Summary of Recent Advances
The elderly face significant risk for susceptibility to infection and cancer due to declining immune function. Various agents used in the setting of bone marrow transplantation and aging studies represent promising approaches to combating T cell defects in the aging population. Preclinical and clinical studies on the T cell reconstitution effects of sex steroid ablation, keratinocyte growth factor, the growth hormone pathway, and the cytokines interleukin-7, interleukin-12, and interleukin-15 indicate that these strategies may be used to alleviate the effects of T cell deficiencies in the elderly.
Introduction
Immune function decreases with age, due to quantitative and qualitative changes in the cells of the immune system and their niches. The deleterious effects of aging on the T cell compartment are well-studied as they lead to increased susceptibility to infection, decreased immunosurveillance of malignant cells, and difficulty in establishing protective immunity via vaccination. Aging reduces the number and T cell potential of hematopoietic precursors, and involution of the thymus renders it less capable of supporting de novo T cell development. Consequently, aging compromises the functional capacity of lymphocytes, resulting in a T cell pool with restricted receptor specificity and fewer naïve T cells [1,2]. These effects are reviewed in depth elsewhere in this issue, and we will concentrate instead on strategies to boost T cell function in aging individuals.
The conditioning regimens for hematopoietic stem cell transplantation (HSCT) cause significant damage to recipient thymi, resulting in prolonged post-transplant T cell deficiency [3]. Studies in experimental mouse models of HSCT have identified agents that are relevant for boosting T cell reconstitution not only after transplant, but also potentially in aging recipients.
Reviewed below are studies in older and transplanted mice, which elucidate mechanisms for improving T cell development.
Sex steroid ablation
Sex steroids are known to negatively regulate development of immune cells. The increase in sex steroids at puberty corresponds to the dramatic involution of the thymus at this time. Accordingly, prepubescent castration to reduce sex steroid levels prevents thymic atrophy and post-pubescent castration reverses thymic atrophy while enhancing T cell numbers in the periphery [4-9]. Murine models of autologous and allogeneic HSCT have indicated that surgical castration of recipients increases T cell reconstitution following transplantation [10,11].
Luteinizing Hormone-Releasing Hormone (LHRH) modulates testicular steroidogenesis, and LHRH agonist treatment results in chemical castration. Administration of the LHRH agonist Leuprolide to aging recipients leads to larger thymus size and improved thymic architecture [4-7]. Administration of Leuprolide following allogeneic HSCT significantly improves thymocyte and T cell reconstitution [12]. In addition to increased T cell numbers, Leuprolide also enhanced T cell function after allogeneic transplant in vitro and in vivo [12]. Leuprolide treatment following allogeneic HSCT leads to enhanced hematopoietic stem cells (lineage-Sca-1+c-kit+) and common lymphoid progenitors (lineage-ckitloIL-7Ra+) in the bone marrow, which may result from blocking deleterious effects of sex steroids on the bone marrow stromal cell compartment [12]. The increase in lymphoid progenitors likely contributes to enhanced T cell reconstitution.
Clinical trials using chemical castration with the LHRH agonist goserelin following autologous and allogeneic HSCT resulted in greater CD4 T cell regeneration, broad TCR repertoire, and enhanced peripheral T cell function [13].
Keratinocyte Growth Factor (KGF)
KGF, also called Fibroblast Growth Factor 7, mediates the proliferation and differentiation of epithelial cells, including thymic epithelial cells (TECs), which express the KGF receptor FGFR2IIIb [14]. Thymic fibroblasts, mesenchymal cells, and thymocytes can produce KGF [15-17]. KGF administration can protect the thymus from damage, and enhance T lymphopoiesis in young and old mice [18].
KGF-/- mice do not have thymic defects, but display impaired T cell reconstitution following sublethal irradiation and/or allogeneic and syngeneic BMT [19-21]. Administration of exogenous KGF results in enhanced thymic reconstitution following HSCT and can augment post-transplant T cell responses to DNA plasmid tumor vaccination [19,22]. HSCT recipients treated with KGF had (a) more T cells before vaccination, (b) greater numbers of tumor-specific T cells following vaccination, (c) a higher T effector-to-regulatory T cell ratio, (d) a larger proportion of memory T cells with central memory phenotype, and (e) effector T cells with a broader T cell receptor repertoire [22]. Pretransplant and peritransplant KGF treatment in rhesus macaques resulted in preservation of thymic architecture, increased thymic output and naïve T cell numbers, and enhanced T cell function [23].
KGF treatment in aged mice leads to improved thymocyte numbers, improved thymic architecture, and enhanced peripheral T cell function [19]. Middle-aged recipients of BMT treated with posttransplant KGF had increased thymopoiesis and peripheral T cell numbers [19].
In addition, KGF treatment can also be combined with sex steroid blockade resulting in preservation of thymic architecture, enhanced thymopoiesis and peripheral T cell reconstitution, and improved T cell repertoire and function [24].
KGF is FDA-approved for the prevention of mucositis in patients receiving high dose therapy (especially HSCT recipients). The proven safety of KGF and the extensive preclinical data regarding its effects on thymopoiesis make KGF and promising strategy to enhance thymopoiesis in the elderly. Clinical trials are on-going to determine if KGF administration to patients undergoing T cell-depleted allogeneic HSCT will enhance T cell reconstitution.
Growth hormone (GH), Insulin Growth Factor-1 (IGF-1), and ghrelin
GH is produced primarily by the pituitary, but it can also be produced by hematopoietic cells [25-28]. GH receptor is expressed on thymocytes, peripheral T cells, and various other cells of hematopoietic origin [29-32]. Aging humans have lower serum levels of GH, as well as IGF-1, a downstream target of GH signaling [33]. GH effects are mediated in large part via IGF-1 induction, although GH may also act directly to improve immune reconstitution [33]. The IGF-1 receptor is expressed on thymocytes and T cells, and may act directly on these cells to stimulate lymphopoiesis by modulating homing receptors on progenitors, or induce IL-7 or stem cell factor production by thymic epithelial cells [33-37]. Administration of GH or IGF-1 can enhance T cell recovery in (a) syngeneic HSCT recipients, (b) allogeneic HSCT recipients, (c) aging mice, and (d) aging BMT recipients [38,39].
Ghrelin is a peptide hormone that can (a) promote the secretion of GH through activation of the growth hormone secretagogue receptor (GHS-R) and (b) exert anti-inflammatory effects by blocking the NFkB pathway, and cytokine secretion in Th1 and Th17 cells [40-43]. Ghrelin is expressed in the thymus and spleen of young mice, and decreases with age [43,44]. Administration of ghrelin can improve thymic organization and thymocyte numbers in aging mice resulting in increased recent thymic emigrants, peripheral T cell populations, and an improved T cell repertoire [44]. Both ghrelin- and GHS-R-deficient mice demonstrate accelerated thymic involution associated with increased adipocyte formation in the thymus [44,45]. Ghrelin affects not only the development of T cells, but also their function in the periphery. Recently, Dixit et al demonstrated that T cell-derived ghrelin is responsible for controlling proinflammatory cytokine expression in aging mice and in humans [43]. Treatment with exogenous ghrelin corrected age-related inflammation [43], which could further contribute to the improved T cell reconstitution in older mice.
Interleukin (IL)-7, IL-12, IL-15
IL-7 and IL-15 are common cytokine γ chain receptor cytokines, with various stimulatory effects on lymphocytes. IL-7 is produced by stromal cells throughout the body, including thymic stroma [46]. Signaling induced by IL-7 supports thymocyte development, and peripheral T cell survival and proliferation [47-51]. The administration of IL-7 to aging individuals and individuals with thymic damage caused by chemotherapeutic or radiation-based conditioning for bone marrow transplantation has been shown to increase thymopoiesis, T cell maturation and function [52-56]. These findings extend to clinical trials, where human IL-7 administration has proven safe and effective in increasing T cell immunity [57]. Administration of recombinant human IL-7 (rhIL-7) to a cohort of metastatic melanoma and metastatic sarcoma patients led to expansion of CD4 and CD8 T cells, with a reduction in the percentage of regulatory T cells [58]. Another clinical study found that patients with nonhematologic, nonlymphoid cancers who received a course of IL-7 treatment had increased circulating CD4 and CD8 T cell numbers [59]. In addition, the T cells in these patients (a) were cycling, (b) up-regulated the anti-apoptotic factor Bcl-2, (c) had a broad TCR repertoire, and (d) had naïve and central memory phenotypes, with lower proportions of regulatory T cells and senescent CD8 effector cells [59]. Treatment with IL-7 also increased numbers of naïve and central memory phenotype cells from CD4 and CD8 lineages in HIV-infected patients, and expanded T cells produced IFNγ and/or IL-2 in response to HIV antigens [60].
IL-15 is secreted by many cell types in the body, ranging from fibroblasts to epithelial cells to immune cells. It signals through the IL-15Rα, IL-2Rβ, and common cytokine receptor γ chain to stimulate proliferation in lymphocytes. Administration of IL-15 following bone marrow transplantation improves peripheral memory CD8 T cell expansion by promoting proliferation and decreasing apoptosis by increasing intracellular Bcl-2 levels [61,62]. IL-15, which is less toxic than IL-2, may also be combined with other treatments such as immunotherapy and tumor vaccination in order to augment their effects on T cell reconstitution [63-65].
IL-12 has been reported to act as an accessory cytokine to IL-7 and IL-2 to support thymocyte proliferation and development [66]. Although IL-12 levels are not decreased in aging wildtype animals, thymic involution was accelerated in aging IL-12b-/- mice, and in vitro stimulation of thymocytes with IL-7 or IL-2 in combination with IL-12 led to synergistic enhancement of proliferation [66]. Additionally, Chen et al recently demonstrated that adenoviral administration of IL-12 (AdIL-12) to aging mice followed by wildtype adenoviral treatment resulted in enhanced cytotoxic lymphocyte (CTL) responses measured in vitro and in vivo [67]. The aging mice (18 months old) treated with AdIL-12 secreted greater levels of IFNγ, showed comparable T cell proliferative responses to those in young mice, and demonstrated efficient antigen-specific killing in vitro. These results indicate that administration of IL-12 to aging individuals may slow or reverse thymic involution by augmenting thymocyte proliferation, which would lead in turn to regenerative crosstalk between thymocytes and the thymic stroma.
Conclusion
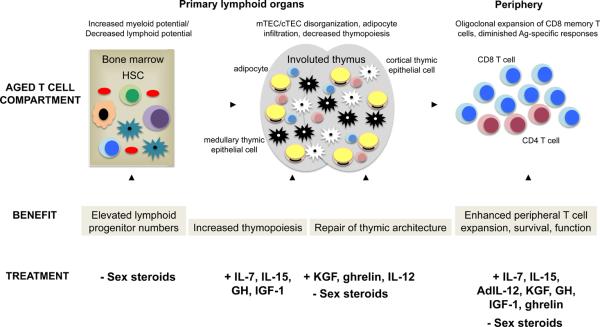
Recent mechanistic and clinical studies have defined strategies that could enhance T cell immunity in aging individuals (summarized in Figure 1). These approaches enhance the number of T cell precursors (sex steroid ablation), promote thymopoiesis (sex steroid ablation, KGF, GH, IGF-1, ghrelin, IL-7, IL-15, and IL-12), and peripheral T cell numbers (sex steroid ablation, KGF, GH, IGF-1, ghrelin, IL-7, IL-15), survival (GH, IGF-1, ghrelin, IL-7, IL-15), repertoire (sex steroid ablation, KGF, ghrelin, IL-7), and function (sex steroid ablation, KGF, GH, IGF-1, ghrelin, IL-7, IL-15, IL-12).
Figure 1. Strategies to enhance immune function in the elderly improve the development, expansion, and function of T cells.
Sex steroid modulation via surgical or chemical castration in aging and transplanted mice or humans enhances lymphoid progenitor generation, thymopoiesis, and peripheral T cell function. Keratinocyte growth factor acts directly on thymic epithelial cells to promote proliferation and differentiation, leading to greater capacity for T cell development and function in the periphery. Members of the growth hormone signaling pathway act on lymphocytes to enhance thymic and T cell function. The cytokines IL-7, IL-12, and IL-15 exert their effects on thymocytes and mature T cells to enhance numbers and function.
Acknowledgements
This research was supported by National Institutes of Health grants RO1-HL069929 (MvdB), RO1-CA107096 (MvdB), RO1-AI080455 (MvdB) and PO1-CA33049 (MvdB). Support was also received from the Ryan Gibson Foundation, the Elsa U. Pardee Foundation, the Byrne Foundation, the Emerald Foundation, and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, The Bobby Zucker Memorial Fund (MvdB) and The Lymphoma Foundation. Additional support was provided by the NIH Grant T32 AIO7621 and the Starr Stem Cell Scholar Fellowship (AMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, Ammatuna P, Fletcher JM, Caruso C, Pawelec G. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol. 2007;42:995–1002. doi: 10.1016/j.exger.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 4.Greenstein BD, Fitzpatrick FT, Adcock IM, Kendall MD, Wheeler MJ. Reappearance of the thymus in old rats after orchidectomy: inhibition of regeneration by testosterone. J Endocrinol. 1986;110:417–422. doi: 10.1677/joe.0.1100417. [DOI] [PubMed] [Google Scholar]
- 5.Greenstein BD, Fitzpatrick FT, Kendall MD, Wheeler MJ. Regeneration of the thymus in old male rats treated with a stable analogue of LHRH. J Endocrinol. 1987;112:345–350. doi: 10.1677/joe.0.1120345. [DOI] [PubMed] [Google Scholar]
- 6.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 8.Windmill KF, Lee VW. Effects of castration on the lymphocytes of the thymus, spleen and lymph nodes. Tissue Cell. 1998;30:104–111. doi: 10.1016/s0040-8166(98)80011-6. [DOI] [PubMed] [Google Scholar]
- 9.Windmill KF, Meade BJ, Lee VW. Effect of prepubertal gonadectomy and sex steroid treatment on the growth and lymphocyte populations of the rat thymus. Reprod Fertil Dev. 1993;5:73–81. doi: 10.1071/rd9930073. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, Hubbard VM, Kochman A, Willis LM, Greenberg AS, et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, Boyd RL. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen-Kiang S, et al. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol. 2009;182:5846–5854. doi: 10.4049/jimmunol.0801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **13.Sutherland JS, Spyroglou L, Muirhead JL, Heng TS, Prieto-Hinojosa A, Prince HM, Chidgey AP, Schwarer AP, Boyd RL. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]; This study demonstrates that the LHRH agonist goserelin signficantly improves T cell reconstitution in human autologous and allogeneic transplantation patients, and increases disease-free survival in autologous recipients.
- 14.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 15.Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, Rubin JS, Rudensky A, Farr AG. Regulation of thymic epithelium by keratinocyte growth factor. Blood. 2002;100:3269–3278. doi: 10.1182/blood-2002-04-1036. [DOI] [PubMed] [Google Scholar]
- 16.Gray DH, Tull D, Ueno T, Seach N, Classon BJ, Chidgey A, McConville MJ, Boyd RL. A unique thymic fibroblast population revealed by the monoclonal antibody MTS-15. J Immunol. 2007;178:4956–4965. doi: 10.4049/jimmunol.178.8.4956. [DOI] [PubMed] [Google Scholar]
- 17.Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR. Clinical strategies to enhance T cell reconstitution. Semin Immunol. 2007;19:289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 21.Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, Krenger W, Hollander GA. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- *22.Jenq RR, King CG, Volk C, Suh D, Smith OM, Rao U, Yim N, Holland AM, Lu SX, Zakrzewski JL, et al. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses following murine allogeneic bone marrow transplantation. Blood. 2008 doi: 10.1182/blood-2008-05-155697. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that KGF can be used as a vaccine adjuvant to enhance tumor-killing and overall survival in tumor-bearing murine recipients of allogeneic BMT. These findings are promising for elderly patients who typically respond poorly to vaccination.
- **23.Seggewiss R, Lore K, Guenaga FJ, Pittaluga S, Mattapallil J, Chow CK, Koup RA, Camphausen K, Nason MC, Meier-Schellersheim M, et al. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a non-human primate model, the authors demonstrate that pre- or peritransplant KGF treatment resulted in maintenance of thymic architecture and enhanced T cell numbers in autologous recipients. This study supports the findings in small animals that FDA-approved KGF may improve T cell reconstitution in humans undergoing HSCT or age-related thymic atrophy.
- *24.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, Blazar BR. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111:5734–5744. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors combine KGF administration with sex steroid blockade in a murine BMT model with additive benefits on T cell reconstitution.
- 25.Hattori N, Shimatsu A, Sugita M, Kumagai S, Imura H. Immunoreactive growth hormone (GH) secretion by human lymphocytes: augmented release by exogenous GH. Biochem Biophys Res Commun. 1990;168:396–401. doi: 10.1016/0006-291x(90)92334-v. [DOI] [PubMed] [Google Scholar]
- 26.Varma S, Sabharwal P, Sheridan JF, Malarkey WB. Growth hormone secretion by human peripheral blood mononuclear cells detected by an enzyme-linked immunoplaque assay. J Clin Endocrinol Metab. 1993;76:49–53. doi: 10.1210/jcem.76.1.8421102. [DOI] [PubMed] [Google Scholar]
- 27.Weigent DA, Baxter JB, Wear WE, Smith LR, Bost KL, Blalock JE. Production of immunoreactive growth hormone by mononuclear leukocytes. Faseb J. 1988;2:2812–2818. doi: 10.1096/fasebj.2.12.3044906. [DOI] [PubMed] [Google Scholar]
- 28.Weigent DA, Blalock JE. Immunoreactive growth hormone-releasing hormone in rat leukocytes. J Neuroimmunol. 1990;29:1–13. doi: 10.1016/0165-5728(90)90142-a. [DOI] [PubMed] [Google Scholar]
- 29.Bresson JL, Jeay S, Gagnerault MC, Kayser C, Beressi N, Wu Z, Kinet S, Dardenne M, Postel-Vinay MC. Growth hormone (GH) and prolactin receptors in human peripheral blood mononuclear cells: relation with age and GH-binding protein. Endocrinology. 1999;140:3203–3209. doi: 10.1210/endo.140.7.6854. [DOI] [PubMed] [Google Scholar]
- 30.Dardenne M, Mello-Coelho V, Gagnerault MC, Postel-Vinay MC. Growth hormone receptors and immunocompetent cells. Ann N Y Acad Sci. 1998;840:510–517. doi: 10.1111/j.1749-6632.1998.tb09589.x. [DOI] [PubMed] [Google Scholar]
- 31.de Mello-Coelho V, Gagnerault MC, Souberbielle JC, Strasburger CJ, Savino W, Dardenne M, Postel-Vinay MC. Growth hormone and its receptor are expressed in human thymic cells. Endocrinology. 1998;139:3837–3842. doi: 10.1210/endo.139.9.6199. [DOI] [PubMed] [Google Scholar]
- 32.Gagnerault MC, Postel-Vinay MC, Dardenne M. Expression of growth hormone receptors in murine lymphoid cells analyzed by flow cytofluorometry. Endocrinology. 1996;137:1719–1726. doi: 10.1210/endo.137.5.8612507. [DOI] [PubMed] [Google Scholar]
- 33.Welniak LA, Sun R, Murphy WJ. The role of growth hormone in T-cell development and reconstitution. J Leukoc Biol. 2002;71:381–387. [PubMed] [Google Scholar]
- 34.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 35.Kooijman R, Willems M, De Haas CJ, Rijkers GT, Schuurmans AL, Van Buul-Offers SC, Heijnen CJ, Zegers BJ. Expression of type I insulin-like growth factor receptors on human peripheral blood mononuclear cells. Endocrinology. 1992;131:2244–2250. doi: 10.1210/endo.131.5.1425423. [DOI] [PubMed] [Google Scholar]
- 36.Stuart CA, Meehan RT, Neale LS, Cintron NM, Furlanetto RW. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab. 1991;72:1117–1122. doi: 10.1210/jcem-72-5-1117. [DOI] [PubMed] [Google Scholar]
- 37.Walsh PT, O'Connor R. The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol. 2000;30:1010–1018. doi: 10.1002/(SICI)1521-4141(200004)30:4<1010::AID-IMMU1010>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 38.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 39.Tian ZG, Woody MA, Sun R, Welniak LA, Raziuddin A, Funakoshi S, Tsarfaty G, Longo DL, Murphy WJ. Recombinant human growth hormone promotes hematopoietic reconstitution after syngeneic bone marrow transplantation in mice. Stem Cells. 1998;16:193–199. doi: 10.1002/stem.160193. [DOI] [PubMed] [Google Scholar]
- 40.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 41.Smith RG, Jiang H, Sun Y. Developments in ghrelin biology and potential clinical relevance. Trends Endocrinol Metab. 2005;16:436–442. doi: 10.1016/j.tem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Dixit VD, Taub DD. Ghrelin and immunity: a young player in an old field. Exp Gerontol. 2005;40:900–910. doi: 10.1016/j.exger.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009 doi: 10.1182/blood-2008-09-181255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe for the first time that ghrelin levels decrease in the aging thymus, and that administration of ghrelin improved thymus structure and thymopoiesis in 14-month-old mice.
- *45.Youm YH, Yang H, Sun Y, Smith RG, Manley NR, Vandanmagsar B, Dixit VD. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem. 2009;284:7068–7077. doi: 10.1074/jbc.M808302200. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that loss of ghrelin leads to a reduction in the number of TEC and an increase in the number of adipocytes in the thymus. These findings suggest a mechanism through which ghrelin directly mediates thymic function by shaping the thymic microenvironment.
- 46.Sakata T, Iwagami S, Tsuruta Y, Teraoka H, Tatsumi Y, Kita Y, Nishikawa S, Takai Y, Fujiwara H. Constitutive expression of interleukin-7 mRNA and production of IL-7 by a cloned murine thymic stromal cell line. J Leukoc Biol. 1990;48:205–212. doi: 10.1002/jlb.48.3.205. [DOI] [PubMed] [Google Scholar]
- 47.Morrissey PJ, McKenna H, Widmer MB, Braddy S, Voice R, Charrier K, Williams DE, Watson JD. Steel factor (c-kit ligand) stimulates the in vitro growth of immature CD3-/CD4-/CD8- thymocytes: synergy with IL-7. Cell Immunol. 1994;157:118–131. doi: 10.1006/cimm.1994.1210. [DOI] [PubMed] [Google Scholar]
- 48.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 49.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 52.Abdul-Hai A, Or R, Slavin S, Friedman G, Weiss L, Matsa D, Ben-Yehuda A. Stimulation of immune reconstitution by interleukin-7 after syngeneic bone marrow transplantation in mice. Exp Hematol. 1996;24:1416–1422. [PubMed] [Google Scholar]
- 53.Alpdogan O, Muriglan SJ, Eng JM, Willis LM, Greenberg AS, Kappel BJ, van den Brink MR. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alpdogan O, Schmaltz C, Muriglan SJ, Kappel BJ, Perales MA, Rotolo JA, Halm JA, Rich BE, van den Brink MR. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 55.Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood. 1996;88:1887–1894. [PubMed] [Google Scholar]
- 56.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 57.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **59.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]; IL-7 administration to patients with nonhematologic, nonlymphoid cancers led to increased T cell production and function, with enhanced proportions of recent thymic emigrants and naïve T cells.
- **60.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Avettand-Fenoel V, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that IL-7 administration to HIV-1-infected patients with profound T cell deficiency leads to increased T cell numbers and enhanced cytokine secretion in response to HIV antigens.
- 61.Alpdogan O, Eng JM, Muriglan SJ, Willis LM, Hubbard VM, Tjoe KH, Terwey TH, Kochman A, van den Brink MR. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–873. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 62.Katsanis E, Xu Z, Panoskaltsis-Mortari A, Weisdorf DJ, Widmer MB, Blazar BR. IL-15 administration following syngeneic bone marrow transplantation prolongs survival of lymphoma bearing mice. Transplantation. 1996;62:872–875. doi: 10.1097/00007890-199609270-00031. [DOI] [PubMed] [Google Scholar]
- 63.Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7:23–35. doi: 10.1080/14653240510018037. [DOI] [PubMed] [Google Scholar]
- 64.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169:4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- 66.Li L, Hsu HC, Stockard CR, Yang P, Zhou J, Wu Q, Grizzle WE, Mountz JD. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J Immunol. 2004;172:2909–2916. doi: 10.4049/jimmunol.172.5.2909. [DOI] [PubMed] [Google Scholar]
- **67.Chen J, Wang J, Li J, Wu Q, Chu Lim F, Yang P, Hsu HC, Curiel DT, Mountz JD. Enhancement of cytotoxic T-lymphocyte response in aged mice by a novel treatment with recombinant AdIL-12 and wild-type adenovirus in rapid succession. Mol Ther. 2008;16:1500–1506. doi: 10.1038/mt.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that administration of IL-12 to 18 month old mice via an adenoviral vector results in enhanced cytotoxic T cell numbers, activity, and proliferation.