Abstract
Pregnancy induces dynamic changes in the maternal environment that include reversible modifications in response to systemic mediators and local signals. The spleen can be used to determine the effects of pregnancy on multiple cellular populations, including those of the erythroid lineage and the immune system. Current evidence suggests that the transient increase in the size of the spleen during pregnancy is due to the expansion of erythroid precursors. However, it is unclear what factors contribute to this increase. Moreover, the additional erythroid cells may compete with neighboring leukocytes for growth factors or space, and this may in turn alter the function of these populations. Therefore, we assessed proliferation and apoptosis throughout gestation using in vivo bromodeoxyuridine incorporation and the TUNEL assay, respectively. Here, we show that erythroid-lineage TER-119+ cells expanded significantly in midgestation because of enhanced proliferation and diminished apoptosis. This correlated with increased expression of the erythropoietin receptor (Epor) and decreased expression of the death receptor Fas, respectively. Leukocytes demonstrated population-specific responses. Natural killer cells proliferated in early pregnancy. Both lymphocytes and CD11B+ cells underwent enhanced proliferation during midgestation. In contrast, neutrophils exhibited augmented proliferation throughout pregnancy. These subset-specific alterations in proliferation and death in the spleen suggest that complex regulation of population dynamics exists during pregnancy.
Keywords: apoptosis, erythrocytes, erythroid lineage, erythropoiesis, immunology, leukocytes, lymphocytes, lymphoid tissues, mouse, pregnancy, proliferation, TER-119
Erythroid lineage cells comprise an increased proportion of spleen cells during midgestation due to enhanced proliferation and decreased apoptosis.
INTRODUCTION
To support pregnancy, numerous adaptations must occur throughout the body, including accelerated erythropoiesis. The differentiation and maturation of erythrocytes from progenitor cells occur predominantly in the bone marrow, but a low level of splenic erythropoiesis takes place in adult mice [1–3]. The production of erythrocytes in the murine spleen is augmented during pregnancy [4–6] and may be mediated by erythropoietin (EPO) signaling [7–9]. A recent study demonstrated that the proportion of erythrocyte precursors in the murine spleen expands during midgestation [10]. However, this study did not address the effects this increase in erythroid cells had on other populations in the spleen.
The development and function of immune cells in lymphoid tissues are influenced by interactions with neighboring cells and the extracellular environment. Like other lymphoid organs, the spleen possesses an intricate architecture that facilitates cellular interactions for the efficient generation of immune responses [11]. During pregnancy, reversible changes occur in lymphoid tissues, including the increased size and cellularity of the spleen [12, 13], involution of the thymus [14, 15], and hyperplasia of the uterine-draining lymph nodes [16, 17]. The integration of systemic hormonal fluctuations and local factors may modify the function, proliferation, trafficking, or death of cell populations. Alterations in the size or activity of specific populations could influence other resident cells through the direct competition for niche or the availability of cytokines and growth factors. Because pregnancy induces characteristic changes in the spleen, we undertook detailed analyses of the proportion, proliferative capacity, and susceptibility to apoptosis of erythroid-lineage cells and leukocytes during normal gestation.
MATERIALS AND METHODS
Mice and Breeding
C57BL/6 (B6) mice (8–10 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained under specific pathogen-free conditions at the University of Vermont. Prior to mating, female mice were housed in groups of five to suppress estrus by the Whitten effect. Three days prior to mating, single female mice were transferred into vacant male cages to stimulate estrous cycling. For timed pregnancies, male mice were introduced in the evening, and the next morning female mice were visually checked for the presence of a copulation plug, which was denoted Day 0 of pregnancy. Male mice were then removed, and plugged female mice were housed in groups of three or four throughout gestation. Mice were euthanized by carbon dioxide treatment followed by cervical dislocation. Animals were used in accordance with the Institutional Animal Care and Use Committee at the University of Vermont.
Antibodies for Flow Cytometry and Cell Sorting
To identify specific erythroid and immune cell populations by flow cytometry, we used a panel of monoclonal antibodies against cell surface proteins that are generally accepted to characterize each cellular subset. Erythroid precursors were distinguished by the presence of the lymphocyte antigen 76 (LY76; TER-119) molecule, which is found on the red cell surface from the proerythroblast to the mature erythrocyte stage [18]. Throughout development, B lymphocytes selectively express the protein tyrosine phosphatase receptor type C (PTPRC; B220) protein, a glycosylated high-molecular weight splice variant of the common leukocyte antigen [19, 20]. The T-cell receptor beta chain (TCRB) is one component of the T-cell antigen receptor signaling complex [21] that is expressed exclusively on the surface of alpha/beta T lymphocytes [22]. The lymphocyte antigen 6 complex, locus G (LY6G; GR1) protein is expressed predominantly by neutrophils in peripheral tissues as well as on the cell surface of granulocytes in the bone marrow [23]. Cells of the monocyte/macrophage lineage can be characterized by expression of integrin alpha M (ITGAM; CD11B) [24]. Tissue resident macrophages in the spleen that are CD11B positive express genes involved in chemotaxis and bacterial degradation, whereas CD11B-negative cells express genes involved in phagocytosis and iron recycling [25]. Natural killer (NK) cells in C57BL/6 mice are identified by the unique expression of the killer cell lectinlike receptor subfamily B member 1C (KLRB1C; NKR-P1C; NK1.1) protein [26, 27].
The following monoclonal antibodies (mAbs) were used: phycoerythrin (PE)-anti-TER-119, PE-Cy5.5-anti-GR1, and PE-anti-NK1.1 (all from eBioscience Inc., San Diego, CA); PE-anti-TCRB and allophycocyanin-anti-B220 (BD Biosciences, San Jose, CA); and PE-Texas Red (PETR)-anti-CD11B (Invitrogen Corp./Caltag Laboratories, Carlsbad, CA).
In Vivo Bromodeoxyuridine Assay
To compare the proportion of proliferating cells from pregnant and nonpregnant mice, a modified bromodeoxyuridine (BrdU) incorporation assay was performed as described previously [28]. Briefly, pregnant mice and corresponding unmated (UM) controls were given four intraperitoneal injections of BrdU at 24, 20, 15, and 1 h prior to euthanasia. Spleens were harvested, and single-cell suspensions were generated in IMDM medium containing 10% fetal bovine serum (FBS; Invitrogen) and 1 μM beta-mercaptoethanol (Bio-Rad Laboratories, Hercules, CA). Cells were enumerated, and nonspecific binding was blocked by treatment with 0.5 μM Fcγ III/II Receptor (CD16/CD32; BD Biosciences). Then, cells were incubated with mAbs against the TER-119, B220, TCRB, GR1, CD11B, and NK1.1 molecules. Cells were next treated with ice-cold 70% ethanol in PBS (Mediatech, Herndon, VA) for 30 min and then washed with PBS, followed by treatment with PBS containing 1% methanol-free formaldehyde (1% form; Ted Pella Inc.; Redding, CA) for 15 min. After washing with PBS, cells were permeabilized with PBS-1% form containing 0.01% Tween-20 (Bio-Rad Laboratories) overnight. The next day, cells were washed with PBS and treated with 50 units of deoxyribonuclease I (Sigma-Aldrich, St. Louis, MO) in buffer containing 0.15 M NaCl and 4.2 mM MgCl2 (Sigma-Aldrich) at pH 5.0 for 15 min at 37°C. Samples were then washed with PBS containing 0.1% bovine serum albumin (PBS-0.1% BSA; Sigma-Aldrich) and were incubated with fluorescein isothiocyanate (FITC)-anti-BrdU (BD Biosciences) for 30 min. After washing with PBS-0.1% BSA, cells were fixed with PBS-1% formaldehyde containing 0.1% BSA (PBS-1% form-0.1% BSA). The BrdU incorporation into each cellular subset was detected by flow cytometry (BD LSRII; BD Biosciences) and quantified using FlowJo software analysis (Tree Star Inc., Ashland, OR).
TUNEL Assay to Detect Apoptosis
A modified version of the previously described [28] TUNEL assay was used to detect apoptotic cells by flow cytometry. Single-cell splenocyte suspensions were generated and surface stained with the same mAbs as in the BrdU assay. Cells were first fixed in PBS-1% form for 15 min, washed with PBS, and then permeabilized by treatment with ice-cold 70% ethanol in PBS for 15 min. After washing with PBS, cells were incubated with 10 units of terminal deoxynuclotidyl transferase (TdT) and 6.25 μM fluorescein-12–2′-deoxy-uridine-5′-triphosphate (FITC-dUTP) in 1× TdT reaction buffer with 2.5 mM cobalt chloride (all from Roche Applied Science, Indianapolis, IN) for 1 h at 37°C. After washing with PBS-0.1% BSA, samples were fixed with PBS-1% form-0.1% BSA, and TUNEL-positive cells from each cellular subset were determined by flow cytometry and FlowJo software analysis.
Fluorescence-Activated Cell Sorting and RT-PCR of Sorted Cells
To isolate TER-119+ cells, spleens from Day 12 pregnant mice and UM controls were harvested sterilely, and single-cell suspensions were generated in IMDM medium containing 10% FBS (Invitrogen). After blocking nonspecific binding by treatment with 0.5 μM Fcγ III/II Receptor (CD16/CD32; BD Biosciences), 30 million cells were stained with the PE-anti-TER-119 antibody for 30 min. After washing with PBS-0.1% BSA, cells were resuspended in IMDM medium without serum. The 100-μM tip of the BD FACSAria cell sorter (BD Biosciences) was used for sorting. Nucleated cells were selected by forward- and side-scatter gating, and aggregates were eliminated by doublet discrimination. Cells positive for PE staining were collected at greater than 90% purity. Total RNA was extracted from at least 50 000 cells using Trizol reagent (Invitrogen) per the manufacturer's guidelines. Samples were quantified by ultraviolet absorbance at 260 nm on a NanoDrop spectrophotometer (ThermoScientific, Wilmington, DE), and RNA integrity was tested using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA).
The iScript cDNA synthesis kit (Bio-Rad Laboratories) was used to synthesize cDNA from 250 ng of RNA template using a mix of random hexamers and oligo(dT)s. From each sample, cDNA was used to amplify the following target genes: Fas (Fas, forward 5′-AACAAAGTCCCAGAAATCGCCTATG-3′, reverse 5′-TCCTGTCTC CTTTTCCAGCACTT-3′); Fas ligand (Fasl, forward 5′-CGGTGGTATTTTTCATGGT TCTGGT-3′, reverse 5′-TACTGGGGTTGGCTATTTGCTTTTC-3′); interleukin 7 receptor (Il7r, forward 5′-ACCCAAGAATCAAGGAGGATGG-5′, reverse 5′-GGCTA AGATGACCAACAAAAACAC-3′); and erythropoietin receptor (Epor, forward 5′-TTCTGGTCCTCATCTCGTTGTTGC-3′, reverse 5′-CCTTGTGGGTGGTGAAGAGAC-3′). For quantification, cDNA was also amplified for five housekeeping genes: hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1, forward 5′-CAGTCCCAGCGTCGTGAT-3′, reverse 5′-CAAGTCTTTCAGTCCTGTCCATAA-3′); tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (Ywhaz, forward 5′-GCAACGATGTACTTGCTCTTTTGG-3′, reverse 5′-GTCCACAATTCCTTTCTTGTCATC-3′); beta-actin (Actb, forward 5′-GAAGATCAAGATCATTGCTCCTCCTG-3′, reverse 5′-CTCATCGTACTCCTGCTTGCTG-3′); beta-2 microglobulin (B2m, forward 5′-CAGTTCAGTATGTTCGGCTTCCC-3′, reverse 5′-ATGCTATCCAGAAAACCCC TCAAA-3′); and succinate dehydrogenase complex, subunit A, flavoprotein (Sdha, forward 5′-ATGCCAGGGAAGATTACAAAGTGC-3′, reverse 5′-GTAACCTTGCCAGTCTTGATGTCC-3′). Each reaction used 1 μl of cDNA, 150 nM forward and reverse primers, and 12.5 μl of Power Sybrgreen Master Mix (Applied Biosystems, Carlsbad, CA) in a 25-μl reaction. The reactions were performed on an ABI Prism 7000 (Applied Biosystems) using an initial denaturation of 10 min at 95°C and 40 cycles of 15 sec at 95°C and 60 sec at 60°C, followed by a melt-curve analysis to ensure that only the correct product was amplified.
Standard curves were generated for all of the target genes as well as the housekeeping genes using a single sample, which was serially diluted over the working range of the assay. Using these standard curves, the relative quantities of each sample were determined. Relative target mRNA values were normalized by dividing the target quantity by the geometric mean of the quantities of the housekeeping genes. Each sample was run in triplicate and averaged. Negative water controls were run for each primer set in the real-time PCR reaction. In each primer set, at least one primer was designed over an exon-exon junction.
Statistical Analysis
For analysis of flow cytometric data, groups of mice from each gestational day were compared to the UM controls using one-way analysis of variance (ANOVA) with Dunnett multiple-comparison test. For analysis of RT-PCR data, Student t-tests were used. In both cases, P values of less than 0.05 were considered significant. All analysis was performed using GraphPad Prism 4 (GraphPad Software, La Jolla, CA).
RESULTS
Cellular Proliferation and Death in the Spleen Changes Throughout Gestation
Consistent with previous observations [12, 13], C57BL/6 mice demonstrated a reversible increase in the weight (data not shown) and cellularity of the spleen during pregnancy. Although the total number of spleen cells remained relatively unchanged during early pregnancy, cell number was significantly elevated on Day 12 of gestation compared with UM mice (Fig. 1A). Splenic cellularity peaked at 300 million cells on Gestational Day 15, which was 2.6-fold higher than UM controls. By Day 18, cell number had decreased and was not significantly different from UM mice.
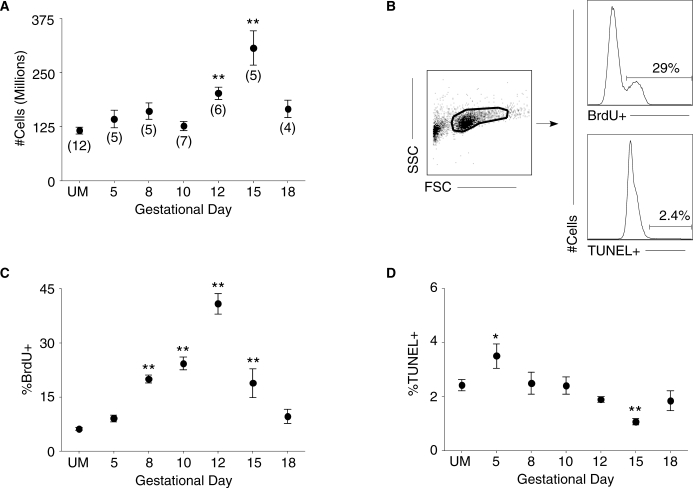
FIG. 1.
Splenic cellularity, proliferation, and apoptosis are affected by pregnancy. A) The total number of spleen cells from UM controls and pregnant mice throughout gestation were counted. Numbers in parentheses represent the total number of mice analyzed from each time point in this and all subsequent experiments. B) Representative analysis of flow cytometric data from a Day 12 pregnant mouse. Left: forward-scatter (FSC) versus side-scatter (SSC) plot of whole spleen. Right: histogram analysis for the proportion of BrdU+ (top) and TUNEL+ (bottom) cells. C) Percentage of total spleen cells that are BrdU+ on each gestational day. D) Percentage of spleen cells positive for TUNEL staining throughout pregnancy. In each graph, symbols and error bars depict mean and SEM. Statistical significance was determined by one-way ANOVA with Dunnett posttest using UM controls for comparison. *P < 0.05; **P < 0.01.
To investigate the contributions of proliferation and death to the changes in splenic cellularity during pregnancy, the in vivo BrdU incorporation and TUNEL assay were used. To analyze cellular proliferation, pregnant mice at various days of gestation and UM controls received intraperitoneal BrdU injections during the 24 h prior to euthanasia. Single-cell suspensions of spleen cells from these mice underwent parallel processing for BrdU incorporation and TUNEL staining, with the representative flow cytometric analysis shown (Fig. 1B). The proportion of proliferating cells in the spleen was significantly elevated on Day 8 of pregnancy compared with UM controls (Fig. 1C). Peak proliferation occurred on Gestational Day 12, when approximately 45% of cells manifested BrdU incorporation. The proportion of proliferating cells remained significantly elevated on Day 15 of pregnancy, but by Day 18 the fraction of BrdU+ cells was similar to that in UM mice.
Compared with UM mice, apoptosis was significantly elevated on Day 5 of pregnancy (Fig. 1D). From Gestational Day 8 to Gestational Day 12 there was a steady decrease in the proportion of apoptotic cells in the spleen, and on Day 15 of pregnancy the fraction of TUNEL+ cells was significantly reduced by 1.5-fold compared with UM controls. By late Gestational Day 18, the proportion of cells undergoing apoptosis was not statistically different from UM mice. Overall, these data suggest that enhanced proliferation coupled with diminished apoptosis contributed to the observed increase in splenic cellularity on Gestational Days 12 and 15.
Increased Proliferation and Decreased Apoptosis of TER-119+ Cells Contribute to the Increased Spleen Size in Pregnancy
Because the spleen is composed of erythroid precursors and multiple classes of leukocytes, we next analyzed the effects of pregnancy on the proliferation and death of each of these populations. Previous studies using histological staining [4, 5] and electron microscopy [6] demonstrated an increase in erythroid populations in the splenic red pulp during normal pregnancy in mice. Consistent with these observations, the proportion of splenic erythroid-lineage cells, as determined by staining for the TER-119 antigen, was significantly higher on Gestational Day 8 (13.5%) compared with the UM level (1.2%; Fig. 2A). The proportion of splenic TER-119+ cells peaked at 45% on Day 12 of pregnancy. On Day 15, the fraction of TER-119+ cells was diminished, and by late Gestational Day 18 it was not different from UM controls.
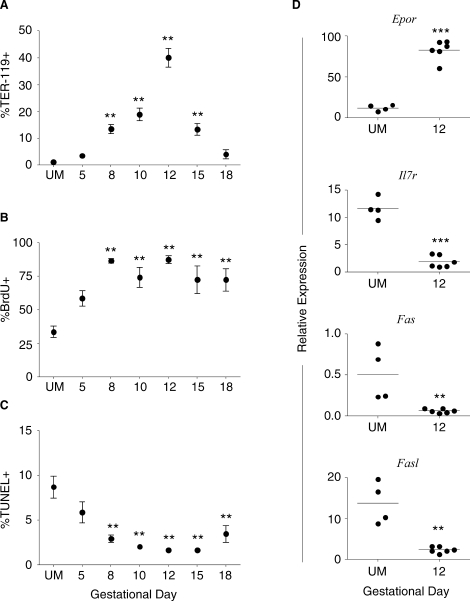
FIG. 2.
Pregnancy induces expansion of the splenic TER-119+ population by increasing proliferation and decreasing apoptotic death of these cells. A) Percentage of TER-119+ spleen cells from UM controls and pregnant mice throughout gestation. B) The proliferation of TER-119+ cells measured by BrdU incorporation during pregnancy. C) Apoptosis of TER-119+ cells throughout gestation determined by TUNEL assay. A–C) Symbols and error bars depict mean and SEM; y axes show percent positive cells; x axes show gestational day. Statistical analysis was performed by one-way ANOVA with Dunnett posttest. D) RT-PCR of sorted TER-119+ cells from UM controls and Day 12 pregnant mice; y axes show relative expression of Epor, Il7r, Fas, and Fasl as determined by standard curve analysis and normalized by dividing by the geometric mean of the housekeeping genes. Statistical significance was determined using the Student t-test to compare Day 12 samples to UM controls. **P < 0.01; ***P < 0.001.
The proliferation of TER-119+ cells in the spleen was significantly elevated beginning on Day 8 of gestation, as demonstrated by BrdU incorporation in more than 85% of cells (Fig. 2B). This enhanced level of proliferation in the TER-119+ population continued throughout pregnancy, with approximately 72% of these cells being BrdU+ on late Gestational Day 18. Concurrently, a reduced proportion of apoptotic TER-119+ cells were present beginning on Gestational Day 8 (Fig. 2C). By Day 10, apoptosis of TER-119+ cells was decreased by 4-fold compared with UM mice, and this reduction of TUNEL+ cells continued until Day 18 of pregnancy. These data suggest that the combination of increased proliferation and decreased death of TER-119+ cells contributed to the midgestational increase in splenic cellularity.
Increased Expression of the Erythropoietin Receptor Contributes to the Enhanced Proliferation of TER-119+ Cells During Midgestation
To determine the proliferative signals that influenced the expansion of TER-119+ cells during pregnancy, TER-119+ cells were sorted from the spleens of UM and Day 12 pregnant mice, and the expression of the erythropoietin receptor (Epor) and interleukin 7 receptor (Il7r) was determined by quantitative RT-PCR. The EPOR mediates the survival and proliferation of red cell precursors [7–9], and TER-119+ cells isolated from Midgestational Day 12 manifested an 8-fold increase in Epor expression compared with cells from UM controls (Fig. 2D). TER-119+ cells have been shown to express the interleukin 7 receptor and proliferate in response to interleukin 7 [29]; however, the relative expression of Il7r was 6-fold lower in TER-119+ cells from Day 12 pregnant mice compared with UM controls (Fig. 2D). These data suggested that increased expression of the erythropoietin receptor contributed to the observed proliferation of TER-119+ cells during midpregnancy.
Reduced Expression of Fas and Fas Ligand Contributes to the Decreased Apoptosis of TER-119+ Cells During Pregnancy
Signaling through the death receptor FAS after the binding of its ligand (FASL) can induce cell death, and the downregulation of these molecules was described recently as a mechanism for enhancing erythropoiesis [30]. To determine whether this pathway was involved in the decreased apoptosis of TER-119+ cells during gestation, the expressions of Fas and Fasl mRNA in TER-119+ cells sorted from UM and Day 12 pregnant mice were quantified. The relative expression of Fas in TER-119+ cells from pregnant mice was diminished by 8.5-fold compared with UM controls (Fig. 2D). Compared with UM mice, the expression of Fasl was also decreased by 6-fold on Day 12 of pregnancy (Fig. 2D). Together, these data suggested that the reduced expression of Fas and Fasl may contribute to the decreased apoptosis of TER-119+ cells during pregnancy. Thus, TER-119+ cells respond to gestational signals that drive a rapid expansion and contraction of this population, and this corresponded with the dynamic changes in the size and cellularity of the spleen during pregnancy.
Pregnancy Induces Dynamic Changes in Lymphocyte Proliferation and Death
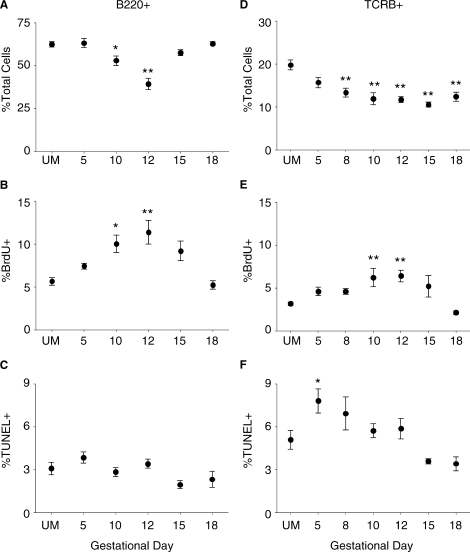
To understand how these dynamic changes in the TER-119+ population during pregnancy affected other cellular subsets, the changes in coexisting leukocyte populations were analyzed. Because lymphocytes represent a large fraction of spleen cells, cellular proportions, proliferative capacity, and susceptibility to apoptotic death of these cells were determined throughout gestation. During early gestation, the proportion of B220+ B cells in the spleen was not different from UM controls (Fig. 3A). However, on Midgestational Days 10 and 12, the fraction of splenic B220+ cells was significantly decreased, which coincided with the expansion of the TER-119+ population. By Day 15 of pregnancy, the proportion of B lymphocytes in the spleen had recovered and was not significantly different from UM mice. In spite of the observed proportional decrease in the B220+ population, the fraction of proliferating B cells was significantly increased on Midgestational Days 10 and 12 (Fig. 3B). B-cell proliferation was diminished on Day 15 of pregnancy, and by Day 18 it was not different from UM controls. There were no significant differences in the proportion of B cells undergoing apoptosis at any gestational day compared with UM mice (Fig. 3C). These data suggested that although there is a decreased proportion of splenic B cells during midgestation, neither the systemic effects of pregnancy nor the local expansion of TER-119+ cells diminished the proliferative capacity of these cells.
FIG. 3.
The effects of pregnancy on the total proportion, proliferation, and apoptosis of B and T lymphocytes. A–C) Pregnancy effects on B220+ B cells in the spleen. A) The proportion of splenic B220+ lymphocytes from UM controls and pregnant mice throughout gestation. B) Proliferation of B220+ cells in the spleen measured by BrdU incorporation. C) Pregnancy effects on the apoptosis of B220+ lymphocytes as determined by the TUNEL assay. D–F) The effects of pregnancy on splenic TCRB+ T-lymphocyte percentage (D), proliferation (E), and apoptosis (F). In all graphs, symbols and error bars depict mean and SEM, and x axes show gestational day. Statistical analysis was done by one-way ANOVA with Dunnett posttest. *P < 0.05; **P < 0.01.
Next, changes in the proportion, proliferation, and death of splenic T lymphocytes identified by the expression of the TCRB were analyzed. The proportion of TCRB+ T cells declined during early gestation and was significantly decreased on Day 8 compared with UM mice (Fig. 3D). This reduction continued through midgestation, and by Day 15 of pregnancy the proportion of TCRB+ cells was 50% lower than in UM controls. On Late Gestational Day 18, the fraction of splenic T cells had not yet recovered to the UM level. Despite their proportional decrease, T lymphocytes demonstrated increased proliferation on Midgestational Days 10 and 12 (Fig. 3E). This proliferation was transient, and by Day 15 the fraction of BrdU+ T cells was not significantly different from UM controls. On Early Gestational Day 5, T lymphocytes demonstrated a 1.5-fold elevation in apoptosis (Fig. 3F). However, by Day 8 of gestation the proportion of TUNEL+ T cells had decreased, and T-cell apoptosis was not significantly different from UM mice for the remainder of pregnancy. Thus, T cells from pregnant animals continued to proliferate during pregnancy, with only a brief period of elevated apoptosis, and responded to endogenous signals during midgestation to increase their proliferative rate.
Pregnancy Effects on Splenic Innate Immune Cell Proliferation and Death are Subset Dependent
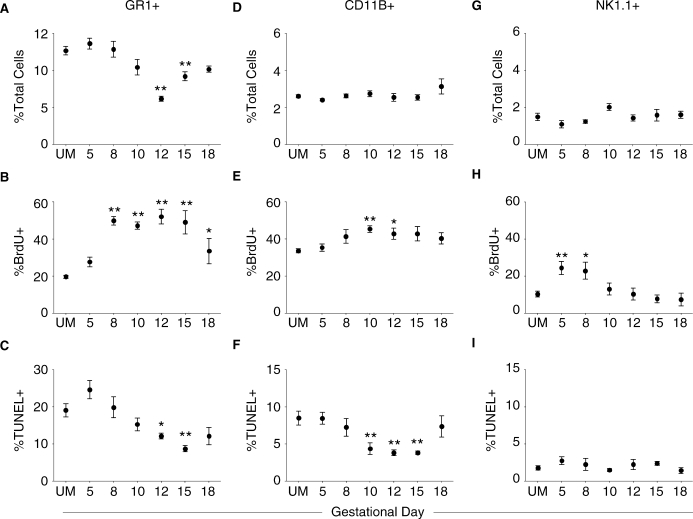
Because the proliferative and apoptotic responses of lymphocytes differed from TER-119+ cells, the effects of pregnancy on other immune cell populations in the spleen were analyzed. The proportion of neutrophils, as identified by the LY6G (GR1) protein, remained unchanged during early pregnancy compared with UM controls (Fig. 4A). However, during midgestation, the proportion of these cells declined, and by Day 12 it was reduced by 50%. The fraction of splenic neutrophils remained significantly decreased on Day 15, but by the end of gestation, the proportion of GR1+ cells had returned to the UM level. Similar to TER-119+ cells, neutrophils demonstrated significantly increased levels of proliferation throughout pregnancy, with nearly half of the GR1+ cells incorporating BrdU on Days 8 through 15 of gestation (Fig. 4B). The proportion of proliferating neutrophils began declining on Day 18, but this fraction was still elevated compared with UM controls. Neutrophils demonstrated slightly elevated apoptosis on Day 5 of pregnancy (Fig. 4C). This was followed by a gradual decline in the proportion of TUNEL+ cells until Gestational Day 12, when the fraction of apoptotic cells was significantly lower than in UM mice. The fraction of apoptotic GR1+ cells had returned to the UM level on Gestational Day 18.
FIG. 4.
Pregnancy effects on the proliferation and death of splenic innate immune cells are subset specific. A–C) Pregnancy effects on GR1+ neutrophils in the spleen. A) The total proportion of neutrophils in UM controls and pregnant mice throughout gestation. B) Proliferation of GR1+ cells during pregnancy measured by BrdU incorporation. C) Pregnancy effects on apoptosis of GR1+ neutrophils determined by the TUNEL assay. D–F) Pregnancy effects on splenic CD11B+ cell percentage (D), proliferation (E), and apoptosis (F). G–I) Pregnancy effects on NK1.1+ NK cell percentage (G), proliferation (H), and apoptosis (I) in the spleen. In all graphs, symbols and error bars depict mean and SEM, and x axes show gestational day. Statistical analysis was done by one-way ANOVA with Dunnett posttest. *P < 0.05; **P < 0.01.
The proportion of splenic macrophages, as identified by the presence of the ITGAM (CD11B) protein, was not significantly altered during pregnancy compared with UM mice (Fig. 4D). Similar to lymphocytes, the proliferation of macrophages was elevated during midgestation and returned to the UM level during late pregnancy (Fig. 4E). Concurrently, the apoptosis of splenic CD11B+ cells was significantly decreased beginning on Midgestational Day 10 (Fig. 4F). Macrophages continued to demonstrate diminished apoptosis on Days 12 and 15 of gestation, but by Day 18 the proportion of TUNEL+ cells was not different from UM controls.
Finally, the effects of pregnancy on NK cells in the spleen was determined by the expression of the KLRB1C (NKR-P1C; NK1.1) molecule. Natural killer cells accounted for approximately 2% of splenocytes in UM mice, and this proportion was not significantly altered throughout pregnancy (Fig. 4G). Unlike the other populations studied, the proportion of proliferating NK cells was 2-fold higher on Early Gestational Days 5 and 8 (Fig. 4H). The proliferation of NK1.1+ cells declined by Day 10 of gestation and was not different from UM controls throughout the remainder of pregnancy. The proportion of apoptotic NK cells in the spleen was not significantly different from that measured in UM mice at any gestational day examined (Fig. 4I). Together, these data demonstrated that the dynamic changes in the proliferation and death of innate immune cells throughout pregnancy were subset specific.
DISCUSSION
These observations highlight the contributions of cellular proliferation and death to the previously reported changes in spleen weight and cellularity throughout normal pregnancy in mice [12, 13]. Our studies showed that the midgestational increase in splenic cellularity is primarily due to a 40-fold increase in TER-119+ erythroid-lineage cells. Increased proliferation and decreased apoptosis beginning in early pregnancy contributed to the expansion of this population. TER-119+ cells isolated on Midgestational Day 12 had increased levels of mRNA for the erythropoietin receptor, suggesting that erythropoietin signaling may contribute to the increased proliferation of these cells. These cells also demonstrated reduced expression the death receptor Fas and its ligand Fasl, providing a possible explanation for their decreased apoptosis. Late in gestation, both the weight of the spleen and the proportion of TER-119+ cells decreased, but these cells continued to manifest elevated BrdU incorporation and decreased apoptosis. Possibilities to explain this observation include enhanced maturation [18] or the release of TER-119+ cells into the bloodstream.
T and B lymphocytes retained the ability to proliferate throughout pregnancy and demonstrated enhanced proliferation during midgestation, with no increase of apoptosis during this period. Thus, although the expansion of the TER-119+ population caused a reduction in the proportion of splenic lymphocytes, it did not directly suppress their proliferation or induce apoptosis. These data along with previous reports demonstrating that the total number [31] and the function of splenic lymphocytes are retained [32–34] suggest the immune system is not globally suppressed during pregnancy, as has been proposed by others [35–38].
Because innate immune cells are critical for the initiation and regulation of adaptive immunity, changes in these populations could influence overall immune responsiveness. In this model, we found that the proliferation and apoptosis of innate immune cells throughout gestation were subset dependent. Similarly to TER-119+ cells, neutrophils demonstrated enhanced proliferation and decreased apoptosis throughout gestation. CD11B+ splenic macrophages underwent increased proliferation and decreased apoptosis during midgestation only, similarly to lymphocytes. Uniquely, splenic NK cells experienced increased proliferation early in pregnancy, with no change in the fraction of apoptotic cells throughout gestation. The differences in these subsets argue against one global systemic signal controlling the proliferation and death of all immune cells during pregnancy.
Proliferation, death, and trafficking all contribute to the size of a cell population. In this study, in vivo BrdU incorporation over a 24-h period was used to determine the proliferation of splenic cells, with positive cells representing either resident cells that proliferated in the spleen or recently divided cells migrating through the spleen at the time of harvest. The TUNEL assay detected the fraction of apoptotic cells present in the spleen, which reflected the balance of apoptotic cell generation and splenic phagocyte clearance. However, neither of these methods addressed the contribution of immune cell trafficking to the reversible changes in the spleen during pregnancy.
Our studies show that alterations in proliferation and apoptosis underlie dynamic shifts in spleen cell populations during murine pregnancy. Understanding the interactions between these populations in the context of systemic and environmental signals may provide insight into human pregnancy and pregnancy-related diseases with immune components.
Acknowledgments
We thank Karen Oppenheimer for assistance with RNA purification and for performing the quantitative RT-PCR reactions, and Colette Charland for cell sorting. We are grateful to Drs. Jonathan Boyson, Matthew Poynter, and Karen Spach for critically reviewing the manuscript.
Footnotes
1Supported by National Institutes of Health grants HD43185, AI36333, AI4566, T32 AI055402, P20 RR021905, and the Department of Obstetrics, Gynecology, and Reproductive Sciences, University of Vermont College of Medicine.
REFERENCES
- Brodsky I, Dennis LH, Kahn SB, Brady LW.Normal mouse erythropoiesis. I. The role of the spleen in mouse erythropoiesis. Cancer Res 1966; 26: 198–201.. [PubMed] [Google Scholar]
- Curry JL, Trentin JJ, Wolf N.Hemopoietic spleen colony studies. II. Erythropoiesis. J Exp Med 1967; 125: 703–720.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich IN, Kubanek B.The ontogeny of erythropoiesis in the mouse detected by the erythroid colony-forming technique. I. Hepatic and maternal erythropoiesis. J Embryol Exp Morphol 1979; 50: 57–74.. [PubMed] [Google Scholar]
- Fruman GJ.Blood formation in the pregnant mouse. Blood 1968; 32: 242–248.. [PubMed] [Google Scholar]
- Fowler JH, Nash DJ.Erythropoiesis in the spleen and bone marrow of the pregnant mouse. Dev Biol 1968; 18: 331–353.. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Matsumura G, Ito T.Effects of pregnancy on erythropoiesis in the splenic red pulp of the mouse: a quantitative electron microscopic study. Arch Histol Jpn 1981; 44: 429–438.. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Miyata K, Yoshimura A.Proliferation and erythroid differentiation through the cytoplasmic domain of the erythropoietin receptor. J Biol Chem 1994; 269: 5976–5980.. [PubMed] [Google Scholar]
- Klingmuller U.The role of tyrosine phosphorylation in proliferation and maturation of erythroid progenitor cells–signals emanating from the erythropoietin receptor. Eur J Biochem 1997; 249: 637–647.. [DOI] [PubMed] [Google Scholar]
- Wojchowski DM, He TC.Signal transduction in the erythropoietin receptor system. Stem Cells 1993; 11: 381–392.. [DOI] [PubMed] [Google Scholar]
- Bustamante JJ, Dai G, Soares MJ.Pregnancy and lactation modulate maternal splenic growth and development of the erythroid lineage in the rat and mouse. Reprod Fertil Dev 2008; 20: 303–310.. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G.Structure and function of the spleen. Nat Rev Immunol 2005; 5: 606–616.. [DOI] [PubMed] [Google Scholar]
- Maroni ES, de Sousa MA.The lymphoid organs during pregnancy in the mouse. A comparison between a syngeneic and an allogeneic mating. Clin Exp Immunol 1973; 13: 107–124.. [PMC free article] [PubMed] [Google Scholar]
- Mattsson R, Nilsson B, Lindahl-Kiessling K.An investigation of splenic enlargement in pregnant mice. Dev Comp Immunol 1979; 3: 683–695.. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW.Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci U S A 1999; 96: 12021–12026.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller AL, Schnell FJ, Kersh GJ.Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology 2007; 121: 207–215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell JD, McDougall CM, Speedy G, Inchley CJ.Changes in lymphocyte accumulation and proliferation in the lymph nodes draining the pregnant uterus. Clin Exp Immunol 1978; 31: 397–407.. [PMC free article] [PubMed] [Google Scholar]
- Hetherington CM, Humber DP.The effect of pregnancy on lymph node weight in the mouse. J Immunogenet 1977; 4: 271–276.. [DOI] [PubMed] [Google Scholar]
- Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y.The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol 2000; 109: 280–287.. [DOI] [PubMed] [Google Scholar]
- Coffman RL.Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev 1982; 69: 5–23.. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Weissman IL.B220: a B cell-specific member of the T200 glycoprotein family. Nature 1981; 289: 681–683.. [DOI] [PubMed] [Google Scholar]
- Kuhns MS, Davis MM, Garcia KC.Deconstructing the form and function of the TCR/CD3 complex. Immunity 2006; 24: 133–139.. [DOI] [PubMed] [Google Scholar]
- Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M.Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol 1989; 142: 2736–2742.. [PubMed] [Google Scholar]
- Fleming TJ, Fleming ML, Malek TR.Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol 1993; 151: 2399–2408.. [PubMed] [Google Scholar]
- Springer T, Galfre G, Secher DS, Milstein C.Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol 1979; 9: 301–306.. [DOI] [PubMed] [Google Scholar]
- Gorgani NN, Ma Y, Clark HF.Gene signatures reflect the marked heterogeneity of tissue-resident macrophages. Immunol Cell Biol; 86: 246–254.. [DOI] [PubMed] [Google Scholar]
- Koo GC, Peppard JR.Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma 1984; 3: 301–303.. [DOI] [PubMed] [Google Scholar]
- Carlyle JR, Mesci A, Ljutic B, Belanger S, Tai LH, Rousselle E, Troke AD, Proteau MF, Makrigiannis AP.Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J Immunol 2006; 176: 7511–7524.. [DOI] [PubMed] [Google Scholar]
- Lens SM, Kataoka T, Fortner KA, Tinel A, Ferrero I, MacDonald RH, Hahne M, Beermann F, Attinger A, Orbea HA, Budd RC, Tschopp J.The caspase 8 inhibitor c-FLIP(L) modulates T-cell receptor-induced proliferation but not activation-induced cell death of lymphocytes. Mol Cell Biol 2002; 22: 5419–5433.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello FB, Keller JR, Klarmann KD, Dranoff G, Mazzucchelli R, Durum SK.IL-7 induces myelopoiesis and erythropoiesis. J Immunol 2007; 178: 1553–1563.. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M.Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood 2006; 108: 123–133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, Jiang SP.The fetus and the maternal immune system: pregnancy as a model to study peripheral T-cell tolerance. Crit Rev Immunol 1999; 19: 461–480.. [PubMed] [Google Scholar]
- Constantin CM, Masopust D, Gourley T, Grayson J, Strickland OL, Ahmed R, Bonney EA.Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol 2007; 179: 4383–4389.. [DOI] [PubMed] [Google Scholar]
- Harrison MR.Maternal immunocompetence. I. The graft-versus-host reactivity of lymphocytes from pregnant rats and the distribution pattern of 51Cr-labeled lymphocytes in pregnant mice. Scand J Immunol 1976; 5: 549–558.. [DOI] [PubMed] [Google Scholar]
- Pavia CS, Stites DP.Humoral and cellular regulation of alloimmunity in pregnancy. J Immunol 1979; 123: 2194–2200.. [PubMed] [Google Scholar]
- Baines MG, Pross HF, Millar KG.Effects of pregnancy on the maternal lymphoid system in mice. Obstet Gynecol 1977; 50: 457–461.. [PubMed] [Google Scholar]
- Hamilton MS, Hellstrom I.Altered immune responses in pregnant mice. Transplantation 1977; 23: 423–430.. [DOI] [PubMed] [Google Scholar]
- Parmiani G, Invernizzi G.Depression of cellular immune response during syngeneic pregnancy as measured by the graft-versus-host reaction. Transplantation 1975; 19: 363–368.. [DOI] [PubMed] [Google Scholar]
- Nicklin S, Billington WD.Impairment of graft versus host reactivity in pregnant mice. Clin Exp Immunol 1982; 49: 135–141.. [PMC free article] [PubMed] [Google Scholar]