Abstract
Conventional antiarrhythmic drugs target the ion permeability of channels, but increasing evidence suggests that functional ion channel density can also be modified pharmacologically. Kv1.5 mediates the ultrarapid potassium current (IKur) that controls atrial action potential duration. Given the atrial specific expression of Kv1.5 and its alterations in human atrial fibrillation, significant effort has been made to identify novel channel blockers. In this study, treatment of HL-1 atrial myocytes expressing Kv1.5-GFP with the class I antiarrhythmic agent quinidine, resulted in a dose-, and temperature-dependent internalization of Kv1.5, concomitant with channel block. This quinidine-induced channel internalization was confirmed in acutely dissociated neonatal myocytes. Channel internalization was subunit-dependent, activity-independent, stereospecific, and blocked by pharmacologic disruption of the endocytic machinery. Pore block and channel internalization partially overlap in the structural requirements for drug binding. Surprisingly, quinidine-induced endocytosis was calcium-dependent and therefore unrecognized by previous biophysical studies focused on isolating channel-drug interactions. Importantly, while acute quinidine-induced internalization was reversible, chronic treatment led to channel degradation. Together, these data reveal a novel mechanism of antiarrhythmic drug action and highlight the possibility for new agents that selectively modulate the stability of channel protein in the membrane as an approach for treating cardiac arrhythmias.
Keywords: Potassium channel, trafficking, cardiac, antiarrhythmic
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and a major risk factor for increased stroke, heart failure, and cardiovascular morbidity. The preferred therapy for AF is sustained sinus rhythm control; however, the efficacy of currently-used antiarrhythmic drugs is diminished by adverse side effects resulting from a lack of ion channel selectivity and nonspecific ventricular activity 1, 2. Due to the frequency of AF and its associated morbidity, development of atrial-specific therapies is a major focus of both industrial and academic research efforts 3, 4.
Kv1.5 (KCNA5) has emerged as a promising pharmacologic target for treatment of AF. In humans, Kv1.5 is selectively expressed in atrial myocytes where it mediates the ultrarapid delayed rectifier current (IKur) that contributes to cellular repolarization and controls action potential (AP) duration 5, 6. Although significant effort has been made to identify novel blockers of Kv1.5, compounds with both atrial selectivity and clinical efficacy remain elusive and highlight the need for new potential therapeutic strategies or targets.
Conventional antiarrhythmic drugs generally target the ion permeability of channels. Increasing evidence, however, suggests that functional ion channel density can be modified pharmacologically in that a drug may both directly block an ion channel and indirectly disrupt normal protein trafficking 7. In fact, one report shows that nearly half of hERG channel pore-blockers tested also decrease anterograde delivery of the channel to the cell surface 8. In addition, two reported cases of disrupted protein trafficking leading to drug-induced prolongation of the cardiac AP highlight that this pleiotropic drug action can modulate cardiac excitability 9, 10. Research into the therapeutic value for antiarrhythmic agents that affect channel trafficking has focused almost exclusively on hERG channel blockers that stabilize misfolded channels and rescue hERG trafficking mutants. To date, no studies have addressed the potential properties of antiarrhythmic drugs to acutely modulate surface density of functional channels that exist on the cardiac myocyte membrane. Here we report, for the first time, a novel paradigm for antiarrhythmic pharmacology in the control of the cell surface stability of Kv1.5 in atrial myocytes and present a new mechanism for the inhibition of ion current through drug-stimulated endocytosis of channel protein.
Materials and Methods
See supplement for detailed methods.
Immunocytochemistry
Immunocytochemistry was performed and all images were collected, quantified, and analyzed as reported earlier and as described in the supplement 11.
Electrophysiology
Whole-cell voltage clamp experiments were performed on HL-1 cells stably expressing Kv1.5-pHluorin as described previously 12. All experiments were performed at room temperature.
Neonatal Myocyte Isolation and Electroporation
Cardiomyocytes from neonatal mice were isolated and cultured according to methods adapted from Zlochiver, et al 13. Electroporation was performed on acutely isolated myocytes before plating as described in supplement.
Results
The antiarrhythmic drug, quinidine, stimulates rapid Kv1.5 internalization in HL-1 atrial myocytes
Multiple antiarrhythmic drugs have been shown to inhibit Kv1.5, including the class I antiarrhythmic drug quinidine, which, like many Kv channel inhibitors, causes an activity-dependent, open-channel block of Kv1.5 (Online Figure I). The present study was designed to investigate the potential pleiotropic effects of antiarrhythmic agents on Kv1.5 using this well characterized drug as a prototype 14. Our studies were initiated in the HL-1 immortalized mouse atrial myocyte cell line in which we previously demonstrated that Kv1.5 undergoes constitutive internalization and recycling to maintain steady-state ion channel surface levels 11. Using an extracellular GFP epitope-tagged Kv1.5 construct that mimicked wild type channel function, we discovered that quinidine triggered Kv1.5 internalization concomitant with block of channel current (Figure 1A, Online Figure I). Exposure to increasing concentrations of quinidine stimulated a dose-dependent increase in internalized Kv1.5 with a corresponding loss of surface protein (Figure 1B). This quinidine-induced internalization culminated in an 80 ± 13% (n = 143; p < 0.001) increase over constitutive channel endocytosis at 100 μmol/L quinidine (EC50 ≈ 1 μmol/L). Quinidine-induced internalization of Kv1.5 was rapid and achieved a maximum within 10 min of treatment (Online Figure I). Electrophysiology experiments confirmed that 10 min quinidine treatment represents steady-state channel block (Online Figure I). Together, these data indicate that the rate-limiting step for this process is equilibration of quinidine across the cell membrane. Following 10 min vehicle treatment, the level of internalized Kv1.5 is minimal and consistent with constitutive endocytosis as previously reported11 (Figure 1A). Importantly, at the 10 min time point quinidine-stimulated internalization was statistically greater compared to control (p <0.001) and could be reliably separated from constitutive endocytosis and was therefore used, unless otherwise stated, in our later studies.
Figure 1. Quinidine stimulates internalization of Kv1.5 in a dose- and temperature-dependent manner.
(A) Representative images of Kv1.5-GFP overexpressed in HL-1 cells, showing total GFP signal (left), surface channel as detected by surface labeling with anti-GFP followed by goat anti-rabbit Alexa Fluor 405 (middle), and internalized channel detected by labeling with goat anti-rabbit Alexa Fluor 647 (right), at 0min (top), 10min at 37°C with vehicle (0.1% DMSO) (middle), and 10min at 37°C with 100 μmol/L quinidine (bottom). (B) Quantification of internalized (top) and surface (bottom) Kv1.5 following treatment with increasing concentrations of quinidine for 10min at 37°C. (C) Comparison of dose response for Kv1.5-GFP internalization following quinidine treatment for 10min at room temperature (25°C) and 37°C (EC50 = 900 nmol/L at 37°C). Scale bars = 10 μm. * indicates p < 0.05; *** indicates p < 0.001 as determined by oneway ANOVA with Tukey post-test.
We also measured a temperature-dependence for the quinidine-induced internalization of Kv1.5 (Figure 1C). The drug-induced internalization was greater than two-fold higher at the physiological temperature of 37°C compared to room temperature (80 ± 13% (n = 143) at 37°C versus 45 ± 11% (n = 130) at 25°C; p < 0.001). Importantly, the EC50 values were identical and only the extent of quinidine-induced internalization was temperature-dependent. It is noteworthy, that previous biophysical studies measuring pore-block with quinidine were performed at room temperature where the effects of internalization would be inadvertently reduced.
To negate the possibility of an antibody-induced artifact we generated a Kv1.5 construct in which GFP was replaced with pHluorin, a GFP variant whose fluorescent properties are sensitive to the pH of the immediate environment 15. Using live-cell imaging of HL-1 cells expressing Kv1.5-pHluorin, we found no difference in the time course, extent, or subcellular localization of quinidine-induced internalized channel compared to results from our antibody-labeling internalization assay (Online Figure II). Together, these results suggest that antiarrhythmic drugs may modulate retrograde trafficking of Kv1.5 as a mechanism contributing to their inhibition of outward K+ current.
Constitutive and quinidine-induced internalization of Kv1.5 is conserved in native dissociated mouse myocytes
We investigated the validity of the quinidine effect in native dissociated mouse myocytes. The live-cell internalization assay was performed on acutely dissociated neonatal mouse myocytes expressing Kv1.5-GFP to probe for alterations in Kv1.5 distribution upon quinidine treatment. Using this technique, we found levels of constitutive and quinidine-induced channel internalization similar to those observed in the HL-1 model (Figure 2A). This drug-induced internalization culminated in an 84 ± 19% (n = 45) (p < 0.001) increase over constitutive channel endocytosis at 100 μmol/L quinidine (n = 52), with a corresponding decrease in surface levels (Figure 2B). These data confirm that the endogenous machinery and mechanistic requirements for constitutive and quinidine-induced Kv1.5 internalization are conserved in native cardiac tissue.
Figure 2. Constitutive and quinidine-induced internalization occur in native mouse myocytes.
(A) Representative images of acutely dissociated neonatal mouse myocytes transiently expressing Kv1.5-GFP by electroporation showing total GFP signal (left), surface channel (left middle), internalized channel (right middle), and anti-troponin (right) signal, at 0min (top), 10min at 37°C with vehicle (middle), and 10min at 37°C with 100 μmol/L quinidine (bottom). Scale bars = 10 μm. (B) Quantification of internalized (left) and surface (right) Kv1.5 following treatment with 100 μmol/L quinidine for 10min at 37°C. *** indicates p < 0.001 as determined by unpaired t-test.
Specificity of quinidine-induced internalization
We investigated the subunit specificity by measuring quinidine effects on internalization of two other prominent cardiovascular potassium channels, Kv4.2 and Kv2.1, expressed in HL-1 cells. Although ion permeability of both channels is blocked by quinidine (IC50 of 10 μmol/L and 20 μmol/L quinidine, respectively) 16, 17, neither Kv4.2 nor Kv2.1 internalized in response to any drug concentration tested over the time course studied (Figure 3A, Online Figure III and IV). Together, these data demonstrate a subunit-dependence for the quinidine-induced internalization of Kv1.5.
Figure 3. Quinidine-induced internalization is subunit-dependent, activity-independent, and stereospecific.
Dose-response for HL-1 cells expressing Kv4.2-GFP or Kv2.1-GFP (A) or Kv1.5-W472F (B) treated with increasing concentrations of quinidine for 10min at 37°C. ** indicates p < 0.01; *** indicates p < 0.001 as determined by one-way ANOVA with Tukey post-test. (C) Quantification of internalized Kv1.5 following treatment with 100 μmol/L quinidine or 200 μmol/L quinine, the diastereomer of quinidine, for 10min at 37°C. *** indicates p < 0.001 as determined by student's unpaired t-test.
Quinidine-induced internalization occurred at resting membrane potentials suggesting that the drug-induced trafficking effects may be conduction-independent. To address this possibility, we measured internalization of the pore-dead mutant Kv1.5-W472F, which efficiently traffics to the myocyte membrane but is incapable of conducting current 18. For both the mutant and wild-type controls, the quinidine-induced internalization resulted in an approximately 90 ± 20% (n = 90) (p < 0.001) increase over constitutive endocytosis, with no difference observed in the EC50 value (Figure 3B). These data confirm that the quinidine-induced internalization of Kv1.5 is independent of channel ion conductance.
Structural requirements of quinidine-induced internalization
To further examine specificity and gain possible insight into the pharmacophore responsible for drug-induced internalization of Kv1.5, we tested quinine, the diastereomer of quinidine that also causes a dose-dependent block of Kv1.5 current 19, 20. We found that, contrary to quinidine, a maximal concentration of quinine was not able to enhance Kv1.5 internalization above constitutive levels (Figure 3C). Quinine was, however, capable of inducing significant, dose-dependent block of Kv1.5 that was reversible upon drug washout (Online Figure V). The inability of quinine to induce channel internalization, despite effective pore block, demonstrates that quinidine-induced internalization of Kv1.5 is stereospecific.
The subunit-dependence and stereospecificity of quinidine-induced internalization of Kv1.5 indicates a reliance of this internalization on the structure of the channel. To determine whether the protein structural requirements for quinidine-induced internalization were the same as those for pore block, we used the live-cell internalization assay to investigate the effect of quinidine on several mutants of Kv1.5. Kv1.5-T480A contains a single point mutation in the putative binding site for quinidine, resulting in a greater than 90% reduction in sensitivity to open-channel blockers of Kv1.5 21, 22. We found that, as with pore block, quinidine-induced internalization was abolished with the T480A mutation (Figure 4A). To further probe the amino acid requirements, we expanded our ala-scanning mutagenesis to include three additional amino acid residues (Ile-508, Leu-510, and Val-512) which are considered part of the highly conserved antiarrhythmic drug binding site within Kv channels 21, 22. We found that as reported for channel block, quinidine-induced internalization was abolished by the Kv1.5-I508A mutation. Surprisingly, however, unlike channel block, internalization was near wild-type for both Kv1.5-L510A and Kv1.5-V512A (Figure 4B). Additionally, we tested Kv1.5-P532L, a naturally occurring mutation reported to cause a significant rightward shift in the dose response curve for quinidine block of channel current 23. We observed a similar decrease in sensitivity to quinidine-induced channel internalization with a shift in the EC50 from approximately 1 μmol/L for wild-type channel to 104 μmol/L for Kv1.5-P532L (n ≥ 90) (Figure 4C). These data show that quinidine is acting directly on the channel and not through off-target effects that may influence trafficking. Importantly, these data also indicate that antiarrhythmic drugs may both block channel current and disrupt protein endocytosis concurrently to alter the functional ion channel density in the cell membrane and that these two effects share partial overlap in the structural requirements for drug binding, but the necessary amino acids are not are not identical.
Figure 4. Structural requirements for quinidine binding are partially conserved for pore block and channel internalization.
(A) Dose-response for HL-1 cells expressing Kv1.5-GFP or the quinidine-insensitive Kv1.5-T480A mutant treated with increasing concentrations of quinidine for 10min at 37°C. (B) Quinidine-induced internalization for HL-1 cells expressing Kv1.5-GFP containing the T480A, I508A, L510A, or V512A mutation treated with 100 μmol/L quinidine for 10min at 37°C. (C) Dose-Response for HL-1 cells expressing Kv1.5-GFP or the Kv1.5-P532L mutant treated as described in (A). * indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001 as determined by one-way ANOVA.
Channel internalization is prevented by disruption of endocytic machinery
Our previous studies demonstrated that constitutive Kv1.5 internalization occurs via a microtubule-dependent, dynein-mediated endocytic pathway 11. To further address specificity and determine if quinidine-induced internalization shares a similar mechanism, we used pharmacological and dominant-negative methods to disrupt the endocytic machinery prior to measuring quinidine-stimulated Kv1.5 internalization. Dynasore, a cell-permeant, potent small molecule inhibitor of dynamin, prevents the budding and pinching off of endocytic vesicles 24, 25, and has been used in neurons to block synaptic vesicle endocytosis 26. Acute pretreatment with this pharmacologic inhibitor abolished quinidine-induced internalization of Kv1.5 in atrial myocytes (Figure 5A). We also observed a nearly 100% increase in surface levels of Kv1.5 over untreated controls indicating that Dynasore prevents constitutive endocytosis of the channel (Figure 5B,C). Additionally, we found that p50-dynamitin overexpression, which we and others have reported blocked constitutive Kv1.5 endocytosis 11, 27, also blocked quinidine-induced internalization with a corresponding increase in surface levels similar to the inhibition measured with Dynasore (Online Figure VI). These data indicate that quinidine-induced internalization of Kv1.5 occurs via a microtubule-dependent, dynein-mediated pathway as previously demonstrated for constitutive endocytosis.
Figure 5. Channel internalization is prevented by pharmacologic disruption of the endocytic machinery.
(A) HL-1 cells expressing Kv1.5-GFP were treated for 1 hour with 80 μmol/L Dynasore at 37°C prior to surface-labeling with anti-GFP antibody. Quantification of internalized Kv1.5 following treatment with 100 μmol/L quinidine for 10min at 37°C in the continued presence of Dynasore. (B) Quantification of surface Kv1.5 for cells treated as described in (A). (C) Representative images of surface (red) and internalized (white) Kv1.5 in cells treated as described in (A). Scale bars = 10 μm. ** indicates p < 0.01; *** indicates p < 0.001 as determined by one-way ANOVA with Tukey post-test.
Quinidine-induced internalization is calcium-dependent
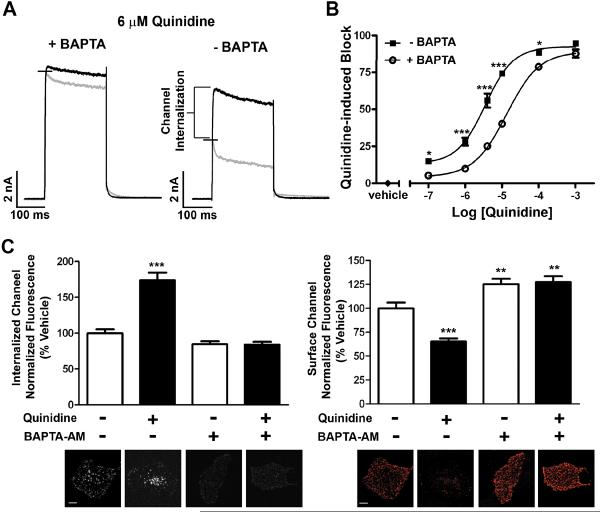
To further investigate the mechanisms controlling quinidine-induced internalization, we tested the calcium dependence of this process. The biophysical properties of Kv1.5 pore-block by quinidine have been previously characterized 14, 20. Our survey of the literature revealed that all of these electrophysiological studies included a calcium-chelating agent in the pipette solution to isolate the drug-channel interaction. Since several intracellular trafficking pathways are known to be calcium-dependent 28, inclusion of a calcium-chelating agent could inhibit the endogenous or drug-induced trafficking pathways. To investigate this possibility, we performed whole-cell patch-clamp recordings on cells stably expressing Kv1.5 both in the presence and absence of the calcium-chelating agent, BAPTA, in the pipette solution. In response to a single depolarizing pulse from −80mV to +60mV, perfusion of cells for 10 min with 6 μmol/L quinidine reduced the peak current and accelerated the time course of inactivation under both conditions. Surprisingly, however, exclusion of BAPTA resulted in a much larger effect on peak current and revealed a significant calcium-dependent decrease. This decrease in Kv1.5 current in the absence of BAPTA was observed over a range of voltages (Online Figure VII). Further, in the absence of BAPTA in the pipette solution, the dose-response for quinidine was significantly leftward shifted with a 3-fold decrease in the IC50 (IC50 = 13 μmol/L + BAPTA and 3.5 μmol/L ‒ BAPTA) (n = 5) (Figure 6B). Conversely, this calcium-dependent decrease in current density did not occur with 20 μmol/L quinine, the diastereomer of quinidine (Online Figure VIII). This is in agreement with our finding that quinine did not induce channel internalization. Together, these data provide functional evidence for quinidine-induced channel internalization through a calcium-dependent mechanism.
Figure 6. Quinidine-induced internalization occurs via a calcium-dependent mechanism.
Whole-cell voltage clamp experiments were performed on HL-1 cells stably expressing Kv1.5-pHluorin. A single depolarizing pulse to +60mV was applied as described in methods. (A) Current traces are shown for a single cell prior to (black) and following (gray) 10min exposure to 6 μmol/L quinidine in the presence (left) or absence (right) of BAPTA in the pipette solution (n=10 cells). (B) Dose-response curve upon treatment with increasing concentrations of quinidine for 10min at room temperature in the presence or absence of BAPTA in the pipette solution (IC50 = 13 μmol/L + BAPTA and 3.5 μmol/L - BAPTA; n=5 cells; Hill slope = 1.042 + BAPTA and 1.135 - BAPTA). (C) HL-1 cells expressing Kv1.5-GFP were pretreated for 1 hour with 10 μmol/L BAPTA-AM before surface-labeling with anti-GFP antibody. Quantification of internalized (left) and surface (right) Kv1.5 following treatment with 100 μmol/L quinidine for 10min at 37°C in the continued presence of BAPTA-AM. Below each bar graph is a representative image for that condition. Scale bars = 10 μm. * indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001 as determined by one-way ANOVA with Tukey post-test.
To determine if these functional measurements are supported by fluorescence imaging data, we measured the quinidine-induced internalization of Kv1.5 in the presence of the cell-permeant compound BAPTA-AM to chelate intracellular calcium. Using this method, pretreatment with 10 μmol/L BAPTA-AM completely blocked quinidine-induced internalization of Kv1.5 with a corresponding increase in surface levels (Figure 6C). These results were nearly identical to those determined after endocytic disruption with Dynasore and p-50 dynamitin (Figure 5C and Online Figure VI). Together, these data demonstrate that quinidine-induced internalization is calcium-dependent and therefore unrecognized by previous biophysical studies focused on isolating channel-drug interactions.
Differential effects of acute versus chronic treatment with quinidine
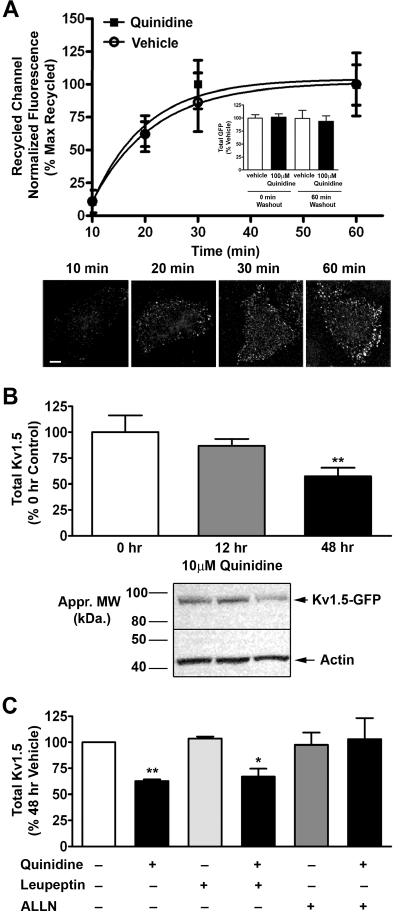
Previously we reported that, following constitutive internalization, a population of Kv1.5 originating on the atrial myocyte cell surface recycled back to the plasma membrane 11. Electrophysiologically, we showed that quinidine-induced block of Kv1.5 current is reversible upon drug washout. Upon quinidine-stimulation, internalized Kv1.5 also co-localized with the early endosomal marker EEA1 (data not shown). Therefore, we measured the intracellular fate of the quinidine-induced, internalized Kv1.5 upon drug washout. Using a modified form of the recycling assay developed previously within our laboratory, and HL-1 cells transiently expressing Kv1.5-GFP, we found that a population of Kv1.5 undergoing quinidine-induced internalization recycled back to the plasma membrane with the same time constant as constitutive recycling (τ =29.06 min and 25.72 min, respectively) (Figure 7A). This channel recycling also occurred on a time scale nearly identical to recovery from current block 14. This suggests that the rate limiting step for recovery of Kv1.5 current is not quinidine dissociation from channel and indicates that quinidine is acting on the internalization pathway and not on the recycling pathway. Importantly, over the time course studied, we detected no loss in total protein, as measured by total GFP fluorescence, indicating that there was no detectable channel degradation (Figure 7A inset).
Figure 7. Acute quinidine-induced internalization is reversible, whereas chronic treatment results in channel degradation.
(A) Quantification of recycled Kv1.5 at 0, 10, 20, 30, and 60 min at 37°C post treatment with 100 μmol/L quinidine for 10 min at 37°C. Vehicle and quinidine treated data sets were each normalized to their own baseline (no washout) and maximum. Corresponding total GFP levels are provided for 0 and 60 min of recycling (inset). Below are representative images showing the increase in recycled Kv1.5-GFP with time, post quinidine treatment. Scale bar = 10 μm. (B) HL-1 cells stably expressing Kv1.5-pHluorin were treated with 10 μmol/L quinidine for 0, 12, or 48 hours at 37°C. Quantification of total Kv1.5 protein levels normalized to actin and control (48 hour DMSO) levels. Below is a representative image of a Western blot for 0, 12, and 48 hours of quinidine treatment. (C) HL-1 cells stably expressing Kv1.5-pHluorin were treated with vehicle, 10 μmol/L quinidine, 10 μmol/L leupeptin, 500 nmol/L ALLN, quinidine and leupeptin, or quinidine and ALLN for 48 hours at 37°C (63% or 67% decrease with 48 hr quinidine or quinidine and leupeptin (n = 8); no statistical decrease for quinidine and ALLN (n = 4)). Quantification was performed as in B. * indicates p < 0.05; ** indicates p < 0.01 as determined by one-way ANOVA with Tukey post-test.
In a clinical setting, however, antiarrhythmic agents are administered chronically, over long periods of time 29−32. To investigate a potential long-lasting effect of chronic quinidine on the dynamic trafficking of Kv1.5, we treated HL-1 cells stably expressing Kv1.5 with a clinically relevant concentration of 10 μmol/L quinidine. Total Kv1.5 protein levels were assessed by Western blot at 0, 12, and 48 hours of quinidine exposure, and were reduced by 13% and 43% at 12 and 48 hours, respectively (n = 9; n = 21, respectively; p < 0.01 at 48hr) (Figure 7B). Immunocytochemistry revealed a corresponding decrease in surface Kv1.5 (Online Figure IX) that was significant at 12 hours of quinidine treatment. As expected, these data indicate that Kv1.5 internalization precedes the loss of total channel protein and likely reflects a delay in the cellular timecourse for the onset of protein degradation. Importantly, the decrease in total Kv1.5 protein at 48 hours of chronic quinidine treatment was blocked by the mild proteasome inhibitor, ALLN, but not the lysosomal inhibitor, leupeptin (Figure 7C). Together, these data reveal that acute quinidine treatment leads to channel internalization that is reversible upon drug washout, whereas chronic quinidine treatment leads to channel degradation.
Discussion
Here, we report a previously unrecognized mechanism of antiarrhythmic drug action in the acute modulation of surface channel density. Using quinidine, an antiarrhythmic agent which has both class Ia actions 33, 34 and class III actions in mammalian atrium and ventricle, we demonstrate that channel blockers can both inhibit ion conduction and regulate the stability of the channel protein within the membrane. These pleiotropic actions, which may be independent, have important implications for antiarrhythmic drug therapy as well as drug safety testing.
The manipulation of ion channel trafficking pathways, particularly those that target functional channels existing in the myocyte membrane represents an alternative and potentially beneficial new therapeutic strategy. There is a clear need for the development of new, longer-term antiarrhythmic drugs to successfully maintain normal atrial sinus rhythm without risking the occurrence of potentially life-threatening, proarrhythmic ventricular side effects. The inhibition of Ito current, through block of Kv4.2/Kv4.3 channels in the atria and ventricle, is a frequent side effect of putative Kv1.5-selective agents. Our data demonstrate that quinidine-induced internalization of Kv1.5 is dose-, and temperature-dependent and, although it inhibited current, did not stimulate internalization of Kv2.1 or Kv4.2 channels. Ultimately, the development of new compounds and their efficacy to selectively modulate trafficking pathways is dependent on the ability to separate the pleiotropic actions and to isolate the pharmacophore responsible for block and/or internalization. In the current study, several lines of evidence indicate that these two processes may be separable. For instance, quinidine-induced internalization was voltage- and activity-independent in that it occurs at resting membrane potentials during immunocytochemistry and when cells are voltage clamped at −80 mV during electrophysiology measurements (Figures 1 and 6). In contrast, open channel block of Kv1.5 was voltage- and activity-dependent (Online Figure I). In addition, there are several pieces of data in this study in which pore block occurred without channel internalization. As mentioned above, both Kv4.2 and Kv2.1 exhibit significant quinidine-mediated current block, however neither underwent drug-induced internalization, despite constitutive endocytosis of Kv4.2 nearly identical to Kv1.5 (Online Figure III). Importantly, drug-induced internalization of Kv1.5 was stereospecific in that quinine, the diastereomer of quinidine, caused current block of Kv1.5, but was incapable of inducing channel internalization even at a maximal dose (Figure 3). Interestingly, ala-scanning mutagenesis of the conserved Kv channel drug binding site showed that not all residues were conserved for both pore block and channel internalization (Figure 4). To facilitate the development of new antiarrhythmic drugs that selectively modulate channel density, future detailed structure-activity studies are required to identify the essential features within the channel and as part of the drug molecule responsible for quinidine's multiple activities. The mechanism of drug-induced internalization most likely involves a conformational change in the channel protein; however, not all of the mutagenesis results in this study can be reconciled with the model that drug block and drug-induced internalization can be separated. For example, if one considers the effects of the T480A mutation, which occurs within the pore helix and diminishes both block and internalization, compared to our results that drug-induced internalization occurs when cells are clamped at −80 mV, it is difficult to envision how quinidine can reach a binding site in the conduction pathway to initiate internalization if the cytoplasmic gate of Kv1.5 is firmly closed. An alternative strategy is the identification of the molecular machinery and further elucidation of the proteins involved in channel targeting, such as the dynein motor complex (Online Figure VI), which may reveal novel, selective auxiliary therapeutic targets. Nonetheless, this study highlights the potential for development of new agents which can selectively affect either ion conduction and/or ion channel trafficking pathways as a new means to gain therapeutic specificity.
The potential for drugs to modulate surface density also raises important issues for drug safety screening. It is well known that non-antiarrhythmic drugs can have proarrhythmic liabilities through the off-target inhibition of ion channels in the heart. This issue has received considerable attention from pharmaceutical companies and regulatory agencies that now mandate cardiac ion channel testing as part of drug safety profiling. Attention to the potential problem of non-antiarrhythmic drugs affecting both pore-block and channel trafficking originated with work related to the hERG channel 7. Data presented in this manuscript advance this concept to include the atrial specific target Kv1.5 and extend this concern to the acute regulation of surface density. Current safety screens focus almost entirely on a drug's capacity to block ion conduction. The calcium-dependence of quinidine-induced internalization of Kv1.5 is important when considering the in vivo effects of this drug and most likely many others. Our results demonstrate that use of compounds that deplete free intracellular calcium block a major component of quinidine action on Kv1.5. This calcium-dependent component is responsible for a significant fraction of the quinidine-mediated decrease in current density and can explain the leftward shift in the EC50 for quinidine from 13 μmol/L in the biophysical studies including BAPTA to 3.5 μmol/L in the absence of BAPTA (Figure 6B). However, it is important to note that the free calcium concentration is likely very high in our electrophysiological experiments performed in the absence of any calcium buffer, while large changes in free calcium are not expected in immunocytochemistry experiments. In addition, the two experiments were performed at different temperatures; therefore, the two conditions may not be identical. It is also possible that what is marked as calcium-dependent channel internalization (Figure 6A) is a mix of fast block and channel internalization. However, separation of these two mechanisms is complicated by our finding that the rate-limiting step for the onset of drug action is equilibration across the membrane and both block and internalization recover upon washout of the drug. Nevertheless, this work implies that antiarrhythmic agents such as quinidine, which affect channel trafficking pathways, may show greater efficacy and potency in the in vivo condition where calcium-dependent pathways are uninhibited. Screens for pore block may simply miss channel trafficking effects and dramatically underestimate drug actions.
Another issue that may compound these concerns is the acute versus chronic effects of altering channel surface density. Our results show that chronic quinidine treatment results in a significant decrease in Kv1.5 channel protein by diverting channel from a recycling to degradation pathway. Recent work suggests that a fraction of internalized Kv1.5 enters proteasomal compartments 35. This is supported by data in this manuscript showing that inhibition of the proteasomal, but not lysosomal, degradation machinery prevented the chronic quinidine-induced decrease in total Kv1.5. The time course of recovery from this repression may precipitate drug-withdrawal side effects while long-term suppression of channel expression may contribute to remodeling of heart tissue. The alternative is that chronic suppression may overcome current antiarrhythmic drug limitations of acute cardioversion and result in the benefit of maintained rhythm control. Nonetheless, together these data give further credence to concerns regarding the comprehensiveness of current ion channel drug safety tests.
In summary, this report reveals a novel mechanism of antiarrhythmic drug action in the modulation of surface channel density. Results of this study highlight the possibility for development of new agents that selectively modulate ion conduction and/or the stability of channel protein in the membrane as an alternative or complementary strategy for treating atrial fibrillation and other potential cardiac arrhythmias.
Supplementary Material
Acknowledgements
We thank Dr. Benedict Lucchesi (University of Michigan) for his insight and discussion regarding this work, and Dr. Tomas Kirchhausen (Harvard Medical School) for the generous gift of Dynasore.
Sources of Funding This work was supported by the Systems and Integrative Biology Training Grant (to S.M.S.) and NIH grant HL0270973 (to J. R. M.).
Footnotes
Disclosures None.
References
- 1.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane database of systematic reviews (Online) 2007:CD005049. doi: 10.1002/14651858.CD005049.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Mahe I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Archives of internal medicine. 2006;166:719–728. doi: 10.1001/archinte.166.7.719. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5:1034–1049. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- 4.Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. American heart journal. 2006;151:771–778. doi: 10.1016/j.ahj.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, Wible B, Li GR, Wang Z, Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- 6.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Human molecular genetics. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 7.van der Heyden MA, Smits ME, Vos MA. Drugs and trafficking of ion channels: a new pro-arrhythmic threat on the horizon? British journal of pharmacology. 2008;153:406–409. doi: 10.1038/sj.bjp.0707618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wible BA, Hawryluk P, Ficker E, Kuryshev YA, Kirsch G, Brown AM. HERG-Lite: a novel comprehensive high-throughput screen for drug-induced hERG risk. Journal of pharmacological and toxicological methods. 2005;52:136–145. doi: 10.1016/j.vascn.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, Wible BA, Brown AM. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Molecular pharmacology. 2004;66:33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Drolet B, Simard C, Roden DM. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation. 2004;109:26–29. doi: 10.1161/01.CIR.0000109484.00668.CE. [DOI] [PubMed] [Google Scholar]
- 11.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. The Journal of biological chemistry. 2007;282:29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- 12.Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of Shaker-like potassium channels to lipid rafts. The Journal of biological chemistry. 2000;275:7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- 13.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophysical journal. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyders J, Knoth KM, Roberds SL, Tamkun MM. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Molecular pharmacology. 1992;41:322–330. [PubMed] [Google Scholar]
- 15.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 16.Caballero R, Pourrier M, Schram G, Delpon E, Tamargo J, Nattel S. Effects of flecainide and quinidine on Kv4.2 currents: voltage dependence and role of S6 valines. British journal of pharmacology. 2003;138:1475–1484. doi: 10.1038/sj.bjp.0705199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T, Snyders DJ, Roden DM. Inhibition of cardiac potassium currents by the vesnarinone analog OPC-18790: comparison with quinidine and dofetilide. The Journal of pharmacology and experimental therapeutics. 1997;280:1170–1175. [PubMed] [Google Scholar]
- 18.Chen FS, Steele D, Fedida D. Allosteric effects of permeating cations on gating currents during K+ channel deactivation. The Journal of general physiology. 1997;110:87–100. doi: 10.1085/jgp.110.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White NJ. Cardiotoxicity of antimalarial drugs. The Lancet infectious diseases. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 20.Snyders DJ, Yeola SW. Determinants of antiarrhythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ Res. 1995;77:575–583. doi: 10.1161/01.res.77.3.575. [DOI] [PubMed] [Google Scholar]
- 21.Decher N, Kumar P, Gonzalez T, Pirard B, Sanguinetti MC. Binding site of a novel Kv1.5 blocker: a “foot in the door” against atrial fibrillation. Molecular pharmacology. 2006;70:1204–1211. doi: 10.1124/mol.106.026203. [DOI] [PubMed] [Google Scholar]
- 22.Decher N, Pirard B, Bundis F, Peukert S, Baringhaus KH, Busch AE, Steinmeyer K, Sanguinetti MC. Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. The Journal of biological chemistry. 2004;279:394–400. doi: 10.1074/jbc.M307411200. [DOI] [PubMed] [Google Scholar]
- 23.Drolet B, Simard C, Mizoue L, Roden DM. Human cardiac potassium channel DNA polymorphism modulates access to drug-binding site and causes drug resistance. The Journal of clinical investigation. 2005;115:2209–2213. doi: 10.1172/JCI23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Developmental cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods in enzymology. 2008;438:77–93. doi: 10.1016/S0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- 28.Smillie KJ, Cousin MA. Dynamin I phosphorylation and the control of synaptic vesicle endocytosis. Biochemical Society symposium. 2005:87–97. doi: 10.1042/bss0720087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbeck G, Remp T, Hoffmann E. Effects of Class I drugs on atrial fibrillation. Journal of cardiovascular electrophysiology. 1998;9:S104–108. [PubMed] [Google Scholar]
- 30.Colatsky TJ, Follmer CH, Starmer CF. Channel specificity in antiarrhythmic drug action. Mechanism of potassium channel block and its role in suppressing and aggravating cardiac arrhythmias. Circulation. 1990;82:2235–2242. doi: 10.1161/01.cir.82.6.2235. [DOI] [PubMed] [Google Scholar]
- 31.Siddoway LA, Roden DM, Woosley RL. Clinical pharmacology of old and new antiarrhythmic drugs. Cardiovascular clinics. 1985;15:199–248. [PubMed] [Google Scholar]
- 32.Camm AJ. Safety considerations in the pharmacological management of atrial fibrillation. International journal of cardiology. 2008;127:299–306. doi: 10.1016/j.ijcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Colatsky TJ. Mechanisms of action of lidocaine and quinidine on action potential duration in rabbit cardiac Purkinje fibers. An effect on steady state sodium currents? Circ Res. 1982;50:17–27. doi: 10.1161/01.res.50.1.17. [DOI] [PubMed] [Google Scholar]
- 34.Slawsky MT, Castle NA. K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. The Journal of pharmacology and experimental therapeutics. 1994;269:66–74. [PubMed] [Google Scholar]
- 35.Zhang L, Foster K, Li Q, Martens JR. S-acylation regulates Kv1.5 channel surface expression. Am J Physiol Cell Physiol. 2007;293:C152–161. doi: 10.1152/ajpcell.00480.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.