Abstract
It is well established that cAMP signaling is an important regulator of the oocyte meiotic cell cycle. Conversely, the function of cGMP during oocyte maturation is less clear. Herein, we evaluated the expression of cGMP-hydrolyzing phosphodiesterases (PDEs) in the somatic and germ cell compartments of the mouse ovarian follicle and demonstrate that PDE5 is preferentially expressed in somatic cells. Cyclic GMP is a potent inhibitor of cAMP hydrolysis from oocyte extracts, with a 50% inhibitory concentration of 97 nM. Luteinizing hormone (LH) stimulation of cultured preovulatory follicles results in a marked decrease in cGMP content, and a nadir is reached in 1.5 h; similarly, oocyte cGMP levels decrease after gonadotropin stimulation in vivo. The LH-dependent decrease in cGMP requires activation of the epidermal growth factor network. Treatment of follicles with a PDE5 inhibitor increases cGMP in the follicle well above unstimulated levels. Although LH causes a decrease in cGMP in follicles preincubated with PDE5 inhibitors, the levels of this nucleotide remain above unstimulated levels. Under these conditions of elevated cGMP, LH stimulation does not cause oocyte maturation after 5 h of incubation. Microinjection of a cGMP-specific PDE into oocytes causes meiotic maturation of wild-type oocytes, suggesting that an intraoocyte pool of cGMP is involved in the maintenance of meiotic arrest. This effect is absent in PDE3A-deficient oocytes. Taken together, these findings provide evidence that cGMP and cAMP signaling cooperate in maintaining meiotic arrest via regulation of PDE3A and that a decrease in cGMP in the somatic compartment is one of the signals contributing to meiotic maturation.
Keywords: cell cycle, cyclic adenosine monophosphate, cyclic guanosine monophosphate, follicle, GVBD, luteinizing hormone, meiosis, meiotic arrest, meiotic maturation, oocyte, ovary, PDE3A, PDE5A
A pool of cGMP is present in the oocyte and maintains meiotic arrest; a decrease in cGMP content in the follicle is required for LH induction of oocyte maturation.
INTRODUCTION
In the female, germ cells begin meiosis soon after colonization of the fetal gonad but then enter a prolonged period of quiescence at late prophase. This stage is characterized by the presence of a morphologically distinguishable nucleus (germinal vesicle [GV]) with a prominent nucleolus and is associated with partial condensation of the chromosomes. For development into a fertilizable egg, oocytes must reenter the meiotic cell cycle and complete the first meiotic division with extrusion of the first polar body. They then arrest in metaphase of the second meiotic division and are ovulated. The physiological stimulus for oocyte meiotic resumption is the preovulatory surge of luteinizing hormone (LH) that triggers reactivation of the meiotic machinery, and its morphological manifestation is the breakdown of the oocyte nuclear envelope, termed GV breakdown (GVBD) [1].
A role for cyclic nucleotides in the maintenance of meiotic arrest was recognized early on, when it was shown that inhibitors of cAMP hydrolysis or membrane-permeant cAMP analogs prevented spontaneous meiotic maturation in oocytes removed from the follicular environment [2–7]. This concept has been further confirmed by recent data demonstrating that high levels of intraoocyte cAMP are essential in vivo for maintaining the meiotic arrest [8–12]. Although it is now widely accepted that a decrease in cAMP is the signal for meiotic resumption in mammals and amphibians, the biochemical steps involved are not well defined. It has been proposed that closure of the extensive network of gap junction communication between the oocyte and somatic cells, or between mural granulosa cells and cumulus cells, is the event that causes a decrease in cAMP in the oocyte [13, 14]. However, several observations are difficult to reconcile with this model, and a positive signal affecting oocyte cAMP, rather than a removal of a negative constraint, has been implicated [15, 16].
Cyclic GMP has also been shown to be involved in the regulation of oocyte maturation, as several studies have indicated that cGMP levels in the somatic compartment of the ovary decrease in response to LH treatment both in vitro and in vivo [17, 18] and that intraoocyte cGMP levels drop during spontaneous oocyte maturation [19]. Conversely, an increase in cGMP in the germ cell compartment has been correlated with a delay in the resumption of meiosis, suggesting that endogenous cGMP may be inhibitory to meiotic resumption [19–21]. Several recent studies [22–24] show that guanylate cyclase activators (such as nitric oxide [NO] donors) inhibit oocyte meiotic resumption. However, in some of these studies [22, 25] NO donors have opposing effects on the resumption of meiosis depending on the dosage, thus complicating the interpretation of the results.
We have recently reported that phosphodiesterase type 3A (PDE3A) is required for resumption of meiosis in mammalian oocytes and that females lacking PDE3A are infertile [26]. The possibility that PDE3A could be regulated by cGMP in the oocyte [27], as well as the data suggesting a role for cGMP in oocyte meiotic resumption, prompted us to further investigate the interplay between ovarian cGMP homeostasis, PDE3A, and oocyte meiotic resumption.
MATERIALS AND METHODS
Materials
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Rockford, IL) unless otherwise specified. Tritiated cyclic nucleotides were from Amersham Healthcare (Piscataway, NJ). The PDE inhibitors UK-090234 and TP-10, demonstrating selectivity for PDE1 and PDE10A, respectively, were generous gifts from Pfizer Inc. (New York, NY). BAY 60–7550 was from Alexis Biochemicals (Plymouth Meeting, PA). Purified sildenafil (Viagra; Pfizer Inc.) and tadalafil (Cialis; Lilly-ICOS Co., Indianapolis, IN) and purified recombinant bovine PDE5 holoenzyme [28] were generously provided by Drs. Jackie Corbin and Sharron Francis (Vanderbilt University, Nashville, TN). The protein kinase A inhibitor (PKI)-tide peptide (Ile-Ala-Ala-Gly-Arg-Thr-Gly-Arg-Arg-Gln-Ala-Ile-His-Asp-Ile-Leu-Val-Ala-Ala) was from AnaSpec Co. (San Jose, CA). Cyclic GMP concentrations in preovulatory follicles (POFs) were determined using the cGMP enzyme immunoassay (EIA) kit (Cayman Chemical Co., Ann Arbor, MI). Phosphate-buffered saline (PBS) without calcium and magnesium was from the University of California, San Francisco, cell culture facility. Ultrapure water, fetal bovine serum (FBS), α minimal essential medium (α-MEM), and Novex Sharp protein ladder were from Invitrogen (Carlsbad, CA). Millicell-CM culture plate insert was from Millipore (Billerica, MA). Protease and phosphatase inhibitor tablets were from Roche Diagnostics (Indianapolis, IN) and were used as directed by the manufacturer. The ECL Plus Western blotting kit was from Amersham Healthcare. Mouse monoclonal antibody against connexin 43 was from Becton Dickinson (Fair Lakes, NJ). Rabbit polyclonal antibodies against phosphorylated serines 255 and 262 of connexin 43 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit IgG from Pierce (Thermo Fisher Scientific). Equine chorionic gonadoptropin (eCG) and diethylamine NONOate were from Calbiochem (San Diego, CA). Recombinant LH (rLH) was from Serono International (Rockland, MA).
Animals
Immature wild-type (WT) female mice (C57BL/6) from Charles River Laboratories (Davis, CA) were stimulated at age 22–23 days with 5 IU of eCG (administered i.p.) to promote the development of ovarian POFs. After 44–48 h, animals were euthanized, and ovaries were excised. Oocytes, cumulus-oocyte complexes (COCs), and POFs were collected as previously described [12]. The Pde3a−/− colony (C57BL/6×129Sv), generated as previously described [26], was established and maintained through heterozygous breeding. Mice were genotyped by PCR using specific primers designed to detect WT and targeted alleles from extracted tail DNA as previously described [26]. All experiments using PDE3A-null oocytes were performed with mice of C57BL/6×129Sv mixed background. Animal procedures and euthanasia were in accord with accepted standards of humane animal care and were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Assay of PDE Activity in Ovaries, Preovulatory Follicles, and Denuded Oocytes
Whole ovaries were washed two times in PBS, frozen in liquid nitrogen, and stored at −80°C until use. After two cycles of freeze thawing, ovaries were homogenized in 500 μl of lysis buffer (LB) at pH 7.4 (50 mM Tris-HCl, 1% NP-40, 150 mM NaCl, 1 mM edetic acid, and protease and phosphatase inhibitors) and then gently rotated at 4°C for 30 min. Lysate was clarified by centrifugation at 4°C for 5 min at 1500 × g, and the protein concentration of the supernatant was determined by bicinchoninic acid (BCA) protein assay. The protein sample was diluted to 0.048 μg/μl in 10 mM potassium phosphate (pH6.8) with 25 mM beta-mercaptoethanol (KPM)-bovine serum albumin (BSA), and 10 μl was assayed for cGMP-PDE activity in a final volume of 100 μl as previously described [29], with 300 nM [3H]cGMP as substrate and 2 mM ethyleneglycoltetracetic acid (EGTA). Under these conditions, the assays were linear with respect to time and tissue amount (data not shown). Characterization of cGMP-PDE activity in POFs was performed as described for whole ovaries. Assay of cGMP-PDEs in denuded oocytes (DOs) was performed as follows: groups of 100 DOs were collected within 15 min of ovary dissection in M2 media (Millipore, Billerica, MA) with 0.4% BSA. The DOs were then washed three times with 2 ml of PBS, the PBS was aspirated, and the DOs were flash frozen in liquid nitrogen and placed at −80°C until use. Oocytes (n = 300) were lysed by addition of 150 μl of LB, followed by brief vortexing and gentle rotation at 4°C for 30 min. Lysate was spun at 4°C at 14 000 rpm for 10 min, the supernatant was diluted 1:1 in KPM-BSA, and 10–30 μl of supernatant was assayed for cGMP-PDE activity as already described. Cyclic AMP PDE activity in DOs was assayed as aforedescribed except that 1 μM cAMP was used as substrate. For follicle and oocyte samples, a subset of assays was also run in the absence of EGTA to determine the effect on the calcium-sensitive PDE1 component.
Culture of Preovulatory Follicles and DOs
POFs were cultured on Millicell-CM culture plate inserts placed in 35-mm tissue culture dishes in 1.4 ml of α-MEM supplemented with 5% FBS, 100 μg/ml penicillin G, and 50 μg/ml streptomycin sulfate. Groups of 10–15 POFs were cultured in 5% CO2 at 37°C. POFs were preincubated with the compounds listed in Results for the specified times before incubation with 5 IU of rLH as indicated. At the end of the incubation, POFs were punctured to release COCs, and meiotic maturation was assessed in the DOs. Progression of meiotic maturation was defined as the disappearance of a visible GV and nucleolus and was expressed as percentage of GVBD. Denuded oocytes or COCs were cultured in M2 medium with 0.4% BSA in 100-μl drops under mineral oil at 37°C. The meiotic maturation rate was recorded at 200× magnification using an inverted microscope (Leica Instruments, Wetzler, Germany) fitted with a modulation contrast system.
Additional POFs were isolated and cultured as previously described [30]. Groups of 10–18 POFs were preincubated in the presence or absence of 1 μM sildenafil for 1 h. Then, DEA (10 μM) and carbenoxolone (100 μM) were added, and follicles were cultured an additional 5 h. At the end of culture, oocytes were released from POFs by needle puncture, denuded of cumulus cells, and scored for GVBD.
Microinjection of WT and Pde3a−/− Oocytes
Denuded oocytes were injected as previously described [11] using a programmable microinjector (Femtojet Express; Eppendorf AG, Hamburg, Germany). Injection volumes were calculated to be between 10 and 15 picoliter (pL) of the sample. Oocytes from WT mice were isolated in M2 media with 0.4% BSA and 3.5 mM hypoxanthine (HX) and were injected 20–30 min after isolation with the reagents indicated in Results. Immediately after injection, oocytes were transferred to a fresh drop of M2 media with HX and placed on a 37°C warming tray, and maturation was scored every 20 min for the first 2.5 h. For incubation times >2.5 h, oocytes were moved into α-MEM medium supplemented with 5% FBS and 3.5 mM HX and were incubated at 37°C in 5% CO2. Meiotic progression was scored for up to 24 h to check for polar body extrusion. Oocytes from Pde3a−/− animals were isolated, injected, and cultured in the same medium as WT without HX. As a control, PDE5 was heat denatured by boiling for 10 min before injection.
Measurement of cGMP Levels
POFs (n = 10–80) were cultured as already described in the presence or absence of treatments as indicated in Results. After incubation, the POFs were collected and washed three times with PBS, and the PBS was aspirated. POFs were immediately snap frozen in liquid nitrogen and stored at −80°C until use. For determination of oocyte cGMP levels, eCG-primed mice were injected i.p. 44–48 h later with vehicle or 5 IU of hCG. At 2 h after injection, animals were euthanized, and oocytes were collected by puncturing of POFs. Denuded oocytes were washed three times in PBS, snap frozen in liquid nitrogen, and stored at −80°C. For the assay of cGMP levels, 80 μl and 160 μl of ultrapure water were added to oocytes and POFs, respectively. Samples were immediately placed in a boiling water bath for 5 min, cooled to room temperature, and spun at 15 000 × g in a tabletop centrifuge for 1 min. Next, 20 μl and 40 μl of 25% trichloroacetic acid (TCA) were added to oocytes and POFs, respectively. The POFs and oocytes were then homogenized by a microcentrifuge tube-compatible dounce homogenizer and were briefly vortexed and spun at 15 000 × g for 10 min. The supernatant was withdrawn and placed in a clean tube, and the TCA was extracted three times with 1 ml of water-saturated diethyl ether by freezing on dry ice. Residual ether was then removed by vacuum centrifugation (Speed Vac; Savant, Hicksville, NY) until dry. Samples were resuspended in 45 μl and 100 μl of buffer provided in the cGMP EIA kit for oocytes and POFs, respectively. Samples and cGMP standards were acetylated and quantified as described in the cGMP EIA kit.
Western Blot Analysis of Phosphorylated and Nonphosphorylated Connexin 43
POFs were isolated and cultured as previously described [30]. POFs were preincubated with or without 1 μM sildenafil for 1 h, after which time DEA (10 μM) and rLH (5 IU) were added. After 2 h, POFs were washed in PBS and homogenized in ice-cold RIPA buffer containing protease and phosphatase inhibitors. Samples were centrifuged for 5 min at 4°C, supernatants were transferred to new Eppendorf tubes, and protein concentrations were assayed using the BCA Protein Assay Kit (Pierce [Thermo Fisher Scientific]). Protein samples (5 μg) were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene fluoride membranes. Membranes were blocked in tris buffered saline with 0.1% Tween20 (TBST) plus 5% nonfat dry milk for 1 h at room temperature and were incubated overnight at 4°C with anti-phospho-connexin 43 (serine 262) or anti-phospho-connexin 43 (serine 255) diluted 1:200 in TBST plus 5% BSA. The next morning, membranes were washed in TBST and incubated with anti-rabbit IgG-HRP for 1 h at room temperature. After washing with TBST, specific signals were detected using ECL Plus reagent and visualized by autoradiography.
Afterward, membranes were incubated for 30 min at 50°C in a solution containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, and 100 mM β-mercaptoethanol to strip away bound antibodies. Membranes were washed with TBST, blocked for 1 h at room temperature in TBST plus 5% nonfat dry milk, and incubated overnight at 4°C with anti-connexin 43 diluted 1:1000 in TBST plus 0.2% milk. The next morning, membranes were washed in TBST and incubated with anti-mouse IgG-HRP for 1 h at room temperature. Specific signals were detected as already described.
Statistical Analysis
Data are expressed as the mean ± SEM. Statistical analysis was performed using ANOVA, followed by Bonferroni posttest for comparisons of multiple groups, or paired Student t-test was used for direct comparison of data derived from two groups. Values with P < 0.05 were considered statistically significant.
RESULTS
Cyclic GMP Is a Potent Inhibitor of Mouse Oocyte PDE3 Activity
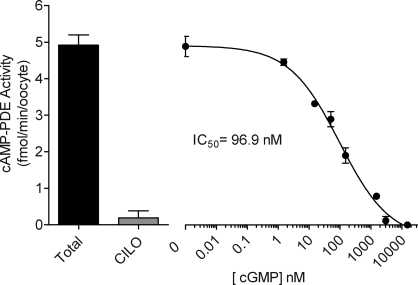
It has previously been shown that PDE3 hydrolytic activity is inhibited by cGMP in platelets and thymocytes [31, 32]. Therefore, we examined whether cGMP could function as a regulator of mouse oocyte PDE3. Phosphodiesterase 3-mediated cAMP hydrolysis was assayed in lysates from isolated GV-stage DOs in the presence or absence of increasing concentrations of cGMP. As previously shown [27], the majority of cAMP hydrolysis in the oocyte is inhibited by the PDE3-selective inhibitor cilostamide (Fig. 1). Most important, we show that cGMP is a potent inhibitor of oocyte cAMP hydrolytic activity, with a 50% inhibitory concentration (IC50) of 97 nM (Fig. 1).
FIG. 1.
Cyclic GMP inhibits PDE3 activity in DOs. Denuded oocytes were isolated from 24-day-old mice after 42–44 h of stimulation with 5 IU of eCG. The cAMP-PDE activity was measured in total oocyte extract as described in Materials and Methods. The equivalent of 20 oocytes was used per point. Activity was measured in the presence of increasing concentrations of cGMP (1.5–15 000 nM), and 4000 nM cilostamide (CILO) was used as a positive control for PDE3 inhibition. Data are the mean ± range of two independent experiments performed in triplicate. Activity is expressed as femtomoles of cAMP hydrolyzed per minute per oocyte.
Characterization of cGMP-Hydrolyzing PDEs in the Somatic and Germ Cell Compartments of the Ovarian Preovulatory Follicle
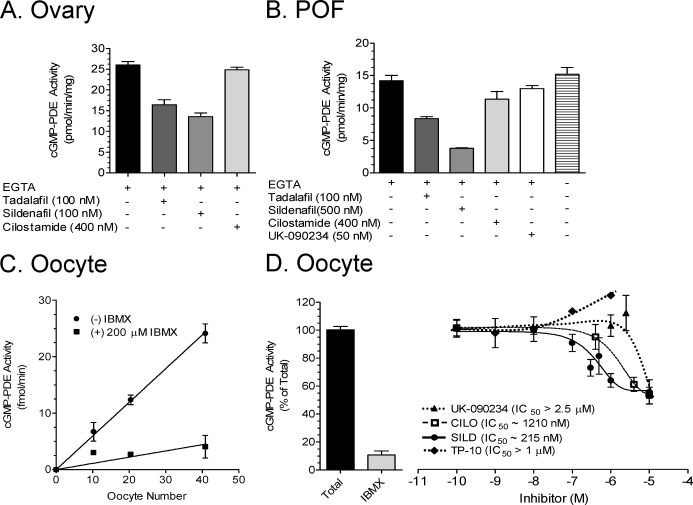
To define the repertoire of cGMP-hydrolyzing PDEs that maintain cGMP homeostasis in the different cellular compartments of the ovarian POF, we measured cGMP hydrolysis in extracts of whole ovaries, follicles, and oocytes in the presence or absence of inhibitors with selectivity for PDE1 (UK-090234), PDE2 (BAY 60–7550), PDE3 (cilostamide), PDE5 (tadalafil and sildenafil), and PDE10 (TP-10). Phosphodiesterase 1, PDE2, PDE3, and PDE10 hydrolyze both cAMP and cGMP, while PDE5 and PDE6 are specific for cGMP hydrolysis [33]. In lysates of whole ovaries, a large component of total cGMP-PDE activity was inhibited by 100 nM of either of the PDE5-selective inhibitors sildenafil and tadalafil (Fig. 2A). Similarly, in isolated POFs, a substantial PDE5 component was observed with the inclusion of 100 nM tadalafil (Fig. 2B). UK-090234 (50 nM) had a minor effect on cGMP-PDE activity in POFs, suggesting the presence of PDE1 (Fig. 2B). This PDE1 component was confirmed by the omission of EGTA from the assay, which slightly increased the total cGMP-PDE activity (Fig. 2B). As a calcium chelator, EGTA acts as a partial inhibitor of calcium-sensitive PDE1 activity. In both whole ovaries and POFs, 400 nM cilostamide had a negligible effect on cGMP hydrolysis (Fig. 2, A and B).
FIG. 2.
Cyclic GMP PDE activity in whole ovary, follicles, and DOs. Ovaries, POFs, and DOs were collected from 24-day-old mice after 42–44 h of stimulation with 5 IU of eCG. The cGMP-PDE activity was measured on total cell extracts as described in Materials and Methods. Final concentrations of inhibitors are indicated. A and B) Activity in the ovary (A) and POFs (B). Activity is expressed in picomoles of cGMP hydrolyzed per minute per milligram of total protein for ovary and POFs. The plus and minus signs indicate the presence (+) and absence (−) of the indicated compounds. C) Cyclic GMP PDE activity in femtomoles of cGMP hydrolyzed per minute in relation to oocyte number. D) The effects of inhibitors on the oocyte cGMP PDE activity. Activity in D is expressed as a percentage, where total activity is 0.39 fmol of cGMP hydrolyzed per minute per oocyte. Data are the mean ± SEM of at least four independent experiments.
Cyclic GMP hydrolytic activity was detected in lysates of DOs. The amount of cGMP hydrolysis was proportional to the number of oocytes used and was inhibited by isobutylmethylxanthine (IBMX), a nonselective inhibitor of all PDEs except PDE8 and PDE9 (Fig. 2C). To further investigate the nature of this oocyte cGMP hydrolytic activity, we used PDE inhibitors with selectivity for all the known cGMP-hydrolyzing enzymes (see above). Using this pharmacological approach, no effect of any of the inhibitors used between 0.1 nM and 100 nM could be observed. These are concentrations sufficient to inhibit >90% of the corresponding recombinant enzymes (data not shown and previous findings [26, 27]). Partial inhibitory effects were observed at higher concentrations of sildenafil, cilostamide, or UK090234 (Fig. 2D). The PDE10-selective inhibitor TP-10 did not inhibit the oocyte cGMP-PDE even at high concentrations (Fig. 2D). BAY 60–7550 at 50 nM and tadalafil at 100 nM had no inhibitory effects (data not shown).
As an alternative approach to identify the PDE genes responsible for the cGMP hydrolytic activity of the oocyte, two public total RNA microarray databases on GV-stage oocytes were examined (GEO Datasets assembled from the Gene Expression Omnibus [GEO] repository, call numbers GDS2300 and GDS1978, found at http://www.ncbi.nlm.nih.gov/pubmed). This analysis yielded positive calls for PDE1A, PDE1B, PDE3A, PDE3B, PDE6A, PDE6B, noncatalytic PDE6D (formerly PrBP), PDE9A, and PDE10A. In addition, seven public expressed sequence tag (EST) datasets were scanned for cGMP-hydrolyzing PDE transcripts from oocytes and unfertilized eggs (http://meg.otago.ac.nz/greeneggdatabase/index.html and http://www.ncbi.nlm.nih.gov/unigene [library numbers Lib. 18552, Lib. 1617, Lib. 16178, Lib. 10029, Lib. 14142, and Lib. 1389]). This approach yielded the following EST hits: one for PDE1B, six for PDE3A, four for PDE3B, one for PDE6B, 11 for PDE6D, and seven for PDE10A. Last, we examined one public microarray database and one unpublished microarray database generated from mRNAs isolated from polysomal fractions of metaphase II and GV-stage oocytes, respectively (GEO Dataset number GDS2387 at http://www.ncbi.nlm.nih.gov/pubmed and M. Conti, unpublished data). The following cGMP-PDEs were present in both databases: PDE1A, PDE1B, PDE1C, PDE2A, PDE3A, PDE6B, PDE6C, PDE6G (noncatalytic), PDE6H (noncatalytic), PDE6D, PDE10A, and PDE11A. Thus, PDE6 and PDE10 were identified as the two most abundant transcripts coding for cGMP-PDEs expressed in the oocyte.
LH Regulates cGMP Concentration in the Follicle via Regulation of the Endothelial Growth Factor Network
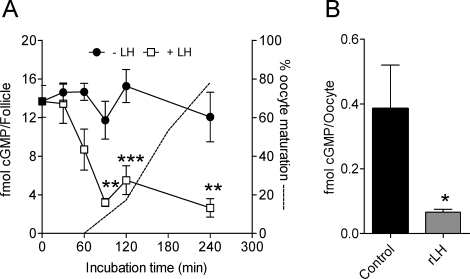
Luteinizing hormone stimulation of cultured POFs produces a marked decrease in cGMP levels (Fig. 3A). This decrease was observed after 1 h of incubation of culture and reached a nadir at 1.5 h. In all experiments performed, there was a 61% decrease in cGMP from 18.3 ± 2.0 to 7.1 ± 1.4 fmol/follicle (n = 11; P < 0.001) after 1-h exposure to LH. This decrease in cGMP concentration in the follicle was associated with an even more profound decrease in cGMP concentration in the oocyte. Cyclic GMP in the oocyte decreased by more than 80% at 2 h after hCG stimulation in vivo (0.40 ± 0.13 to 0.07 ± 0.01 fmol/oocyte; n = 4) (Fig. 3B).
FIG. 3.
Time course of cGMP accumulation in the preovulatory follicle exposed to LH and effect of hCG on oocyte cGMP levels. A) Preovulatory follicles were dissected from eCG-primed immature mice. After 30 min of equilibration in vitro under the conditions detailed in Materials and Methods, rLH at a final concentration of 5 IU was added at Time 0. At the times indicated on the abscissa, follicles were harvested, frozen in liquid nitrogen, and stored until use. For the cGMP assay, extracts were processed as detailed in Materials and Methods, and the cGMP content was measured by ELISA with acetylated samples. Filled and open squares represent follicles with and without LH treatment, respectively. The dotted line represents the time course of oocyte maturation as determined by percentage of GVBD. Asterisks denote statistical significance by two-way ANOVA, followed by Bonferroni posttest, where *** is P < 0.001, and ** is P < 0.01. Each point is the mean ± SEM of three independent experiments conducted on different days. B) Groups of 50 oocytes were dissected from eCG-primed immature mice 2 h after injection of vehicle or 5 IU of hCG. For the cGMP assay, oocytes were processed as detailed in Materials and Methods, and the cGMP content was measured by ELISA with acetylated samples. The asterisk indicates statistical difference, where P < 0.05.
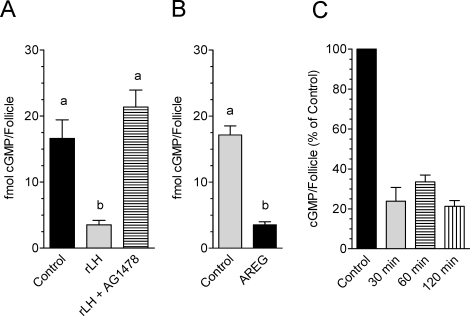
Because many of the LH effects at the time of ovulation are mediated by activation of the epidermal growth factor (EGF) network [34, 35], we tested whether the inhibitory effects of LH on cGMP production also involve this intermediate pathway. The decrease in cGMP that follows LH exposure is prevented when follicles are incubated with the EGF receptor (EGFR) kinase inhibitor AG1478 (Fig. 4A). Moreover, recombinant amphiregulin (AREG) at concentrations that induce oocyte maturation also causes a decrease in cGMP identical to that induced by LH (Fig. 4B). Epiregulin, another EGF-like growth factor, had an effect identical to that of AREG (data not shown). Finally, when the time course of cGMP accumulation in follicles exposed to AREG was investigated, there was no apparent time lag before a decrease was detected. Cyclic GMP was reduced by 80% within 30 min of AREG stimulation (Fig. 4C). Together with the observation that EGF-like growth factor effects are blocked by treatment with a PDE5 inhibitor (Supplemental Fig. S1 available at biolreprod.org), these findings demonstrate that activation of the EGF network is required for the inhibitory effects of LH on cGMP accumulation.
FIG. 4.
Activation of the EGF network is required for the LH-induced drop in follicular cGMP. Groups of 10–20 POFs were cultured with the specified treatments for the indicated times, and cGMP levels were determined as described in Materials and Methods. Different letters at the top of the bars indicate statistical difference, where P < 0.05. A) POFs were preincubated in the presence or absence of 500 nM AG1478 and then stimulated for 2 h with either 5 IU or rLH. B) POFs were treated with or without 100 nM AREG for 2 h. C) POFs were cultured in the presence or absence of 100 nM AREG for the indicated times. Data are expressed as percentage of cGMP per follicle compared with the control for the indicated time. The data represent the mean ± range of two independent experiments.
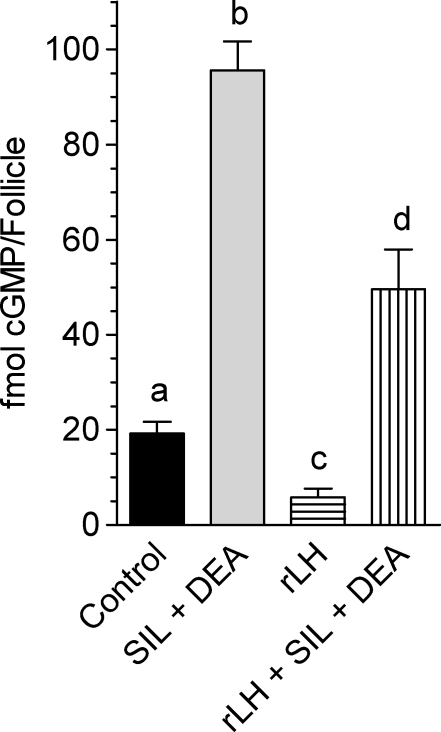
Preincubation of POFs with a cGMP-PDE inhibitor such as sildenafil together with a guanylyl cyclase stimulator (DEA) produced a 5-fold increase in basal cGMP levels (Fig. 5). When LH was added together with sildenafil and DEA to these cultured POFs, cGMP levels were decreased but remained well above the unstimulated levels in the absence of PDE5 inhibitors (Fig. 5).
FIG. 5.
Luteinizing hormone decreases cGMP content in cultured follicles, while sildenafil and DEA increase cGMP. Groups of 20–30 POFs were incubated in the presence or absence of rLH for 1 h with or without preincubation with 1 μM sildenafil (SIL) and 10 μM DEA for a total culture time of 2 h. Cyclic GMP levels were determined as described in Materials and Methods. Data shown are femtomoles of cGMP per follicle ± SEM of at least three independent experiments. Different letters at the top of the bars indicate statistical difference, where P < 0.05.
Induction of Oocyte Maturation Is Prevented by Increasing cGMP in the Preovulatory Follicle
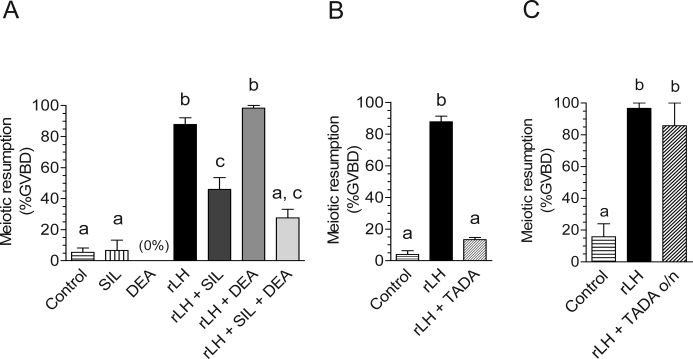
Assuming that the communication between somatic cells and oocytes is intact before LH stimulation and given the decrease in cGMP measured in the oocyte, we hypothesized that preincubation of isolated POFs with PDE5-selective inhibitors would have a significant effect on oocyte meiotic resumption. Stimulation of POFs with LH induced meiotic maturation in 88% of the oocytes, whereas pretreatment with sildenafil (1 μM) for 1 h reduced it to 46% (Fig. 6A). Exposure of POFs to 10 μM NO donor DEA before LH stimulation had no effect by itself but led to a modest potentiation (P > 0.05) of the sildenafil effect (Fig. 6A). Tadalafil, a structurally unrelated PDE5 inhibitor, showed effects comparable to those of sildenafil in preventing LH-induced oocyte maturation (data not shown). When the preincubation time was increased to 1.5 h, tadalafil (Fig. 6B) and sildenafil (data not shown) decreased LH-induced GVBD to 20% and 14%, respectively. However, LH induction of oocyte meiotic resumption in POFs after overnight culture in the presence of tadalafil (Fig. 6C) or sildenafil (data not shown) resulted in GVBD rates similar to those of the control LH-stimulated groups. Given the absence of detectable PDE5 cGMP-hydrolyzing activity in the oocyte, these results suggest that an increase of cGMP in the somatic compartment interferes with the normal time course of LH-induced maturation.
FIG. 6.
In cultured follicles, PDE5 inhibitors prevent LH-induced maturation, and DEA augments this effect. Groups of 10–20 POFs were used for each experimental condition. A) Follicles were preincubated with or without 1 μM sildenafil (SIL) for 1 h, followed by 10 μM DEA for 5 min when indicated. POFs were stimulated with 5 IU of rLH for 4 h; follicles were then punctured, and oocytes were scored for signs of meiotic resumption (GVBD). B) Follicles were preincubated in the presence of 1 μM tadalafil (TADA) for 1.5 h and then stimulated with rLH. C) Follicles were preincubated for 1.5 h in the presence of 1 μM tadalafil with or without LH stimulation, and meiotic resumption was scored at the end of overnight culture. The graphs show the mean ± SEM of at least three independent experiments. Different letters at the top of the bars indicate statistical difference, where P < 0.01.
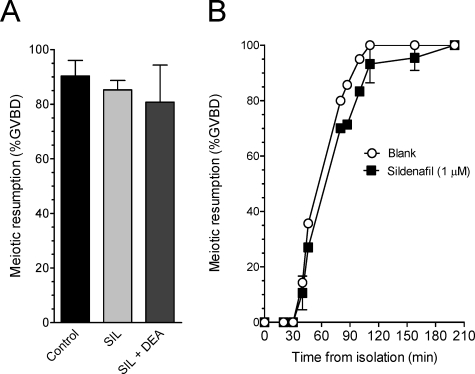
To determine whether PDE5 inhibitors act directly on the oocyte, COCs and DOs were isolated within 15 min of removal of the ovary and transferred to culture dishes with or without 1 μM sildenafil (COCs and DOs) or 1 μM sildenafil plus 10 μM DEA (COCs). Spontaneous oocyte meiotic resumption was assessed after 2.5 h of incubation of COCs or at various time points for up to 4 h for DOs. No effect of sildenafil treatment on GVBD was observed in COCs compared with the control (Fig. 7A). In addition, we observed no effect of DEA plus sildenafil on oocyte maturation in COCs (Fig. 7A). As shown in Figure 7B, there was also no effect of sildenafil on the time course of GVBD in DOs. Preincubation of oocytes with sildenafil for 1.5 h also had no significant effect on the time course of spontaneous maturation (data not shown). Thus, these findings are consistent with the absence of significant PDE5 activity in the oocyte (Fig. 2D) and demonstrate that the inhibition of follicle-enclosed oocyte maturation using sildenafil or tadalafil reflects an increase of cGMP in the somatic compartment.
FIG. 7.
Sildenafil and DEA do not affect spontaneous maturation in mouse COCs and DOs. A) Groups of 20–30 COCs were incubated in 100-μl drops of media under mineral oil for 2.5 h in the presence or absence of 1 μM sildenafil (SIL) alone or with 10 μM DEA plus sildenafil. The maturation stage of oocytes was scored after removal of the granulosa cells and is expressed as percentage of GVBD. B) The DOs were collected in media with or without 1 μM sildenafil and were placed in 100-μl drops of media under mineral oil. The DOs were scored for meiotic maturation as described in Materials and Methods, with Time 0 representing the time of DO isolation. The data represent the mean ± SEM of five independent experiments for A and two independent experiments for B.
An alternative explanation of this finding is that cGMP could affect the functionality of the gap junctions and, indirectly, oocyte maturation [24]. Therefore, we tested whether treatment of POFs with sildenafil and DEA has an effect on LH-induced phosphorylation of connexin 43 on specific serine residues shown to be associated with gap junction closure [16]. No effects were observed on LH-dependent phosphorylation of connexin 43 on serines 262 and 255 for up to 2 h, suggesting that neither drug affects the LH-induced gap junction closure under the experimental conditions tested (Supplemental Fig. S2). In addition, sildenafil plus DEA did not affect the induction of oocyte maturation in POFs cultured in the presence of the gap junction blocker carbenoxolone (Supplemental Fig. S3).
Meiotic Maturation of Isolated Oocytes Is Induced by cGMP Removal Only in the Presence of PDE3A
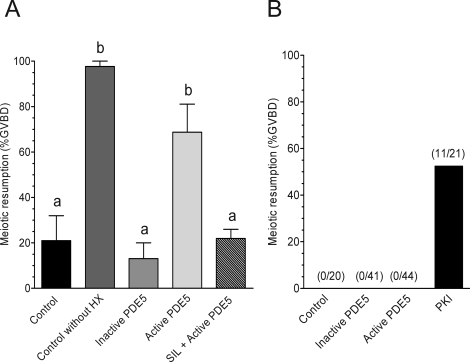
Based on calculations from our measurements in vivo and those of others during spontaneous maturation [19], the intraoocyte cGMP concentration is within the range for inhibition of the cAMP catalytic activity of PDE3A (Fig. 1). Given the presence of an extensive network of gap junctions connecting oocytes to somatic cells, cGMP may equilibrate in the oocyte from the somatic compartment [16, 36, 37], reaching a concentration in the oocyte sufficient to maintain PDE3A in the inhibited state. To test this hypothesis, we injected oocytes with a purified cGMP-specific PDE to deplete the intraoocyte cGMP pool. Wild-type oocytes in HX media were microinjected with 10–15 pL (4.5–6.8 pg) of purified recombinant PDE5 and scored for signs of meiotic progression 2.5 h later. As shown in Figure 8A, there was a significant increase in GVBD with PDE5 injection over control noninjected oocytes. This effect on GVBD required the catalytic activity of PDE5, as denaturation of PDE5 by heat inactivation or preincubation of PDE5 with sildenafil (1 μM) before injection abrogated this effect (Fig. 8A). Conversely, injection of PDE5 into Pde3a−/− oocytes had no effect on GVBD (Fig. 8B), demonstrating that the presence of PDE3A is required for the cGMP depletion to be effective. In addition, the experiment with PDE3A-null oocytes demonstrates that cAMP is not hydrolyzed by PDE5 because injection of a cAMP-PDE (PDE3A) induces maturation [38]. As shown in Figure 8B, injection of 10–15 pL (60–90 pg) of PKI-tide caused a significant increase in GVBD, demonstrating the meiotic competence of the Pde3a−/− oocytes.
FIG. 8.
A pool of cGMP in the oocyte contributes to meiotic arrest through PDE3A. A) Wild-type DOs were microinjected in the presence of 3.5 mM HX with inactive PDE5 or active PDE5 or with active PDE5 preincubated with 1 μM sildenafil (SIL) for 1 h. B) Pde3a−/− oocytes were injected in the absence of HX with inactive PDE5, active PDE5, or PKI-tide. For both experiments, GVBD was scored 2.5 h after injection. The number of injected oocytes that underwent meiotic resumption out of the total number of injected oocytes are listed at the top of each bar. The graphs show the mean ± SEM of at least two independent experiments. Different letters at the top of the bars indicate statistical difference, where P < 0.05.
DISCUSSION
Recent studies [8, 10, 12, 39] have strengthened the hypothesis that endogenous oocyte cAMP is essential for maintaining the meiotic arrest in mammalian oocytes. Results of biochemical and genetic studies [26, 27] have suggested that PDE3A has a critical role in the control of cAMP and the resumption of meiosis in the mammalian oocyte. Herein, we provide evidence that cAMP and cGMP signaling is integrated in the POF and that regulation of both second messengers contributes to the control of oocyte meiotic arrest and to the LH-induced reentry into the meiotic cell cycle. Indeed, through activation of the EGF network, LH causes a major decrease in cGMP accumulation in the follicle before oocyte reentry into meiosis; preventing this decrease significantly delays meiotic maturation. In vivo administration of gonadotropin also causes a significant reduction in intraoocyte cGMP levels. Manipulation of cGMP in the oocyte further shows that a pool of cGMP is involved in the maintenance of meiotic arrest. Finally, our data indicate that a point of convergence of the two signaling pathways is PDE3A in the oocyte.
More than two decades ago, it was proposed that cGMP, in addition to cAMP, may have a role in meiotic resumption: a study [40] investigating oocyte metabolism initially reported that disruption of specific purine metabolic pathways in vivo induces the prompt resumption of oocyte maturation. Moreover, Törnell et al. [19, 20] provided evidence that cAMP and cGMP decrease at the same time during spontaneous oocyte maturation in the rat and that microinjection of cGMP delays meiotic resumption. With the present study, we significantly extend these previous observations by demonstrating a role for cGMP in the control of mouse oocyte maturation and show that a generalized decrease in cGMP concentration in the follicle precedes oocyte maturation.
The expression of PDE5 in granulosa cells of the POF is demonstrated by several findings. Using a pharmacological approach, PDE5 was identified as a major component of the cGMP hydrolytic activity in POFs. Consistent with this conclusion, levels of cGMP in cultured POFs were increased ∼5-fold above basal levels when treated with a selective PDE5 inhibitor (sildenafil) and DEA. This finding is in agreement with a recent study [41] demonstrating immunoreactive PDE5 protein in the mouse POF. Conversely, PDE activity with the pharmacological properties of a PDE5 could not be detected in DOs. When cGMP is increased in the somatic compartment by blocking PDE5 activity, a reversible blockade in meiotic maturation is observed. The effects on meiotic progression are likely indirect because oocytes do not express significant levels of PDE5 and PDE5 inhibitors used at concentrations that block only PDE5 activity have a negligible effect on the maturation of DOs or COCs.
In cultured mouse follicles, cGMP levels were significantly decreased 1 h after rLH stimulation, with a time course that precedes oocyte maturation. The decrease in follicular cGMP induced by LH reaches a nadir at 1.5 h, whereas the increased phosphorylation of connexin 43 reaches a maximum at 2 h (data not shown). It has been shown that phosphorylation of connexin 43 closely parallels the permeability of the gap junctions between granulosa cells and oocytes [16]. Thus, our data suggest that the decrease in cGMP may precede, or perhaps coincides with, the closure of the gap junctions. Although closure of these junctions is sufficient to induce oocyte maturation [24], it has recently been shown that maturation can be induced even in the presence of open junctions [16], and findings in our laboratory are consistent with this conclusion (Vaccari et al., unpublished results). Therefore, it is possible that a decrease in cGMP induced by LH in granulosa cells is the signal that causes meiotic maturation when the gap junctions are prevented from closing.
All experiments we have performed are consistent with the conclusion that LH effects on cGMP accumulation in granulosa cells are indirect and require activation of the EGF network. AREG and EREG mimic the LH effects on cGMP, and the time course is faster than that produced by LH. Inhibition of the EGFR kinase also blocks the LH-dependent decrease in cGMP. Given these observations using cGMP as an end point, our data reassert the obligatory role of the EGF network in LH-mediated meiotic resumption and add strength to the idea that the gonadotropin-dependent decrease in cGMP is an important event in oocyte maturation.
The decrease in cGMP in the follicle does not appear to be associated with follicular activation of cGMP-PDEs, as we were unable to detect increased activity of these enzymes over a period of 2 h of LH stimulation (data not shown), consistent with a previous study [42] in the rabbit. Results of another study [43] have shown that gonadotropins do not affect cGMP hydrolysis in cultured granulosa cells. Furthermore, LH still causes a decrease in cGMP in the presence of PDE5 inhibitors, further excluding the possibility that LH acts through activation of a cGMP-PDE. In several species, LH modulates NO production [23, 44, 45], which may in turn modulate guanylate cyclase activity [42]. Therefore, it is possible that the mechanism by which LH regulates cGMP in the mouse POF is via the NO-guanylate cyclase pathway.
We measured an average of 0.40 fmol of cGMP per oocyte isolated from eCG-primed immature mice. Assuming an average oocyte volume of 280 pL, our calculated intraoocyte cGMP concentration is 1.4 μM. The reported concentration of cGMP in GV-stage oocytes measured by others is 1–2 μM [19], which agrees well with our measurement. These concentrations of cGMP are sufficient to significantly inhibit PDE3, as demonstrated in previous studies [46, 47] with recombinant PDE3A and PDE3 from platelets and confirmed herein using oocyte extracts. An intraoocyte concentration of 1–2 μM cGMP is sufficient to inhibit PDE3A by ≥80%. Luteinizing hormone stimulation causes an 80% decrease in cGMP in the oocyte, which should cause a 3-fold increase in PDE3A activity. Our data are thus consistent with a model in which the somatic compartment provides a tonic source of cGMP that is in dynamic equilibrium with the oocyte and holds it in meiotic arrest by inhibiting the cAMP hydrolytic activity of PDE3A. Luteinizing hormone inhibition of cGMP production in the somatic compartment leads to a decrease in cGMP in the oocyte, confirmed by our measurements, and an increase in PDE3A activity. Indeed, we have reported activation of PDE3A before oocyte maturation [27]. However, it is unlikely that this reported increase in activity is exclusively the consequence of cGMP decrease because the oocyte cGMP is diluted in the assay of PDE activity. Thus, it is possible that additional modes of regulation of PDE3A (such as phosphorylation) contribute to the overall increase in PDE3A activity before meiotic maturation. These findings underscore the central role of PDE3A in meiotic maturation, a role predicted by complete meiotic arrest and infertility of PDE3A-null females [26].
Injection of a cGMP-specific PDE (PDE5) into DOs induces meiotic maturation. This likely occurs via depletion of the oocyte cGMP pool, resulting in activation of PDE3A. This meiotic resumption is not due to direct cAMP hydrolysis by PDE5 because PDE5 is highly specific for cGMP [33] and injection of PDE5 into PDE3A-deficient oocytes does not cause meiotic resumption. This latter finding also indicates that PDE3A is required to translate the effects of a decrease in intraoocyte cGMP. As a caveat, a complication in the interpretation of the injection data is that this experiment is possible only when hypoxanthine, which partially blocks PDE3A, is used to hold WT oocytes in the GV stage. Thus, the function of the cGMP pool could be monitored only on a background of decreased PDE activity. In addition, we cannot formally exclude the possibility that an increase in cGMP in the somatic compartment produces additional effects in granulosa cells that prevent oocyte maturation. However, LH signaling is not globally perturbed by an increase in cGMP, as demonstrated by the efficient LH-dependent phosphorylation of gap junctions under conditions in which cGMP is increased 4-fold. In addition, potential off-target toxic effects of the compounds used on oocyte maturation are inconsistent with the observation that DOs mature normally in the presence of sildenafil and DEA. Finally, a cGMP effect through protein kinase G (PKG), rather than PDE3A as we have proposed, is unlikely in view of the reported observation that PKG inhibitors do not prevent oocyte maturation [41].
Cyclic GMP hydrolytic activity was readily detected in oocyte extracts, pointing to a regulation of cGMP concentration in the oocyte in addition to diffusion. This activity is inhibited by 200 μM IBMX, confirming that a PDE is responsible for the hydrolysis of this cyclic nucleotide. In addition, PDE10A, PDE6B, or PDE3A mRNAs were detected in mouse oocyte public microarray and EST databases. However, the pharmacological screening with available cGMP-PDE inhibitors did not produce a result consistent with mRNA expression. Inhibitors of PDE1, PDE2, PDE3, PDE5, and PDE10 do not affect the oocyte cGMP hydrolysis at concentrations inhibitory to the activity of the recombinant enzymes. In addition, the presence of PDE9 may be excluded because it is considered insensitive to IBMX. Phosphodiesterase 5 was excluded as a candidate for the oocyte cGMP PDE activity because of the absence of an effect of sildenafil and tadalafil at concentrations that completely inhibit purified PDE5 (100 nM) and the absence of ESTs corresponding to PDE5 transcripts. Sildenafil is a potent inhibitor of rod and cone PDE6 enzyme, with IC50 values ranging from 5 to 30 nM [48, 49]. Given the partial effect of sildenafil (at a concentration >100 nM) on the oocyte cGMP PDE activity, it is possible that the oocyte contains a PDE6 with a different subunit composition that results in an enzyme with decreased sensitivity to these inhibitors. Indeed, the inclusion of PDE6-specific inhibitory subunits has been shown to decrease the potency of certain inhibitors for PDE6 [48]. Along these lines, a recent study [50] demonstrates the presence of the PDE6C catalytic subunit in the porcine COC. It is also possible that multiple PDE genes contribute to the cGMP hydrolysis of the oocyte, such that the nature of the PDE could not be unequivocally identified by the pharmacological criteria used. The ambiguous nature of the oocyte cGMP PDE activity may also be reconciled by proposing that a new PDE with unique pharmacological properties is expressed in the oocyte; the oocyte enzyme is perhaps a unique PDE splicing variant or a novel PDE gene code for this enzyme. Finally, it cannot be formally excluded that oocyte extracts sequester or inactivate the inhibitors tested. This latter possibility is unlikely given that cilostamide remains a potent inhibitor of cAMP hydrolysis in oocyte lysates in experiments performed under conditions similar to those of the oocyte cGMP PDE assay (Fig. 1).
In summary, our data show that the cAMP and cGMP pathways are integrated and involved in the control of meiotic maturation. Thus, both cAMP signaling and cGMP signaling cooperate to maintain the meiotic arrest in the oocyte, and a decrease in either one of the cyclic nucleotides is a signal for meiotic maturation. According to our hypothesis, the cGMP effects on the cell cycle machinery are indirect and mediated by inhibition of PDE3A activity in the oocyte. The observed decrease in cGMP concentration in the somatic compartment induced by LH is conveyed to the oocyte, as a decrease in cGMP was also observed in this compartment 2 h after stimulation. It is likely that this decrease functions as an initial trigger for oocyte maturation. The demonstration that EGF-like growth factors are intermediates in the LH-dependent decrease in cGMP in the follicle further strengthens the overall hypothesis. Finally, it should be noted that the PDE5 expressed in the somatic compartment is a potential target for manipulation of fertility, as PDE5 inhibitors significantly delay gonadotropin-induced maturation. Moreover, selective inhibitors of the cGMP PDE enzymes expressed in the oocyte may have contraceptive properties.
Acknowledgments
We kindly acknowledge Pfizer Inc. for compounds UK-090234 and TP-10; Drs. Sharron Francis and Jackie Corbin for purified PDE5 holoenzyme and the PDE5-selective inhibitors sildenafil and tadalafil; and Dr. Laurinda Jaffe for helpful discussions.
Footnotes
1Supported by grants NIH R01 GM080527 and HD052909 (to M.C.) and by fellowships from the Lalor Foundation (to S.V.) and the American Heart Association (to J.L.W.).
These authors contributed equally to this work.
REFERENCES
- Eppig JJ.Oocyte-somatic cell interactions during oocyte growth and maturation in the mammal. Dev Biol (N Y 1985) 1985; 1: 313–347.. [DOI] [PubMed] [Google Scholar]
- Hillensjö T, Ekholm C, Ahrén K.Role of cyclic AMP in oocyte maturation and glycolysis in the pre-ovulatory rat follicle. Acta Endocrinol (Copenh) 1978; 87: 377–378.. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Freter RR, Ward-Bailey PF, Schultz RM.Inhibition of oocyte maturation in the mouse: participation of cAMP, steroid hormones, and a putative maturation-inhibitory factor. Dev Biol 1983; 100: 39–49.. [DOI] [PubMed] [Google Scholar]
- Vivarelli E, Conti M, De Felici M, Siracusa G.Meiotic resumption and intracellular cAMP levels in mouse oocytes treated with compounds which act on cAMP metabolism. Cell Differ 1983; 12: 271–276.. [DOI] [PubMed] [Google Scholar]
- Magnusson C, Hillensjö T.Inhibition of maturation and metabolism in rat oocytes by cyclic amp. J Exp Zool 1977; 201: 139–147.. [DOI] [PubMed] [Google Scholar]
- Cho WK, Stern S, Biggers JD.Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool 1974; 187: 383–386.. [DOI] [PubMed] [Google Scholar]
- Dekel N, Lawrence TS, Gilula NB, Beers WH.Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol 1981; 86: 356–362.. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M.Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol 1996; 178: 393–402.. [DOI] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M.Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol 2003; 258: 385–396.. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA.The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 2004; 306: 1947–1950.. [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M.The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 2005; 287: 249–261.. [DOI] [PubMed] [Google Scholar]
- Vaccari S, Horner K, Mehlmann LM, Conti M.Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol 2008; 316: 124–134.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N.Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 2005; 146: 1236–1244.. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N.Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 2006; 147: 2280–2286.. [DOI] [PubMed] [Google Scholar]
- Downs SM, Daniel SA, Eppig JJ.Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 1988; 245: 86–96.. [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA.Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 2008; 135: 3229–3238.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CJ, Greenwald GS.Cyclic nucleotides, DNA, and steroid levels in ovarian follicles and corpora lutea of the cyclic hamster. Biol Reprod 1982; 26: 230–240.. [DOI] [PubMed] [Google Scholar]
- Ratner A.Effects of follicle stimulating hormone and luteinizing hormone upon cyclic AMP and cyclic GMP levels in rat ovaries in vitro. Endocrinology 1976; 99: 1496–1500.. [DOI] [PubMed] [Google Scholar]
- Törnell J, Billig H, Hillensjo T.Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′,5′-cyclic monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand 1990; 139: 511–517.. [DOI] [PubMed] [Google Scholar]
- Törnell J, Brännström M, Hillensjö T.Different effects of cyclic nucleotide derivatives upon the rat oocyte-cumulus complex in vitro. Acta Physiol Scand 1984; 122: 507–513.. [DOI] [PubMed] [Google Scholar]
- Hubbard CJ, Terranova PF.Inhibitory action of cyclic guanosine 5′-phosphoric acid (GMP) on oocyte maturation: dependence on an intact cumulus. Biol Reprod 1982; 26: 628–632.. [DOI] [PubMed] [Google Scholar]
- Bu S, Xie H, Tao Y, Wang J, Xia G.Nitric oxide influences the maturation of cumulus cell-enclosed mouse oocytes cultured in spontaneous maturation medium and hypoxanthine-supplemented medium through different signaling pathways. Mol Cell Endocrinol 2004; 223: 85–93.. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamagata Y, Sugino N, Takayama H, Kato H.Nitric oxide inhibits oocyte meiotic maturation. Biol Reprod 2002; 67: 1588–1592.. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Galiani D, Nevo N, Dekel N.Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol Reprod 2008; 78: 1111–1118.. [DOI] [PubMed] [Google Scholar]
- Bilodeau-Goeseels S.Effects of manipulating the nitric oxide/cyclic GMP pathway on bovine oocyte meiotic resumption in vitro. Theriogenology 2007; 68: 693–701.. [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V.Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 2004; 114: 196–205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M.Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 2001; 65: 1444–1451.. [DOI] [PubMed] [Google Scholar]
- Corbin J, Blount MA, Weeks JL, Beasley A, Kuhn KP, Ho SJ, Saidi LF, Hurley JH, Kotera J, Francis SH.[3H]sildenafil binding to phosphodiesterase-5 is specific, kinetically heterogenous, and stimulated by cGMP. Mol Pharmacol 2003; 63: 1364–1372.. [DOI] [PubMed] [Google Scholar]
- Weeks JL, Zoraghi R, Francis SH, Corbin JD.The N-terminal domain of phosphodiesterase-11A4 (PDE11A4) decreases affinity of the catalytic site for substrates and tadalafil, and is involved in oligomerization. Biochemistry 2007; 46: 10353–10364.. [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M.Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 2008; 22: 924–936.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DH, Haslam RJ.Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol 1990; 37: 671–681.. [PubMed] [Google Scholar]
- Marcoz P, Prigent AF, Lagarde M, Nemoz G.Modulation of rat thymocyte proliferative response through the inhibition of different cyclic nucleotide phosphodiesterase isoforms by means of selective inhibitors and cGMP-elevating agents. Mol Pharmacol 1993; 44: 1027–1035.. [PubMed] [Google Scholar]
- Francis SH, Turko IV, Corbin JD.Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol 2001; 65: 1–52.. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SLC, Conti M.EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004; 303: 682–684.. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M.Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 2007; 27(5):1914–1924.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnell J, Billig H, Hillensjö T.Regulation of oocyte maturation by changes in ovarian levels of cyclic nucleotides. Hum Reprod 1991; 6: 411–422.. [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Wert SE, Brunner GD.A dramatic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol 1986; 113: 517–521.. [DOI] [PubMed] [Google Scholar]
- Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M.Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J 2006; 25: 5716–5725.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A.Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol 2002; 187: 153–159.. [DOI] [PubMed] [Google Scholar]
- Downs SM, Eppig JJ.Induction of mouse oocyte maturation in vivo by perturbants of purine metabolism. Biol Reprod 1987; 36: 431–437.. [DOI] [PubMed] [Google Scholar]
- Wang S, Ning G, Chen X, Yang J, Ouyang H, Zhang H, Tai P, Mu X, Zhou B, Zhang M, Xia G.PDE5 modulates oocyte spontaneous maturation via cGMP-cAMP but not cGMP-PKG signaling. Front Biosci 2008; 13: 7087–7095.. [DOI] [PubMed] [Google Scholar]
- Patwardhan VV, Lanthier A.Cyclic GMP phosphodiesterase and guanylate cyclase activities in rabbit ovaries and the effect of in-vivo stimulation with LH. J Endocrinol 1984; 101: 305–310.. [DOI] [PubMed] [Google Scholar]
- Conti M, Kasson BG, Hsueh AJ.Hormonal regulation of 3′,5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology 1984; 114: 2361–2368.. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Nakamura Y, Sugino N, Harada A, Takayama H, Kashida S, Kato H.Alterations in nitrate/nitrite and nitric oxide synthase in preovulatory follicles in gonadotropin-primed immature rat. Endocr J 2002; 49: 219–226.. [DOI] [PubMed] [Google Scholar]
- Mitsube K, Mikuni M, Matousek M, Brannstrom M.Effects of a nitric oxide donor and nitric oxide synthase inhibitors on luteinizing hormone-induced ovulation in the ex-vivo perfused rat ovary. Hum Reprod 1999; 14: 2537–2543.. [DOI] [PubMed] [Google Scholar]
- Shakur Y, Holst L, Landstrom T, Mozsesian M, Degerman E, Manganiello V.Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol 2001; 66: 241–277.. [DOI] [PubMed] [Google Scholar]
- Zhang W, Colman RW.Conserved amino acids in metal-binding motifs of PDE3A are involved in substrate and inhibitor binding. Blood 2000; 95: 3380–3386.. [PubMed] [Google Scholar]
- Zhang X, Feng Q, Cote RH.Efficacy and selectivity of phosphodiesterase-targeted drugs in inhibiting photoreceptor phosphodiesterase (PDE6) in retinal photoreceptors. Invest Ophthalmol Vis Sci 2005; 46: 3060–3066.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM.Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol 1998; 159: 2164–2171.. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Cote N, Gagnon MC, Richard FJ.Up-regulation of 3′5′-cyclic guanosine monophosphate-specific phosphodiesterase in the porcine cumulus-oocyte complex affects steroidogenesis during in vitro maturation. Endocrinology 2008; 149: 5568–5576.. [DOI] [PubMed] [Google Scholar]