients with prehepatic portal hypertension, especially in children.
Peer review
The study by Dr. Wen et al, examines the natural history of portal hypertension in a rat model subjected to partial portal vein occlusion. It may be worth the authors effort as the paper provides valuable information on a common animal model of portal hypertension.
Supported by National 10th 5-year Science Research Plan of China, No. 2001BA705B10-15
Peer reviewers: Naofumi Mukaida, MD, PhD, Chairperson and Professor, Division of Molecular Bioregulation, Cancer Research Institute, Kanazawa University, 13-1 Takara-machi, Kanazawa 920-0934, Japan; Mercedes Susan Mandell, MD, PhD, Department of Anesthesiology, University of Colorado Health Sciences Ctr., 12401 E. 17th Ave, B113 Aurora, CO 80045, United States
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
References
- 1.Abraldes JG, Pasarin M, Garcia-Pagan JC. Animal models of portal hypertension. World J Gastroenterol. 2006;12:6577–6584. doi: 10.3748/wjg.v12.i41.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CT, Kuang YL, Chen ZP. [Hyperdynamic status in a partial portal vein ligated (PVL) rat's portal hypertension model] Zhonghua Waike Zazhi. 1994;32:573–575. [PubMed] [Google Scholar]
- 3.Halvorsen JF, Myking AO. Prehepatic portal hypertension in the rat. Immediate and long-term effects on portal vein and aortic pressure of a graded portal vein stenosis, followed by occlusion of the portal vein and spleno-renal collaterals. Eur Surg Res. 1979;11:89–98. doi: 10.1159/000128055. [DOI] [PubMed] [Google Scholar]
- 4.Myking AO, Halvorsen JF. Repreoducibility of a method for a graded stenosis in tubes and vessels of small calibres. An in vitro and in vivo experiment. Eur Surg Res. 1979;11:81–88. doi: 10.1159/000128054. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L, Li ZD, Yu ZW, Sun JS, Zhong ZY. Experience of producing a prehepatic portal hypertension model in dog. Zhonghua Xiaoer Waike Zazhi. 2001;22:42–44. [Google Scholar]
- 6.Polat D, Rizalar R, Tander B, Yildiz L, Ariturk E, Bernay F. Effects of splenohepatopexy and omentopexy in experimentally induced infrahepatic portal hypertension in rats. Pediatr Surg Int. 2004;20:434–438. doi: 10.1007/s00383-004-1171-1. [DOI] [PubMed] [Google Scholar]
- 7.Halvorsen JF, Myking AO, Tvete S. Portohepatic bypass by splenohepatopexy in rats with prehepatic portal hypertension. A long-term (12-month) study of the development of splenohepatic collaterals and their effect on portal vein pressure. Eur Surg Res. 1982;14:409–419. doi: 10.1159/000128315. [DOI] [PubMed] [Google Scholar]
- 8.Orda R, Ellis H. Self-established porto-caval and porto-pulmonary shunts in mechanically induced portal hypertension. An experimental study. Eur Surg Res. 1978;10:172–183. doi: 10.1159/000128006. [DOI] [PubMed] [Google Scholar]
- 9.Canty TG, Jauregizar E, Fernandez-Cruz L. Experimental portal hypertension in the rat. J Pediatr Surg. 1980;15:819–826. doi: 10.1016/s0022-3468(80)80287-9. [DOI] [PubMed] [Google Scholar]
- 10.Sikuler E, Kravetz D, Groszmann RJ. Evolution of portal hypertension and mechanisms involved in its maintenance in a rat model. Am J Physiol. 1985;248:G618–G625. doi: 10.1152/ajpgi.1985.248.6.G618. [DOI] [PubMed] [Google Scholar]