Abstract
Factor IX is a key component of the plasma system that forms a fibrin clot at a site of vascular injury. Activation of factor IX by factor XIa is required in certain situations to prevent bleeding from premature clot degradation. Factor XIa is a coagulation protease comprised of two identical subunits. The biochemical and physiologic implications of this unusual structural feature are being actively investigated. Congenital factor XI deficiency causes a mild-to-moderate bleeding disorder, with hemorrhage typically involving the oral/nasal cavities and the urinary tract. Current treatment recommendations take this tissue-specific bleeding pattern into account and target factor replacement to certain types of procedures and clinical situations. Results from animal models and human population studies indicate that factor XI contributes to thromboembolic disease. This protease may therefore be a legitimate therapeutic target.
Keywords: factor IX, factor VIIa, factor XIa, tissue factor
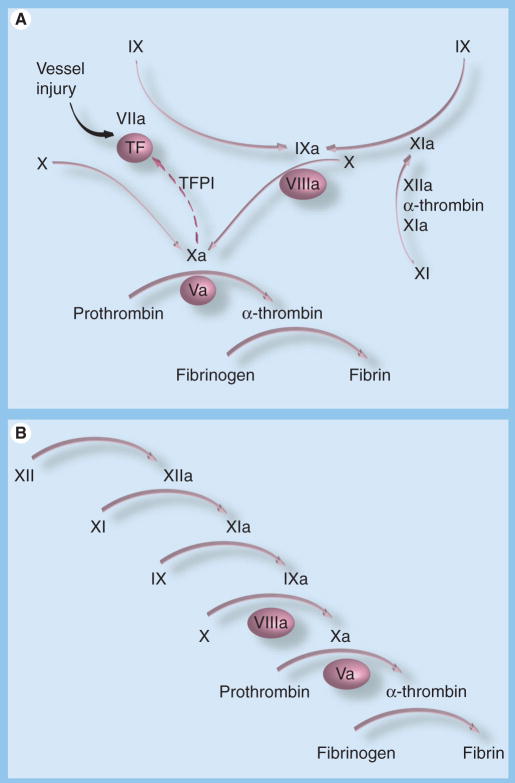
Formation of a clot to stem bleeding at a site of blood vessel injury involves the coordinated activity of a group of plasma proteins that initiate and propagate fibrin formation and subsequently protect fibrin from premature degradation [1]. Factor IX, the precursor of the trypsin-like enzyme factor IXaβ, is an important component of this system, as demonstrated by the severe bleeding disorder associated with congenital factor IX deficiency (hemophilia B) [2]. It is currently thought that the process leading to fibrin generation is initiated by formation of a complex between the plasma protease factor VIIa and a membrane protein expressed on cells underlying the blood vessel endothelium known as tissue factor (TF) (Figure 1A) [3,4]. The factor VIIa/TF complex activates factor X through proteolytic cleavage to factor Xa, which in turn cleaves prothrombin to generate the enzyme α-thrombin. α-thrombin has many activities in hemostasis, including conversion of plasma fibrinogen to fibrin to form a clot [5].
Figure 1. Activation of factor IX in models of plasma coagulation.
(A) Factor VIIa/TF-initiated coagulationIn this model, factor IX (IX) is primarily activated by the factor VIIa/TF complex. Factor IXaβ (IXa) is required to sustain factor X activation after factor VIIa/TF is inhibited by the TF pathway inhibitor (TFPI). TFPI-mediated inhibition of factor VIIa/TF is enhanced by binding of factor Xa to the inhibitor. In this system, factor IX activation by factor XIa is required in certain situations to supplement factor IXaβ produced by factor VIIa/TF. The mechanism for factor XI activation in this model could involve multiple proteases. (B) Factor XII-initiated coagulation. In this model, which describes the main reactions occurring during coagulation in the partial thromboplastin time assay, factor XI is activated by factor XIIa, and factor IX activation is exclusively through factor XIa. In both models, unactivated factors are indicated by Roman numerals, and activated factors by Roman numerals followed by a lower case ‘a’. Nonenzyme cofactors are shown in ovals. Arrows indicate protease-mediated reactions, and the broken arrow indicates inhibition of the factor VIIa/TF complex by TFPI.
TF: Tissue factor.
Factor VIIa/TF also activates factor IX to factor IXaβ [1]. In fact, factor IX is a major substrate for factor VIIa/TF [6]. The main function of factor IXaβ in coagulation is to activate factor X (Figure 1A) [1]. Thus, there are two mechanisms for converting factor X to its active form. When the hemorrhagic syndromes associated with deficiency of factors VII or IX are considered, it is clear that both pathways for factor X activation are required for normal hemostasis. The activity of the factor VIIa/TF complex may be temporally limited at a wound site due to the presence of inhibitors such as the TF pathway inhibitor (Figure 1A) [4] and by accumulation of clot that effectively covers the complex. Factor IXaβ would be required in this scenario for sustained activation of factor X, and bleeding in hemophilia would reflect a failure of this consolidation process, rather than a defect in initiation of coagulation. Phenotypic features of hemophilia, such as the propensity to bleed in tissues low in TF [7] and the requirement for prolonged factor replacement to prevent bleeding after surgery [2], support this interpretation.
It has long been recognized that factor IX can be activated by a factor VIIa/TF-independent process [1]. Indeed, factor IX activation by the protease factor XIa was reported nearly 20 years before it was appreciated that factor IX was activated by factor VIIa/TF. In the partial thromboplastin time (PTT) assay used in clinical laboratories to assess plasma coagulation, factor IX is activated by factor XIa (Figure 1B). The PTT is not sensitive to factor VIIa-mediated factor IX activation. Factor IX activation by factor XIa may represent a relatively new addition to the vertebrate hemostatic mechanism from an evolutionary perspective. Genomic studies of teleost fish have identified genes for homologs of prothrombin and factors VII, IX and X but not for factor XI, the precursor of factor XIa [8].
While factor XIa is required for normal initiation of fibrin formation in the PTT assay, the relatively mild bleeding diathesis associated with factor XI deficiency, when compared with factor IX deficiency, indicates that this protein serves a more limited role in hemostasis [9–12]. From this, it is clear that the PTT does not adequately assess the physiologic function of factor XI. The prothrombin time (PT) assay is usually performed in conjunction with the PTT when assessing patients with a bleeding disorder. While this assay is triggered by addition of TF to plasma, it is not affected by the absence of factor IX or its cofactor factor VIII because of the supraphysiologic concentration of TF used. Thus, our current clinical screening strategy for plasma coagulation overemphasizes the importance of factor XI in the case of the PTT, and fails to assess a major mechanism for activation of factor IX in the case of PT.
Probably as a consequence of the modest role that factor XI plays in hemostasis in vivo, the contribution of factor IX activation by factor XIa to thrombotic disease has received relatively little attention until recently. Presently, work with a variety of animal models and epidemiologic studies with human populations suggest that the contribution of factor XI to thrombosis may be disproportionately greater than its role in hemostasis. This has important implications for development of novel anticoagulant strategies. This review will cover recent advances in our understanding of:
The molecular biology and enzymology of factor IX activation by factor XIa
The bleeding disorder associated with factor XI deficiency and its treatment
The role of factor XI in thromboembolic disease in humans and animal models
Factor IX structure
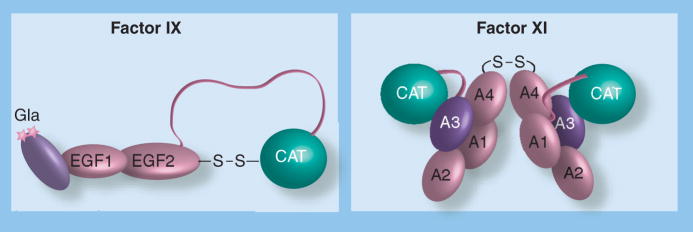
Factor IX is a 57,000-Da single-chain glycoprotein synthesized in hepatocytes and circulates in plasma at a concentration of approximately 90 nM [13]. The protein is structurally similar to factors VII and X, and the regulatory protease protein C [1]. These proteins are precursors of trypsin-like enzymes, with the catalytic domains at the C-termini of the molecules. Starting from the N-terminus, factor IX is composed of a γ-carboxy glutamic acid (Gla) domain, two epidermal growth factor (EGF1 and EGF2) domains, an activation peptide and a catalytic domain (Figure 2) [13]. Factor IX is converted to the active protease factor IXaβ by proteolytic cleavage after amino acids Arg145 and Arg180, releasing the activation peptide (Figure 3). The resulting polypeptides that comprise factor IXaβ, referred to as the light chain (Gla domain and the EGF1 and EGF2 domains) and the heavy chain (catalytic domain), are held together by a disulfide bond. Epitopes on the light chain are required for binding interactions with other components of the coagulation mechanism, including factor VIIa/TF and factor XIa.
Figure 2. Schematic diagrams of factor IX and factor XI.
(A) Factor IX Factor IX is homologous to other vitamin K-dependent coagulation proteins. The Gla domain and EGF domains are in a linear arrangement, with the serine protease (CAT) domain nearly 90 Angstroms from the membrane binding Gla [16]. Activation of factor IX to factor IXaβ involves two proteolytic cleavages at either end of the activation peptide (designated by the ribbon connecting EGF2 to CAT). (B) Factor XI. Factor XI appears to have evolved separately from the vitamin K-dependent proteases. The protein is a disulfide-linked dimer composed of two identical subunits [20]. Dimerization occurs in hepatocytes as a post-translational step. Each 80-kDa subunit contains four apple domains, designated A1 through A4 starting at the N-terminus, with the C-terminal serine protease (CAT) domain following the A4 domain. The four apple domains form a planar structure, with A4 making contact with A1. Factor XI is activated to factor XIa by cleavage after Arg369, which lies between A4 and the catalytic domain. Activation results in a conformational change that allows factor IX to bind to the factor XIa A3 domain. It is likely that an epitope in the C-terminal region of the factor IX Gla domain interacts with the factor XIa A3 domain.
A: Apple; CAT: Catalytic; GLA: γ-carboxy glutamic acid.
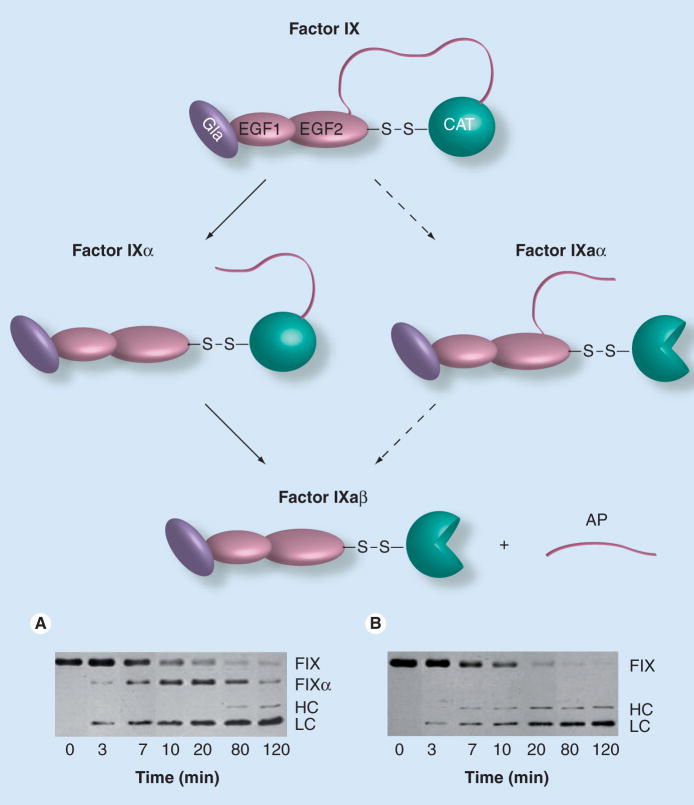
Figure 3. Factor IX activation.
Possible pathways involved in factor IX activation to the active protease factor IXaβ (top) and two western immunoblots of time courses of factor IX activation by (A) factor VIIa/tissue factor (TF) or (B) factor XIa. Factor IX is converted to factor IXaβ by cleavage after Arg145 and Arg180, releasing the activation peptide (the ribbon connecting EGF2 to CAT) that connects the CAT domain, also known as the heavy chain, to the noncatalytic light chain (Gla and EGF domains). The heavy chain and light chains of factor IXaβ remain attached by a disulfide bond. Factor VIIa/TF initially cleaves factor IX after Arg145, forming the intermediate factor IXα (A), which is not an active enzyme because the activation peptide remains attached to the catalytic domain. Factor VIIa/TF subsequently cleaves factor IXα after Arg180 to form factor IXaβ. The active protease domain of factor IXaβ is indicated by the three-quarter circle. Factor XIa also cleaves factor IX initially after Arg145, however, no intermediate is observed in time-course experiments (B) because the subsequent cleavage after Arg180 must be relatively rapid. Initial cleavage of factor IX after Arg180 to form the partially active intermediate factor IXaα is a minor reaction during normal coagulation, but is the preferred pathway of activation by a protease found in the venom of Russell’s viper (Daboia russelli). The western blots in (A & B) are of reduced factor IX proteins. The abbreviations to the right of each panel indicate the positions of uncleaved factor IX (FIX), the large chain of factor IXα that is comprised of the catalytic domain and activation peptide (FIXα), the heavy chain (HC) of factor IXaβ that is the active catalytic domain, and the light chain (LC – the Gla and EGF domains) that is a component of both factor IXα and factor IXaβ.
CAT: Catalytic; GLA: γ-carboxy glutamic acid; TF: Tissue factor.
Gla domains are located at the N-termini of all vitamin K-dependent coagulation proteases (prothrombin, factors VII, IX and X, and protein C) and facilitate assembly of enzyme/substrate complexes on procoagulant phospholipid surfaces such as membranes of activated platelets [1,14]. Gla domains are rich in the amino acid glutamic acid, each of which is modified by addition of a carboxyl group to the γ-carbon by a reaction catalyzed by the vitamin K-dependent enzyme γ-glutamyl-carboxylase [14]. γ-carboxylated glutamic acid residues coordinate binding of calcium, which is required for normal Gla domain conformation and function. The Gla domain mediates binding of factor IX to phospholipids such as phosphatidylserine in cell membranes, likely through a combination of hydrophobic interactions involving residues in the omega loop (Gly4–Gln11), van der Waals forces and charge interactions between calcium ions and phospholipid head groups [15]. The Gla domain of factor IX contains 12 γ-carboxylated glutamic acid residues. In addition to its role in binding to phospholipid membranes, the factor IX Gla domain appears to interact directly with factor VIIa/TF [16] and factor XIa [17].
Recombinant factor IX in which the Gla-domain has been replaced with the Gla-domain from factor VII [17] or protein C [18] is activated normally by factor VIIa/TF, but not by factor XIa. This suggests that a unique epitope that binds to factor XIa is present within the factor IX Gla domain. The N-terminal portion of the Gla domains are involved in phospholipid binding. Phospholipid does not interfere with factor XIa activation of factor IX, indicating that the factor XIa-binding epitope is in the C-terminal portion of the Gla domain. An additional binding site for the factor VIIa/TF complex is present in the factor IX EGF1 domain [16,19]. This site is not required for binding to factor XIa [19], reinforcing the impression that the two activators of factor IX interact with their substrate by distinct mechanisms.
Factor XI structure
Factor XI is a 160,000-Da protein that is a homdimer of two identical 80,000-Da subunits (Figure 2) [9,10]. The molecule, which is synthesized in hepatocytes, circulates in plasma at a concentration of approximately 30 nM in complex with the glycoprotein high-molecular-weight kininogen. Each subunit contains four N-terminal ‘apple domains’ (A1 through A4) and a C-terminal trypsin-like catalytic domain [20]. The A4 domain mediates dimer formation, with residues Leu284, Ile290 and Tyr329 forming the hydrophobic interface [20,21]. In nearly all mammalian species, Cys321 in A4 forms an interchain disulfide bond [9,20]. Interestingly, this bond does not appear to be necessary for the protein to exist as a dimer, as rabbit factor XI, which has histidine at position 321, forms a noncovalent dimer in solution [22], as does recombinant human factor XI in which Cys321 is replaced with another amino acid [23].
The dimeric structure of factor XI is unique among coagulation proteases and is very unusual for a trypsin-like protease in general. The physiologic importance of this structural peculiarity is an area of active investigation. While some naturally occurring mutations in the A4 domain of the human factor XI gene interfere with dimer formation and result in poor secretion from hepatocytes, dimer formation does not appear to be an absolute prerequisite for protein secretion. As will be discussed, the dimeric structure is also not a requirement for normal activation of factor IX. Most coagulation proteases must bind to an activated platelet or other surface to be activated and to express maximal activity, and there is some evidence that this is the case for factor XI/XIa [24]; however, there is conflicting data regarding whether or not the dimeric structure is required for normal activity on platelets [25,26].
The physiologic activator of factor XI has not been definitively identified. Factor XIIa, α-thrombin, factor XIa (autoactivation) and trypsin all activate factor XI [9]. Activation of each factor XI subunit involves cleavage after Arg369, resulting in formation of a heavy chain (apple domains) and light chain (catalytic domain) connected by a disulfide bond [9]. Once activated, the primary substrate of factor XIa during coagulation appears to be factor IX. Trypsin and other degradative enzymes typically cleave multiple substrates, contain epitopes necessary for substrate recognition near their active sites and recognize sites on the substrates near the points of cleavage. By contrast, the trypsin-like coagulation proteases recognize a relatively restricted group of substrates and have one or more substrate-binding epitopes that are remote from the catalytic active site [1]. These binding epitopes, referred to as exosites, are required for initial substrate recognition by the enzyme and are largely responsible for specificity of the enzyme towards the substrate [27–29]. Conversion of factor XI to factor XIa results in the formation or exposure of an exosite on the factor XIa heavy chain that binds factor IX [30]. Studies with recombinant factor XI containing mutations in individual apple domains indicate that this exosite is in the A3 domain [31]. The heavy chain exosite is critical for recognition of factor IX, and there is evidence that it forms a binding site for the factor IX Gla domain (discussed below). Recently, Sinha and colleagues presented evidence for another factor IX-binding exosite in the catalytic domain of factor XIa, distinct from the protease active site [32]. The relative importance of the different factor IX-binding exosites is currently under investigation.
Factor IX activation by factor XIa
The activation of factor IX by factor XIa is a potentially complex process involving a protease with two active sites and a substrate that is cleaved at two precise locations. Hypothetically, factor IX could be cleaved initially either after Arg145 or Arg180, resulting in an intermediate species in which the activation peptide is still attached to the heavy chain (factor IXα) or to the light chain (factor IXaα) (Figure 3). When factor IX is activated by factor VIIa/TF there is accumulation of factor IXα over time prior to formation of factor IXaβ (Figure 3A) [13], suggesting that factor VIIa/TF preferentially cleaves factor IX after Arg145 and releases factor IXα. Factor IXα must then rebind to factor VIIa/TF, possibly in a new conformation, for cleavage after Arg180 to release the activation peptide. This second cleavage appears to be the rate-limiting step for factor IX activation.
By contrast, factor XIa converts factor IX to factor IXaβ with minimal intermediate accumulation (Figure 3B) [33]. Two mechanisms could explain this. Factor IX activation by factor XIa could proceed to completion without releasing an intermediate (a processive mechanism). Alternatively, the process may resemble activation by factor VIIa/TF, with formation of an intermediate that must rebind to the enzyme to be cleaved at the second site. In the latter case, and in contrast to the factor VIIa/TF-mediated process, the rate of the second cleavage by factor XIa would need to be at least as fast as the first to account for the lack of intermediate accumulation. The dimeric conformation of factor XIa adds an additional level of complexity to these considerations. The two active sites of a factor XIa dimer could facilitate a processive mechanism involving simultaneous or sequential cleavage of the two activation sites on one factor IX molecule. Recent work indicates that a conformational change occurs when factor XI is converted to factor XIa that may bring the two catalytic domains into closer proximity [34], possibly facilitating this process.
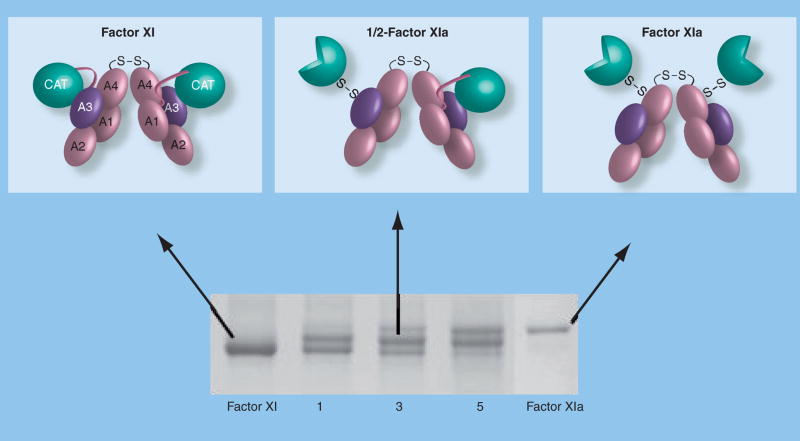
We addressed the importance of the dimeric structure of factor XIa to factor IX activation by preparing and studying factor XIa species containing only one catalytic active site per dimer [35]. When factor XI is activated in solution by factor XIIa or α-thrombin, the reaction proceeds through an intermediate in which only one subunit is activated (Figure 4). We call this protease 1/2-FXIa, and observed its formation in plasma, indicating it is a physiologically relevant species. Using 1/2-FXIa as a starting point, we prepared a second factor XIa species in which both subunits are activated but one active site is irreversibly inhibited (FXIa-1/2i). Both 1/2-FXIa and FXIa-1/2i activate factor IX in a manner indistinguishable from factor XIa, with no factor IX intermediate accumulation. In addition, a recombinant single-chain factor XIa prepared by replacing the A4 dimerization domain (factor XIa/PKA4) [23] activated factor IX without intermediate accumulation, confirming that a single active site is sufficient for normal factor IX activation, and demonstrating that each factor XIa subunit likely behaves as a distinct enzyme.
Figure 4. Activation of factor XI.
Sequential activation of factor XI subunits (top) and an SDS-polyacrylamide gel of a time course of factor XI activation by α-thrombin. The identical 80-kDa subunits of the factor XI dimer interface with each other through the A4 domains. A disulfide bond forms between Cys321 on each A4 domain. Each factor XI subunit is activated by cleavage after Arg369 between the A4 and catalytic domain. The resulting heavy chain (domains A1–A4) remains connected to the active catalytic domain (light chain – indicated by three-quarter circles) by a disulfide bond. Note on the 6% nonreducing polyacrylamide gel that factor XIa migrates through the gel slightly more slowly than factor XI. This is likely related to a conformational change associated with activation that exposes a factor IX-binding exosite on the A3 domain. The activation time course shown in the lower panel clearly demonstrates that conversion of factor XI to factor XIa proceeds through an intermediate that migrates between the substrate and final product. Purification and analysis of this intermediate demonstrated that it is a species with one cleaved subunit and one uncleaved subunit (1/2-FXIa).
A: Apple; CAT: Catalytic.
We also demonstrated that factor XIa, like factor VIIa/TF, cleaves factor IX initially after Arg145, releasing factor IXα [35]. The absence of intermediate accumulation, therefore, must be due to rapid conversion of factor IXα to factor IXaβ relative to the initial cleavage at Arg145. So, in contrast to factor IX activation by factor VIIa/TF, cleavage after Arg145 by factor XIa is likely the rate-limiting step. Interestingly, the factor IX-binding exosite on the factor XIa heavy chain discussed previously appears to be a determinant of the relative rates of cleavage of the two factor IX bonds. As expected, activation of factor IX by the isolated catalytic domain of factor XIa (no heavy chain) [35] or by factor IX in the absence of calcium (disrupts the interaction of factor IX with the heavy chain) [32] is slower than for factor XIa in the presence of calcium. In addition, there is factor IXα accumulation, indicating that the absence of the exosite interaction affects cleavage at Arg180 to a greater degree than cleavage at Arg145.
Other thoughts on the dimeric structure of factor XI
The work discussed in the previous section leaves open the question of the functional importance of the dimeric structure of factor XI/XIa. Recently, Wu et al. presented data indicating that factor XI needs to be a dimer to be activated properly [21]. This group prepared recombinant factor XI monomers by substituting amino acids in the dimer interface in the A4 domain. The monomers were activated poorly by factor XIIa or α-thrombin but, once activated, had normal activity toward factor IX. This suggests a trans-activating mechanism in which the activating protease binds to one subunit of the factor XI dimer and cleaves the opposite subunit after Arg369. This model is not consistent with results for monomeric factor XI/PKA4 (discussed above), which is activated normally by factor XIIa. In factor XI/PKA4, the factor XI A4 domain is replaced by the A4 domain from the homologous protein prekallikrein (PK) [9]. PK, which is a monomer, is also activated by factor XIIa. The discrepancy between the results for the monomers described by Wu et al. and those for factor XI/PKA4 could be explained by a binding site for factor XIIa on the PK A4 domain, which confers normal activation to monomeric factor XI/PKA4.
The observation that 1/2-FXIa is generated in plasma suggests that it may form in vivo. Factor XI and factor XIa bind different proteins, and 1/2-FXIa may have binding properties common to both. For example, there are approximately 1500 binding sites for factor XI on an activated platelet that appear to represent a subpopulation of platelet glycoprotein 1bα (GP1bα) [36]. Factor XI binds to GP1bα through the A3 domain. By contrast, there are far fewer binding sites (~200 per platelet) for factor XIa, and binding does not involve GP1bα or the factor XIa A3 domain [37]. It is possible that 1/2-FXIa binds to a receptor such as GP1bα through the A3 domain of the unactivated subunit, leaving the activated subunit free to activate factor IX. The poor binding of factor XIa to GP1bα would prevent the active subunit from binding to the receptor, preventing formation of an inactive complex. In support of this model, we showed that factor XIa tethered to an acrylamide bead by one subunit is fully active toward factor IX [35].
Molecular biology of factor XI deficiency
Factor XI deficiency was first reported in 1953 by Rosenthal et al. as an autosomal bleeding disorder associated with a prolonged PTT [9]. The incidence in the general population has not been firmly established. Severe deficiency (<15% of normal) has been estimated to occur in approximately one per million of the population; however, the incidence may be considerably higher when symptomatic patients with mild deficiency are included. Severe deficiency is particularly common in Ashkenazi Jews, with an incidence of one in 450 persons, and a carrier (heterozygous) frequency estimated at 5–11% [10]. The molecular biology of factor XI deficiency in Jewish patients is well described. A mutation causing Glu117 in the A2 domain to be replaced by a stop codon (type II mutation) and a Leu substitution for Phe283 in the A4 domain (type III mutation) represent more than 90% of the abnormal alleles in this population [10]. Phe283Leu causes a partial defect in protein dimerization, with monomeric protein being retained within the synthesizing cell.
Approximately 150 other human factor XI gene mutations associated with factor XI deficiency have been identified [38,101–103]. Most are missense or nonsense mutations that result in failure of protein to be secreted from cells (crossreactive material negative mutations [CRM−]). A few are worth noting. Cys38Arg is common in French Basques [39] (allele frequency of ~0.5%), while Cys128Stop accounts for 10% of abnormal alleles in Great Britain [40]. For many CRM− deficiencies of plasma proteins, the mutation either prevents protein synthesis or leads to synthesis of an abnormal protein that is not secreted from the cell. In general, such mutations are associated with a recessive pattern of inheritance because the product of the normal allele is not affected by the mutation. This seems to be the case for the types II and III factor XI mutations. The dimeric structure of factor XI, like that of other multimeric proteins such as von Willebrand factor and fibrinogen, allows for an additional mechanism for CRM− deficiency. Mutations such as Gly400Val, Trp569Ser, Ser225Phe and Cys389Tyr do not prevent protein synthesis or interfere with dimer formation but do prevent secretion from cells [41,42]. In a cell harboring one normal factor XI allele and one allele with such a mutation, normal and abnormal factor XI subunits are synthesized and form dimers. The product of the abnormal allele may form a nonsecretable heterodimer with a normal subunit, effectively trapping a fraction of normal subunits in the cell. This ‘dominant negative’ effect may account for individuals who are heterozygous for a factor XI mutation with plasma factor XI levels that are lower than expected (occasionally within the range for severe deficiency).
Factor XI gene mutations that are associated with dysfunctional factor XI molecules that circulate in plasma (CRM+ deficiency) are rare, in contrast to the great number of CRM+ variants associated with deficiencies of the vitamin K-dependent coagulation proteases. Most CRM+ mutations causing factor XI deficiency are in the catalytic domain (e.g., Pro520Leu, Gly555Glu and Thr575Met), and cause a defect in factor IX activation characterized by a reduced turnover number (Kcat) and a normal Michaelis constant (Km) [43,44]. This is because Km is heavily influenced by the exosite-mediated binding interaction on the heavy chain, which is unaffected by mutations in the catalytic domain. Recently, an Arg184Gly CRM+ mutation in the A3 has been described that interferes with factor IX activation [45]. Previously, we presented evidence that Arg184 lies within the factor IX-binding exosite on the A3 domain, and this naturally occurring mutation reaffirms the importance of this residue to substrate recognition [31].
Clinical phenotype of factor XI deficiency
The bleeding diathesis associated with factor XI deficiency varies considerably between affected individuals, and even in the same patient with different hemostatic challenges. This has caused some difficulty in establishing the inheritance pattern for this disorder, and in formulating concrete treatment recommendations. Bleeding in severe factor XI deficiency (plasma level <15% of normal) is usually the result of trauma or surgery, and is rarely spontaneous [9–12]. Plasma factor XI levels tend to correlate relatively poorly with bleeding phenotype, when compared with bleeding in factor IX- or VIII-deficient patients. Many patients with severe deficiency may not bleed excessively even during surgery without factor replacement. Indeed, some studies report that patients with mild deficiency (15–50% of normal) cannot be distinguished clinically from those with severe deficiency. A number of factors probably contribute to this situation, including the relatively mild bleeding associated with factor XI deficiency, the co-inheritance of other bleeding disorders, differences in the types of hemostatic challenges involved and the nature of the underlying mutations.
A series of studies from Israel of patients with the type II and/or type III factor XI mutations have been key to our understanding of factor XI deficiency, and are a major source for treatment recommendations that can be applied to factor XI-deficient patients in general. Bleeding frequency and severity are highest when trauma involves tissues of the oral cavity, nasopharynx and urinary tract [10]. A propensity for excessive bleeding with trauma from these tissues has been observed in other inherited coagulopathies, likely due to high levels of fibrinolytic activity in these tissues. In a study of Jewish factor XI-deficient patients, injury to the oral cavity or urinary tract was associated with hemorrhage in two-thirds of patients with severe deficiency, regardless of genotype [10]. Bleeding with surgery or injury at other locations was less frequent, and was more common in homozygotes for the Glu117Stop (type II) mutation who have no plasma factor XI compared with those with less-severe deficiency, such as homozygotes for Phe283Leu (type III) who have approximatley 10% of the normal level [10].
Although some studies describe minimal bleeding in heterozygotes for the type II or III mutations even with relatively highrisk procedures, such as tooth extraction, tonsillectomy and urologic surgery [10], others reported difficulty in distinguishing severe and mild deficiency based on bleeding tendency [11]. An assessment of bleeding in 45 families with factor XI deficiency determined that the odds ratios for bleeding were 13.0 and 2.6 for homozygotes and heterozygotes, respectively, compared with controls [10]. It would appear, therefore, that partial factor XI deficiency is a mild risk factor for bleeding, but with a significantly smaller risk than for severe deficiency.
In the PTT assay, factor XI is activated by factor XIIa. However, in contrast to factor XI deficiency, there is a puzzling absence of excessive bleeding associated with total factor XII deficiency, even with major surgery [9]. This could be explained by factor XI activation by other proteases, as discussed earlier. Alternatively, it has been proposed that factor XII deficiency, in addition to causing a defect in factor XI activation, is also associated with a defect in activation of the fibrinolytic system that might counter any propensity to bleed due to decreased factor XI activation [46]. More work is required to determine if one or both of these mechanisms are relevant to hemostasis in vivo.
Treating factor XI-deficient patients
Recommendations for the treatment and prevention of bleeding in factor XI-deficient patients have been refined over the years based on several key observations, including [47,48]:
Bleeding tends to occur most often in certain tissues and with certain procedures
Bleeding is rare with some types of surgery
Antifibrinolytic agents can control bleeding in some situations without factor XI replacement
Patients with very low (<1%) plasma factor XI levels are at high risk of developing inhibitory antibodies when exposed to plasma products
Replacement therapy either with fresh frozen plasma or a factor XI concentrate is the mainstay of therapy. The plasma factor XI level does not increase in response to infusion or inhalation of L-desamino-8-D-arginine vasopressin, and cryoprecipitate does not contain factor XI.
Most major surgery (CNS, cardiothoracic, vascular, head and neck and major abdominal surgery) or procedures involving tissues with high fibrinolytic activity (oropharynx or urinary tract) should be covered with factor replacement to keep plasma levels at 40–45% for 10–14 days in patients with severe deficiency. More than half of patients will bleed if not treated. The plasma half-life of factor XI is approximately 45 h, and once-daily administration of a factor XI-containing product is usually sufficient. Replacement therapy to reach appropriate target levels may be required in patients with milder deficiency (plasma factor XI level 15–40% of normal). In general, patients with factor XI levels greater than 40% do not require replacement, and a history of excessive bleeding suggests other hemostatic abnormalities are present. While surgery at sites other than those described above were often treated in the past with factor replacement (e.g., target level of ~30% for 5–7 days), a retrospective analysis indicates that circumcisions, orthopedic surgery and appendectomies are associated with a low bleeding risk (<5%) [49] and replacement therapy can be withheld unless bleeding occurs. Limiting exposure to plasma products has become an important goal in factor XI deficiency, as antibody inhibitors to factor XI are a common consequence of replacement therapy in patients with severe deficiency. About a third of homozygotes for the Glu117Stop mutation develop neutralizing alloantibodies with replacement therapy, often after their first exposure to a factor XI-containing product [50,51].
Withholding treatment unless bleeding occurs is also recommended in pregnancy for normal vaginal deliveries and after labor, because of the relatively low incidence of excessive bleeding [52]. While a similar approach may be acceptable for Caesarean section, there is limited data on which to base a strong recommendation. Factor replacement should be used for epidural anesthesia, as it is not clear that this procedure is safe in the absence of coverage. Dental procedures such as tooth extraction and minor surgical procedures can be covered with antifibrinolytic therapy (ε-amino caproic acid 5–6 g four-times daily or tranexamic acid 1 g four-times daily), starting 12 h prior to the procedure and continuing for 7 days, without factor replacement [47,48]. Prolonged therapy with antifibrinolytic agents must be used with caution in patients who are immobile or have a history of thromboembolism because of the risk of thrombus formation. These drugs inhibit urokinase and fibrinolytic activity, and their use in patients with bleeding from the urinary tract can lead to renal outflow obstruction from occlusion of the ureter. Aspirin or clopidogrel can be used in factor XI-deficient patients as prophylaxis or to treat manifestations of atherosclerosis. These drugs should be stopped 1 week before a surgical procedure if possible. Atrial fibrillation or venous thromboembolism should be treated with warfarin, with the goal of keeping the international normalized ratio at 2.5 or less to minimize bleeding risk [47,48].
Factor XI concentrates have shown eff icacy in factor XI-deficient patients, but have not been available in the USA in recent years. Hemoleven®, a concentrate of plasma factor XI prepared by the French company LFB, was granted orphan-designation by the US FDA for use in the USA in November of 2007. A concentrate prepared from cryosupernatant by Bioproducts Laboratories (BPL) in the UK has been available on a compassionate-use basis in some countries since 1985. Gascoigne et al. presented a retrospective analysis of 724 infusions of this product in 213 patients [53]. In general, infusions were well tolerated and efficacious. There was an 8.9% incidence of adverse side effects, including a 3.3% incidence of thrombotic events, and it was recommended that the target factor XI level should be higher than 70% to achieve efficacy, but lower than 100% to avoid an increase in thrombotic risk. In the past, use of factor XI concentrate was linked to intravascular coagulation and thrombotic events, particularly in elderly patients with cardiovascular disease receiving doses greater than 30 U/kg. This led to steps in the manufacturing process to inhibit factor XIa in the preparations. Hemoleven contains anti-thrombin and heparin, while the BPL product contains anti-thrombin.
Factor XI-deficient patients who develop inhibitors do not usually have an increased bleeding tendency. Recently, recombinant factor VIIa has been used successfully for major surgery in patients with inhibitors [54]. Concomitant use of antifibrinolytic agents with recombinant factor VIIa may result in a particularly high risk of thrombus formation.
Factor IX & factor XI in thrombotic disease in humans
Despite the relatively limited role of factor XI in hemostasis, there is growing evidence that this protease contributes to thromboembolism in humans. Sustained α-thrombin generation through factor IX activation by factor XIa may potentiate several pro-thrombotic processes including platelet activation, fibrin formation and fibrin modification that enhances resistance to fibrinolysis [55,56]. The evidence for a relationship between plasma levels of factor IX and factor XI and thrombosis is strongest for venous thromboembolism (VTE). Levels of factor IX [57] or factor XI [58] in the upper 10% of the normal population distribution were associated with an approximately twofold increased risk of VTE compared with the remainder of the population in the Leiden Thrombophilia Study. This work is consistent with a recent genetic analysis suggesting a linkage between several single nucleotide DNA polymorphisms, plasma factor XI level and deep vein thrombosis [59]. These results are supported by anecdotal observations that venous thrombosis is rare in patients with factor IX deficiency, and perhaps factor XI deficiency [55,56].
The association between the factor XIa–factor IX pathway and arterial thrombosis in humans appears to be complex. While a link between plasma factor IX levels and risk of arterial disease has not been clearly established, longitudinal studies demonstrated a lower incidence of myocardial infarction (MI) in hemophiliacs compared with controls, with an 80% reduction in mortality from coronary artery disease [55,56]. This suggests that significant factor IX deficiency limits acute thrombus formation at sites of plaque rupture. In the Study of Myocardial Infarction – Leiden (SMILE), men with plasma factor XI levels in the highest quintile had an approximately twofold increased risk of MI compared with those with levels in the lowest quintile [60]. However, Jewish patients with severe factor XI deficiency do not appear to have a lower incidence of MI compared with the general population [61]. A recent study of patients with severe factor XI deficiency, however, identified a significantly lower risk for ischemic stroke compared with age- and sex-matched controls [62]. It is possible that factor XI may be more important for formation of thrombi that affect the CNS than for those within the coronary arteries.
Factor IX & factor XI in animal models of thrombosis
Results from several animal models address the contributions of factor IX and factor XI to thrombosis. The work of Gruber and Hanson in baboons (Papio anubis) first demonstrated a contribution of factor XI to thrombus propagation. These investigators showed that inhibiting factor XI with an antibody limited thrombus growth in TF-coated polytetrafluoro-ethylene grafts introduced into chronic femoral arteriovenous shunts [63]. Initiation of thrombus formation on the graft surface was not affected by the antibody, however, 3D thrombus growth was inhibited with an efficacy comparable to a large dose of heparin. More recently, these workers demonstrated that thrombin–anti-thrombin complex (TAT; a marker of α-thrombin generation) and β-thromboglobulin (a marker of platelet activation) levels were significantly reduced downstream of a growing thrombus when factor XI was inhibited, showing that factor XI contributes to plasma coagulation and platelet activation in this model [64].
Mice with deletions of coagulation factor genes have been instrumental in changing our perspective on the importance of factor IX and factor XI to thrombotic disease. Studies with these animals have been covered in several recent reviews [55,56]. Application of ferric chloride (FeCl3) to the exterior of the carotid artery causes significant damage to the endothelial lining and rapid formation of a platelet-rich occlusive thrombus in wild-type mice. Factor IX or XI deficiency prevents vessel occlusion in response to FeCl3 similarly to a supratherapeutic dose of heparin [65]. Studies using intravital microscopy demonstrated a pronounced reduction in platelet accumulation into arterial thrombi after FeCl3 or laser-induced injury in factor XI-deficient mice [66,67]. The major implication of this work is that the contribution of factor XI to hemostasis does not seem to reflect its importance to thrombosis. This premise was reinforced by the observation that factor XII-deficient mice are also protected from arterial occlusion [67]. Recall that factor XII deficiency is not associated with excessive bleeding.
Deficiency or inhibition of factors IX or XI in mice is also protective in a model of CNS ischemia-reperfusion injury induced by transient occlusion of the middle cerebral artery. Choudri et al. examined the effects of factor IX inhibition on infarct size, fibrin and platelet deposition, and cerebral hemorrhage in this model using active site-inhibited factor IXa (factor IXai) as a competitive inhibitor of factor IXa [68]. Treated animals had major reductions in infarct size and fibrin and platelet deposition compared with controls. In contrast to mice treated with tissue plasminogen activator or heparin, mice treated with factor IXai did not develop reperfusion-associated intracranial bleeding. Kleinschnitz et al. examined the importance of factors XII and XI in this model [69]. Deficiency of either protein reduced ischemic injury compared with wild-type mice, with significantly lower mortality and residual neurologic deficit. Post-reperfusion intracerebral hemorrhage was observed in wild-type mice but not in factor XII- or factor XI-deficient mice. These studies raise the intriguing possibility that interfering with factor IX activation by blocking either factor XIa or factor XIIa might be beneficial in stroke, without the associated risk of therapy-associated hemorrhage that currently limits application of anticoagulation or antifibrinolytic therapy in this setting.
Disseminated intravascular coagulation (DIC) is a thrombo-hemorrhagic syndrome seen in a number of clinical settings including sepsis, trauma and cancer. Tucker et al. recently showed that factor XI plays a role in development of DIC during sepsis in mice [70]. In this study, the cecum was ligated and punctured, resulting in polymicrobial peritonitis and sepsis. Most wild-type mice (87%) died 2–4 days after injury with clear evidence of DIC 24 h after injury (decreased platelet counts and fibrinogen and increased in TAT levels). Mortality was reduced in factor XI-deficient mice (54%) compared with wild-type controls (p = 0.0001), and length of survival among animals that died was greater (p < 0.001), with most deaths occurring between days 3 and 6. Factor XI-deficient mice had smaller changes in platelet count and TAT complexes, and an increase in plasma fibrinogen (probably in response to inflammation), indicating a milder consumptive coagulopathy. The use of anticoagulation therapy to treat the thrombotic component of DIC in humans is often complicated by the adverse effect treatment has on the hemorrhagic component of the syndrome. This study raises the possibility that blocking factor XIa may interfere with a positive-feedback loop that is critical to development of the DIC syndrome.
Expert commentary
Factor IX activation by factor XIa appears to serve a secondary role in normal hemostasis that is most important for preventing bleeding when trauma involves tissues with high intrinsic levels of fibrinolytic activity, such as the oral cavity or the urinary tract. Currently available coagulation assays do not adequately assess this contribution to hemostasis. While factor IX activation by factor XIa plays a relatively specific and limited role in coagulation, it may contribute substantially to formation of pathologic thrombi. Targeting factor IX activation by factor XIa may, therefore, turn out to be useful for treating or preventing thromboembolic diseases.
Five-year view
The next 5 years should see advances in our understanding of the physiologic and pathologic mechanisms that trigger the activation of factor IX by factor XIa. This will facilitate the development of clinical assays that more accurately demonstrate the contributions of factor IX and factor XI to hemostasis and pathologic coagulation. Availability of such assays may allow therapies to be tailored so that factor replacement may be applied more sparingly. One or more factor XI concentrates should be available in the USA to replace fresh frozen plasma for treatment of patients with congenital factor XI deficiency. Finally, active site protease inhibitors that target factor XIa and factor XIIa will be introduced into clinical trials for treatment or prevention of thrombosis. This could lead to anticoagulant strategies that are associated with a relatively low bleeding risk.
Key issues
The coagulation protease factor IX is activated during hemostasis by the factor VIIa/tissue factor complex and by factor XIa.
The two most commonly used assays to assess plasma coagulation (the partial thromboplastin time [PTT] and prothrombin time assays) do not require factor IX activation by factor VIIa/TF. Therefore, it is possible that some abnormalities of factor IX that cause bleeding are missed by these assays, and specialized tests based on them.
In the PTT assay activation of factor IX by factor XIa is essential for formation of a fibrin clot, but this does not accurately reflect the physiologic activity of factor XIa, which is to sustain α-thrombin generation after a clot has formed.
Factor IX activation by factor VIIa/TF and factor XIa involve different mechanisms.
Congenital factor XI deficiency causes a bleeding disorder that is typically inherited as an autosomal recessive disorder, but may behave as a dominant trait in some families.
Current treatment recommendations for patients with factor XI deficiency have been refined to take into account the tissue-specific nature of bleeding and the propensity of patients to develop alloantibody inhibitors when exposed to plasma products.
A factor XI concentrate has been given orphan designation by the US FDA, and can be obtained for treating factor XI-deficient patients.
factor IX activation by factor XIa may contribute to thrombosis, ischemia-reperfusion injury in the CNS and disseminated intravascular coagulation.
Inhibitors of factor XIa and factor XIIa are being developed as potential components of novel anticoagulant strategies that would not be associated with a high risk of bleeding.
Acknowledgments
Financial & competing interests disclosure
The authors wish to acknowledge the generous support of the National Heart, Lung and Blood Institute (grants HL58837 and HL81326) and the American Heart Association (grants 0455245B and 0655311B). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Stephen B Smith, Department of Pathology, Vanderbilt University, 777 Preston Research Building, 2220 Pierce Ave, Nashville, TN 37232-6307, USA.
David Gailani, Division of Hematology/Oncology, Vanderbilt University, 777 Preston Research Building, 2220 Pierce Avenue, Nashville, TN 37232-6307, USA, Tel.: +1 615 936 1505, Fax: +1 615 936 3853, dave.gailani@vanderbilt.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Furie B, Furie BC. Molecular basis of blood coagulation. In: Hoffman R, Benz E, Shattil S, et al., editors. Hematology: Basic Principles and Practice. 4. Churchill Livingstone; New York, NY, USA: 2005. p. 1931. [Google Scholar]
- 2.Lozier JN, Kessler CM. Clinical aspects and therapy of hemophilia. In: Hoffman R, Benz E, Shattil S, et al., editors. Hematology: Basic Principles and Practice. 4. Churchill Livingstone; New York, NY, USA: 2005. p. 2047. [Google Scholar]
- 3.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 4.Monroe DM, Key NS. The tissue factor–factor VIIa complex: procoagulant activity, regulation, and multitasking. J Thromb Haemost. 2007;5:1097–1105. doi: 10.1111/j.1538-7836.2007.02435.x. [DOI] [PubMed] [Google Scholar]
- 5.Davie EW, Kulman JD. An overview of the structure and function of thrombin. Semin Thromb Hemost. 2006;(Suppl 1):3–15. doi: 10.1055/s-2006-939550. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, Broze GJ, Jr, Krishnaswamy S. Formation of factors IXa and Xa by the extrinsic pathway: differential regulation by tissue factor pathway inhibitor and antithrombin III. J Biol Chem. 2004;279:17241–17249. doi: 10.1074/jbc.M312827200. [DOI] [PubMed] [Google Scholar]
- 7.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–2281. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Doolittle RF. The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci USA. 2003;100:7527–7532. doi: 10.1073/pnas.0932632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gailani D, Broze G. Factor XI and the contact system. In: Scriver C, Beaudet A, Sly W, et al., editors. Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York, NY, USA: 2001. pp. 4433–4453. [Google Scholar]
- 10.Seligsohn U. Factor XI in haemostasis and thrombosis: past, present and future. Thromb Haemost. 2007;98:84–89. [PubMed] [Google Scholar]
- 11.Gomez K, Bolton-Maggs P. Factor XI deficiency. Haemophilia. 2008 doi: 10.1111/j.1365-2516.2008.01667.x. In Press. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell NM. Factor XI deficiency. Semin Hematol. 2004;41(1 Suppl 1):76–81. doi: 10.1053/j.seminhematol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt AE, Bajaj SP. Structure–function relationships in factor IX and factor IXa. Trends Cardiovasc Med. 2003;13:39–45. doi: 10.1016/s1050-1738(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 14.Hansson K, Stenflo J. Post-translational modifications in proteins involved in blood coagulation. J Thromb Haemost. 2005;3:2633–2648. doi: 10.1111/j.1538-7836.2005.01478.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang M, Rigby AC, Morelli X, et al. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 16.Chen SW, Pellequer JL, Schved JF, Giansily-Blaizot M. Model of a ternary complex between activated factor VII, tissue factor and factor IX. Thromb Haemost. 2002;88:74–82. [PubMed] [Google Scholar]
- 17.Aktimur A, Gabriel MA, Gailani D, Toomey JR. The factor IX γ-carboxyglutamic acid (Gla) domain is involved in interactions between factor IX and factor XIa. J Biol Chem. 2003;278:7981–7987. doi: 10.1074/jbc.M212748200. [DOI] [PubMed] [Google Scholar]
- 18.Ndonwi M, Broze GJ, Jr, Agah S, Schmidt AE, Bajaj SP. Substitution of the Gla domain in factor X with that of protein C impairs its interaction with factor VIIa/tissue factor: lack of comparable effect by similar substitution in factor IX. J Biol Chem. 2007;282:15632–15644. doi: 10.1074/jbc.M701908200. [DOI] [PubMed] [Google Scholar]
- 19.Ndonwi M, Broze G, Jr, Bajaj SP. The first epidermal growth factor-like domains of factor Xa and factor IXa are important for the activation of the factor VII-tissue factor complex. J Thromb Haemost. 2005;3:112–118. doi: 10.1111/j.1538-7836.2004.01051.x. [DOI] [PubMed] [Google Scholar]
- 20•.Papagrigoriou E, McEwan PA, Walsh PN, Emsley J. Crystal structure of the factor XI zymogen reveals a pathway for transactivation. Nat Struct Mol Biol. 2006;13:557–558. doi: 10.1038/nsmb1095. Description of the crystal structure of factor XI, clearly demonstrating the unusual features of this dimeric trypsin-like serine protease. [DOI] [PubMed] [Google Scholar]
- 21•.Wu W, Sinha D, Shikov S, et al. Factor XI homodimer structure is essential for normal proteolytic activation by factor XIIa, thrombin and factor XIa. J Biol Chem. 2008;283:18655–18664. doi: 10.1074/jbc.M802275200. Demonstration of a biochemical defect associated with a failure of factor XI dimer formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha D, Marcinkiewicz M, Gailani D, Walsh PN. Molecular cloning and biochemical characterization of rabbit factor XI. Biochem J. 2002;367:49–56. doi: 10.1042/BJ20020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Q, Sun MF, Kravtsov DV, Aktimur A, Gailani D. Factor XI apple domains and protein dimerization. J Thromb Haemost. 2003;1:2340–2347. doi: 10.1046/j.1538-7836.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Baird TR, Walsh PN. The interaction of factor XIa with activated platelets but not endothelial cells promotes the activation of factor IX in the consolidation phase of blood coagulation. J Biol Chem. 2002;277:38462–38467. doi: 10.1074/jbc.M205902200. [DOI] [PubMed] [Google Scholar]
- 25.Gailani D, Ho D, Sun MF, Cheng Q, Walsh PN. Model for a factor IX activation complex on blood platelets: dimeric conformation of factor XIa is essential. Blood. 2001;97:3117–3122. doi: 10.1182/blood.v97.10.3117. [DOI] [PubMed] [Google Scholar]
- 26.Sinha D, Marcinkiewicz M, Lear JD, Walsh PN. Factor XIa dimer in the activation of factor IX. Biochemistry. 2005;44:10416–10422. doi: 10.1021/bi050361x. [DOI] [PubMed] [Google Scholar]
- 27.Krishnaswamy S. Exosite-driven substrate specificity and function in coagulation. J Thromb Haemost. 2005;3:54–67. doi: 10.1111/j.1538-7836.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- 28.Page MJ, Macgillivray RT, Di Cera E. Determinants of specificity in coagulation proteases. J Thromb Haemost. 2005;3:2401–2408. doi: 10.1111/j.1538-7836.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- 29.Bock PE, Panizzi P, Verhamme IM. Exosites in the substrate specificity of blood coagulation reactions. J Thromb Haemost. 2007;5:81–94. doi: 10.1111/j.1538-7836.2007.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa T, Verhamme IM, Sun MF, Bock PE, Gailani D. Exosite-mediated substrate recognition of factor IX by factor XIa. The factor XIa heavy chain is required for initial recognition of factor IX. J Biol Chem. 2005;280:23523–23530. doi: 10.1074/jbc.M500894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun MF, Zhao M, Gailani D. Identification of amino acids in the factor XI apple 3 domain required for activation of factor IX. J Biol Chem. 1999;274:36373–36378. doi: 10.1074/jbc.274.51.36373. [DOI] [PubMed] [Google Scholar]
- 32.Sinha D, Marcinkiewicz M, Navaneetham D, Walsh PN. Macromolecular substrate-binding exosites on both the heavy and light chains of factor XIa mediate the formation of the Michaelis complex required for factor IX-activation. Biochemistry. 2007;46:9830–9839. doi: 10.1021/bi062296c. [DOI] [PubMed] [Google Scholar]
- 33.Wolberg AS, Morris DP, Stafford DW. Factor IX activation by factor XIa proceeds without release of a free intermediate. Biochemistry. 1997;36:4074–4079. doi: 10.1021/bi962274y. [DOI] [PubMed] [Google Scholar]
- 34.Samuel D, Cheng H, Riley PW, et al. Solution structure of the A4 domain of factor XI sheds light on the mechanism of zymogen activation. Proc Natl Acad Sci USA. 2007;104:15693–15698. doi: 10.1073/pnas.0703080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Smith SB, Verhamme IM, Sun MF, Bock PE, Gailani D. Characterization of novel forms of coagulation factor XIa: independence of factor XIa subunits in factor IX activation. J Biol Chem. 2008;283:6696–6705. doi: 10.1074/jbc.M707234200. The factor XI dimer does not need to have two active protease domains. Evidence is presented that the species with one active protease site may be a contributor to blood coagulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baglia FA, Gailani D, López JA, Walsh PN. Identification of a binding site for glycoprotein Ibα in the Apple 3 domain of factor XI. J Biol Chem. 2004;279:45470–45476. doi: 10.1074/jbc.M406727200. [DOI] [PubMed] [Google Scholar]
- 37.Miller TN, Sinha D, Baird TR, Walsh PN. A catalytic domain exosite (Cys527– Cys542) in factor XIa mediates binding to a site on activated platelets. Biochemistry. 2007;46:14450–14460. doi: 10.1021/bi701310x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dossenbach-Glaninger A, Hopmeier P. Coagulation factor XI: a database of mutations and polymorphisms associated with factor XI deficiency. Blood Coagul Fibrinolysis. 2005;16:231–238. doi: 10.1097/01.mbc.0000169214.62560.a5. [DOI] [PubMed] [Google Scholar]
- 39.Zivelin A, Bauduer F, Ducout L, et al. Factor XI deficiency in French Basques is caused predominantly by an ancestral Cys38Arg mutation in the factor XI gene. Blood. 2002;99:2448–2454. doi: 10.1182/blood.v99.7.2448. [DOI] [PubMed] [Google Scholar]
- 40.Bolton-Maggs PH, Peretz H, Butler R, et al. A common ancestral mutation (C128X) occurring in 11 non-Jewish families from the UK with factor XI deficiency. J Thromb Haemost. 2004;2:918–924. doi: 10.1111/j.1538-7836.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 41.Kravtsov DV, Wu W, Meijers JC, et al. Dominant factor XI deficiency caused by mutations in the factor XI catalytic domain. Blood. 2004;104:128–134. doi: 10.1182/blood-2003-10-3530. [DOI] [PubMed] [Google Scholar]
- 42.Kravtsov DV, Monahan PE, Gailani D. A classification system for cross-reactive material-negative factor XI deficiency. Blood. 2005;105:4671–4673. doi: 10.1182/blood-2004-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt AE, Ogawa T, Gailani D, Bajaj SP. Structural role of Gly(193) in serine proteases: investigations of a G555E (GLY193 in chymotrypsin) mutant of blood coagulation factor XI. J Biol Chem. 2004;279:29485–29492. doi: 10.1074/jbc.M402971200. [DOI] [PubMed] [Google Scholar]
- 44.Gailani D, Schmidt A, Sun MF, Bolton-Maggs PH, Bajaj SP. A crossreactive material positive variant of coagulation factor XI (FXIP520L) with a catalytic defect. J Thromb Haemost. 2007;5:781–787. doi: 10.1111/j.1538-7836.2007.02390.x. [DOI] [PubMed] [Google Scholar]
- 45.Guella I, Soldà G, Spena S, et al. Molecular characterization of two novel mutations causing factor XI deficiency: a splicing defect and a missense mutation responsible for a CRM+ defect. Thromb Haemost. 2008;99:523–530. doi: 10.1160/TH07-12-0723. [DOI] [PubMed] [Google Scholar]
- 46.Pedicord DL, Seiffert D, Blat Y. Feedback activation of factor XI by thrombin does not occur in plasma. Proc Natl Acad Sci USA. 2007;104:12855–12860. doi: 10.1073/pnas.0705566104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salomon O, Seligsohn U. New observations on factor XI deficiency. Haemophilia. 2004;10(Suppl 4):184–187. doi: 10.1111/j.1365-2516.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 48.Seligsohn U, Zivelin A, Inbal A. Williams Hematology. 7. McGraw-Hill; NY, USA: 2006. Inherited deficiencies of coagulation factors II, V, VII, X, XI, XIII and combined deficiencies of factor V and VIII and of the vitamin K-dependent factors; p. 1887. [Google Scholar]
- 49•.Salomon O, Steinberg DM, Seligsohn U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement. Haemophilia. 2006;12:490–493. doi: 10.1111/j.1365-2516.2006.01304.x. Makes a strong case for tailoring the use of plasma products in factor XI-deficient patients to situations with the highest likelihood of hemorrhagic complications. [DOI] [PubMed] [Google Scholar]
- 50.Salomon O, Zivelin A, Livnat T, et al. Prevalence, causes, and characterization of factor XI inhibitors in patients with inherited factor XI deficiency. Blood. 2003;101:4783–4788. doi: 10.1182/blood-2002-09-2794. [DOI] [PubMed] [Google Scholar]
- 51.Zucker M, Zivelin A, Teitel J, Seligsohn U. Induction of an inhibitor antibody to factor XI in a patient with severe inherited factor XI deficiency by Rh immune globulin. Blood. 2008;111:1306–1308. doi: 10.1182/blood-2007-08-108449. [DOI] [PubMed] [Google Scholar]
- 52.Salomon O, Steinberg DM, Tamarin I, Zivelin A, Seligsohn U. Plasma replacement therapy during labor is not mandatory for women with severe factor XI deficiency. Blood Coagul Fibrinolysis. 2005;16:37–41. doi: 10.1097/00001721-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Gascoigne EW, Dash CH, Gillanders KR. Clinical experience with a factor XI (FXI) concentrate. J Thromb Haemost. 2005;5(Suppl 2):P-S-208. [Google Scholar]
- 54.Bern MM, Sahud M, Zhukov O, Qu K, Mitchell W., Jr Treatment of factor XI inhibitor using recombinant activated factor VIIa. Haemophilia. 2005;11:20–25. doi: 10.1111/j.1365-2516.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 55.Gailani D, Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 56.Gailani D, Renné T. Intrinsic pathway proteases in arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 57.Van Hylckama Vlieg A, van der Linden IK, Bertina RM, Rosendaal FR. High levels of factor IX increase risk of venous thrombosis. Blood. 2000;95:3678–3682. [PubMed] [Google Scholar]
- 58.Meijers J, Tekelenburg W, Bouma B, Bertina R, Rosendaal F. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 59.Bezemer ID, Bare LA, Doggen CJ, et al. Gene variants associated with deep vein thrombosis. JAMA. 2008;299:1306–1314. doi: 10.1001/jama.299.11.1306. [DOI] [PubMed] [Google Scholar]
- 60••.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. Results from the Study of Myocardial Infarction – Leiden (SMILE) suggest that targeted inhibition of factor XI may be useful as a preventative measure for myocardial infarction. [DOI] [PubMed] [Google Scholar]
- 61.Salomon O, Steinberg D, Dardik R, et al. Inherited factor XI deficiency confers no protection against acute myocardial infarction. J Thromb Haemost. 2003;1:658–661. doi: 10.1046/j.1538-7836.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- 62••.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. These results suggest the use of targeted inhibition of factor XI should be investigated as a prophylactic treatment to prevent or lessen the morbidity of stroke. [DOI] [PubMed] [Google Scholar]
- 63•.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. Elegant demonstration of the effect of factor XI inhibition on thrombus formation in a primate. [DOI] [PubMed] [Google Scholar]
- 64.Tucker EI, Marzec UM, Gruber A, Hanson SR. Inhibition of factor XI decreases thrombin production and prevents vascular occlusion in experimental thrombosis in primates. Blood. 2007;110:A763. [Google Scholar]
- 65.Wang X, Cheng Q, Xu L, et al. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 66.Baird TR, Gailani D, Furie B, Furie B. Factor XI deficient mice have reduced platelet accumulation and fibrin deposition after laser injury. Blood. 2004;104:66a. [Google Scholar]
- 67•.Renne T, Pozgajova M, Gruner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. Demonstration of the anti-thrombotic effects of factor XII and factor XI deficiency in arterial thrombosis models in mice. The results are counterintuitive, given the lack of bleeding symptoms in factor XII-deficient humans and animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choudhri TF, Hoh BL, Prestigiacomo CJ, et al. Targeted inhibition of intrinsic coagulation limits cerebral injury in stroke without increasing intracerebral hemorrhage. J Exp Med. 1999;190:91–99. doi: 10.1084/jem.190.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleinschnitz C, Stoll G, Bendszus M, et al. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Tucker EI, Gailani D, Hurst S, Cheng Q, Hanson SR, Gruber A. Survival advantage of coagulation factor XI deficient mice in peritoneal sepsis. J Infect Dis. 2008;15:271–274. doi: 10.1086/589514. Anticoagulation therapy in disseminated intravascular coagulation is applied sparingly because of bleeding complications. This study suggests that interfering with the consumptive process at the level of factor XI may be of benefit, while probably having relatively modest effects on hemostasis in general. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.International Society on Thrombosis and Haemostasis . www.med.unc.edu/isth.
- 102.HGMD Cardiff. www.hgmd.org.
- 103.Factor XI deficiency database . www.factorXI.com.