Abstract
Adult female rats were exposed to lead-free sodium acetate via gavage [0 mg (vehicle control)] or to 16 mg lead as lead acetate for 30 days prior to breeding. Following confirmation of breeding, the female animals continued to be exposed to their respective doses throughout gestation and lactation. When weaned, 16 control and 16 lead-exposed offspring were placed on regular water and food (lead-exposure was discontinued) until postnatal day (PND) 70. At this time, one-half of the control animals and one-half of the lead-treatment animals received intraperitoneal (i.p.) injections of the vehicle (saline) for 10 successive days and the remaining animals in each exposure conditions received daily injections of 1.0 mg/kg (+)-methamphetamine (METH) for 10 days (N=8/group). Locomotion in automated chambers was monitored daily for 45 min post-injection. Subsequently, during dose-effect testing, all animals received consecutive daily i.p. injections of 0, 1.0, 2.0, and then 4.0 mg/kg METH. The results of the experiment showed that both control and lead-exposed animals exhibited heightened locomotor activity (i.e. behavioral sensitization) to the repeated administration of 1.0 mg/kg METH. More importantly, animals developmentally (perinatally) exposed to lead showed more rapid sensitization than did their control counterparts. These data indicate that early lead exposure increases sensitivity to the locomotor-stimulating effects of METH. In contrast, identically exposed lead animals exhibit diminished METH dose-effect responding when tested in an intravenous (i.v.) self-administration paradigm (Rocha et al., 2008a; 2008b).
Keywords: lead, methamphetamine, sensitization
Introduction
Although atmospheric levels of lead in North America have fallen dramatically over the last several years, exposure to lead continues to be problematic. Especially in the inner cities and among minorities, an alarmingly high percentage of children register blood lead levels that exceed the allowable limits set forth by the Centers for Disease Control and Prevention in inner-urban areas (Kemp et al., 2007; Mielke, 1999; Pirkle et al., 1998). Inasmuch as blood lead levels within the so-called “safe” range (less than 10 μg/dl) have been shown to be associated with neurobehavioral impairment (cf, Hubbs-Tait et al., 2005; Lamphear et al., 2005) and deranged cognitive function (Bellinger, 2006; Canfield et al., 2003), there continues to be concern in the scientific community about the potential adverse effects of lead exposure.
In addition to the potential cognitive effects of lead, there is evidence to suggest that lead exposure may result in a predisposition to develop drug abuse. For several years, this laboratory has conducted a series of studies to characterize the interactive relations between developmental lead exposure and changes in sensitivity to psychoactive drugs. It has been observed that perinatal (gestation/lactation) lead exposure enhances locomotor activity (sensitization) to effects of cocaine even when lead exposure had been discontinued at weaning and testing did not occur until 60 days later (Nation et al., 2000). Consistent with these findings, and perhaps more importantly, perinatal lead exposure produces a leftward displacement in the dose-effect for intravenous (i.v.) self-administration responding for cocaine (Nation et al., 2004; Valles et al., 2005), and increases the chances for relapse in a reinstatement paradigm (Nation et al., 2003). Finally, Rocha et al. (2005) report that responding for self-administered cocaine is acquired more quickly in a preparation that combines noncontingent cocaine deliveries for 3 hrs prior to a 3 hr contingent operant testing procedure. Thus, the overall pattern between prenatal lead and cocaine is one of lead-induced augmentation.
A key component of cocaine’s biobehavioral effects relates to antagonism of membrane transporters for dopamine (Lin and Uhl, 2002; Rothman et al., 2001). In contrast, methamphetamine (METH) is a substrate for the dopamine transporters and induces the transporter to run in reverse, thereby increasing synaptic dopamine levels (Howell et al., 2008; Rothman et al., 2001). The use and abuse of METH appears to be a growing issue in the United States (Cho and Melega, 2002; Crèvecoeur et al., 2007) as well as worldwide (Anglin et al., 2000; Rawson and Condon, 2007). With regard to lead exposure and METH use, it is of concern that low socioeconomic status is significantly related to METH use (Iritani, et al., 2007) and to lead exposure within the inner city (Ensminger et al., 1997; Kemp et al., 2007; Mielke, 1999; Mielke, et al., 2008; Pirkle et al., 1998). Of specific interest herein was whether perinatal lead exposure would augment the locomotor effects of METH in a manner consistent with its effects on cocaine-induced hyperlocomotion. Accordingly, offspring from dams repeatedly exposed to 0 mg lead daily or 16 mg lead daily were tested during adulthood regarding changes in locomotor activity following repeated daily intraperitoneal (i.p.) administration of vehicle or 1.0 mg/kg METH, a protocol known to induce sensitization. This METH dose increases locomotion in rats, but it does not induce stereotypy (Hall et al., 2008). Following this sensitization test period, all test animals were administered 0.0, 1.0, 2.0, and then 4.0 mg/kg METH (i.p) on consecutive locomotion trials.
Methods
Animals
Animal maintenance and research were conducted in accordance with the guidelines provided by the Texas A&M University Laboratory Animal Care Committee, and the Public Health Service Policy outlined in the publication of the Guide for the Care and Use of Laboratory Animals (1996).
Lead Exposure Regimen
For 30 consecutive days, adult female (200–225 g) Sprague-Dawley rats (Harlan; Houston, TX) were exposed daily to 0-mg lead (sodium acetate) or 16-mg lead(as lead acetate) daily using a 18 ga gavage needle to administer the respective solutions in a volume of 1.0 ml of pH adjusted deionized water. This procedure has been used in previous developmental lead studies to ensure stable blood/tissue lead levels (cf. Nation et al., 2000; Nation et al., 2003; 2004; Rocha et al., 2004). The present lead dose was selected based on previous studies that found it produces differential behavioral effects while not altering dam body weight or the locomotor ability of pups (see Nation et al., 2000). Following the initial 30-day lead exposure period, females were bred to non-lead exposed males. Once females tested positive for copulatory plugs, the males were removed from the home cage. Rate of pregnancy did not differ between control and lead groups. Females continued to receive their daily doses of the control solution or lead acetate solution throughout the gestation and lactation periods. Standard rat chow (Teklad; Madison, WI) and tap water were continuously available for dams in the home cage. Litters were culled to a maximum of eight pups on PND 2 with the proviso that each group retained the maximum number of male pups. On PND 21, the litters were shifted to housing in groups of 2–3 per cage (males only). Only one pup from each litter was used to form the four groups of the experiment in order to avoid confounds that are sometimes evident in studies involving toxic exposure (Holson and Pearce, 1992). Starting on PND 70, rats were individually housed in a colony room with a 12-hour light/dark cycle (lights off at 1000 hrs). Behavioral testing commenced on PND 70. Locomotion was measured at approximately 10:00 hr, at the start of the dark cycle.
Apparatus
The assessment of locomotion was made in a set of 8 automated optical beam activity monitors (Model RXYZCM-16; Accuscan Instruments, Columbus, OH, USA). Each monitor was housed within a 40 × 40 × 30.5 cm acrylic cage. Activity monitors and cages were located in a sound-proof room with a 40 dB [SPL] white noise generator operating continuously. A multiplexor-analyzer monitored beam breaks from the optical beam activity monitors and tracked the simultaneous interruption of beams. The multiplexor-analyzer updated the animal’s position in the acrylic cage every 10 msec using a 100% real-time conversion system. Computerized integration of the data obtained from the monitor afforded the recording of general activity using total distance traveled scores (in cm) as the primary dependent measure (Sandberg et al., 1987).
Procedure
Test animals were randomly selected from a given litter and then randomly assigned to one of four test groups. One-half of the control animals and one-half of the lead-treatment animals received intraperitoneal (i.p.) injections of the vehicle (saline) for 10 successive days and the remaining animals in each exposure conditions received daily injections of 1.0 mg/kg (+)-methamphetamine (METH) for 10 days (N=8/group). Animals receiving methamphetamine were administered daily i.p. injections of 1.0 mg/kg methamphetamine HCl expressed as the salt, while vehicle controls received saline (1.0 ml/kg volume). Methamphetamine was provided by Dr. Kevin Gormley of the Basic Research Division of NIDA. In this initial phase of the project, animals were tested during 1 hr sessions each day for 10 successive days, in four squads of 8 rats (total =32) counterbalanced by group (i.e. two rats from each lead-drug exposure condition were run in each squad). With the room lights off, animals were placed in their respective test chambers for a 15-min baseline-recording period on each test trial prior to receiving either a methamphetamine (1.0 mg/kg METH) or vehicle (0 mg/kg METH) injection. At the point of the injection, the room lights were turned on and the animal was placed back in the chamber immediately following the injection, at which time the room lights again were turned off. This procedure was employed in order to increase the discriminatory properties of the injections. Previous cocaine investigations (e.g., Post et al., 1981) have shown that contextual cues contribute to augmented responding associated with repeated drug administration. Insofar as administering the injections, placement in the test chambers, turning off the test room lights and other pre-injection correlates serve as conditioned stimuli, it is reasonable to assume that reinstatement of such events could alter locomotor responding (in the absence of the drug). We tested for such a possibility by administering a vehicle only (0 mg/kg METH) injection following initial sensitization testing (see procedures for Day 11 of testing). In all tests conducted in this study, total distance traveled (cm) was recorded preinjection for 15 min and for post-injection across successive 5-min intervals for 45 min. The 45 min test period used herein has been sufficient to detect dose-dependent differences between analogues of amphetamine that act on monoamine transporters (Wellman et al., 2009). On Days 11–14, all animals within each of the four groups received successive daily i.p. injections of 0, 1.0, 2.0, and 4.0 mg/kg METH.
Tissue Sampling and Analyses
For control and lead-exposed dams, 100–150 μl of tail-blood was drawn at breeding, parturition (PND 2), and weaning (PND 21) and analyzed for lead levels. In addition, at the point of termination of the experiment, blood was taken from test animals for lead concentration analyses. Littermates of test animals were sacrificed on PND 2, and blood samples were collected for subsequent analyses. Dams were sacrificed at weaning with blood and tissue (brain, kidney, liver and bone) samples collected for subsequent analyses. The body weights (Mean ± SEM) of the control dams on PND 21 were 308 ± 4 g whereas those of the lead dams were 291 ± 8 g.
Blood lead and tissue residues were measured by inductively coupled plasma - mass spectroscopy on a Perkin Elmer DRC 2 instrument following acid digestion in a microwave. The 208Pb isotope and 209Bi were used as internal standards. Weighted linear calibration was performed with a blank and three external standards (0.05, 20, and 200 parts per billion) and was verified by analyzing NIST SRM 1640 (trace elements in water). Data were acquired in peak hopping mode, using the autolens feature and three replicate reads per determination. Verification of the calibration and baseline were performed after every group of 10 samples and at the end of the analytical run.
Data Analyses
The overall design for the sensitization phase of the study was a split-plot (mixed) factorial consisting of the between-group factors of lead exposure (control versus lead) and methamphetamine dose (0 versus 1 mg/kg) and a within-group factor of time after injection (5 min bins over a 45 min period). The dose-effect behavioral data were analyzed using a split-plot (mixed) factorial design consisting of the between-group factors of lead exposure (control versus lead) and METH pretreatment dose (0 versus 1 mg/kg) and within-group factors of METH treatment dose (0, 1.0, 2.0, and 4.0 mg/kg) and time after injection (5 min bins over a 45 min period as well as total locomotion scores). The body weight and blood/tissue lead level data were analyzed using a complete factorial consisting of the between-group factors of lead exposure (control versus lead) and METH dose (0 versus 1.0 mg/kg). Statistical significance was deemed to be p < 0.05 and the Bonferroni procedure was used to examine mean group differences.
Results
Body Weights
Body weights were averaged for each animal in each group over the final seven days prior to commencing activity testing (data not shown). ANOVA of these data revealed a significant effect of lead treatment (F (1,28) = 7.94, p < 0.009). Lead-pretreated rats weighed an average (± SEM) of 366 g (± 5.1 g), whereas control rats weighed an average of 386 g (± 5.1 g). There were no significant effects of METH treatment nor was there an interaction between lead treatment and METH treatment factors on body weight.
Blood and Tissue Lead Levels
As expected, dams exposed to lead exhibited a considerable body lead burden (Table 1). At the time of weaning, dams exhibited significant levels of lead in tibia, kidney, liver and brain as well as blood. Similarly, littermates showed significant blood levels of lead at PND 2 and PND 22. By the end of this experiment, blood lead levels in both control and lead groups were not different. Thus, the behavioral disturbances associated with perinatal lead exposure occurred at a time point far beyond that at which the lead burden had cleared from blood.
Table 1.
Mean (SEM) blood and tissue lead concentration values for dams, littermates, and test animals.
| Blood lead concentration (μg/dl) | ||
|---|---|---|
| Group 0-mg | Group 16-mg | |
| Dams | ||
| Postnatal Day 2 | 2.53 (2.1) | 77 (15.0) * |
| Postnatal Day 21 | 1.96 (0.48) | 44.1 (6.08) * |
| Littermates | ||
| Postnatal Day 2 | 0.76 (0.2) | 75.5 (11.0) * |
| Blood Post-Experiment | ||
| Methamphetamine | 0.19 (0.007) | 0.24 (0.04) |
| Vehicle Only | 0.19 (0.01) | 0.28 (0.013) |
| Tissue Concentrations of Dams at Weaning (μg/g) | ||
| Brain | 0.006 (0.001) | 0.6 (0.07) * |
| Kidney | 0.04 (0.034) | 11.51 (0.57) * |
| Liver | 0.007 (0.002) | 0.87 (0.15) * |
| Tibia | 0.11 (0.01) | 127.2 (15.18) * |
The symbol indicates that control and lead-exposed animals were significantly different (p < 0.05).
Methamphetamine Sensitization
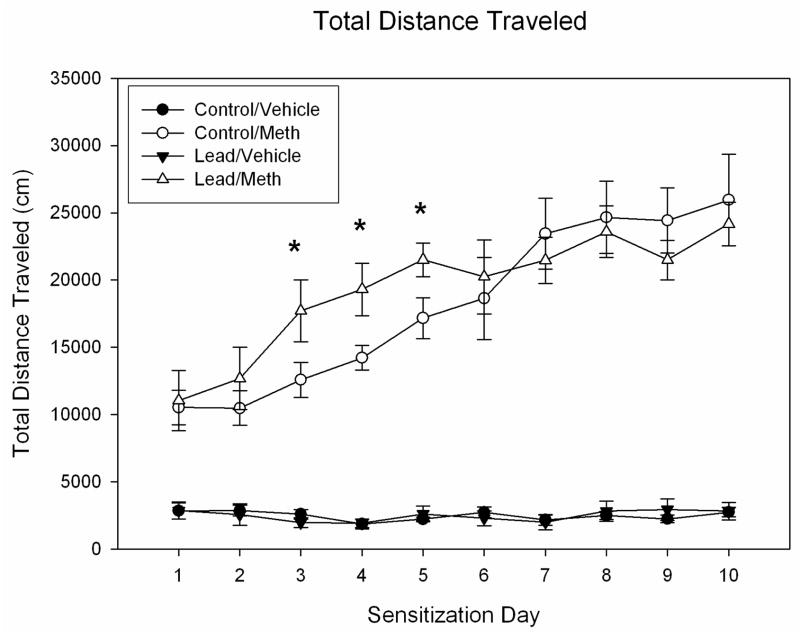
Figure 1 depicts the changes in total distance traveled scores for rats treated on Days 1–10 with either vehicle or 1.0 mg METH. On Day 1, the METH groups exhibited a significant increase in locomotion that was identical for both control and lead-pretreated rats. Repeated administration of this fixed dose of METH resulted in significant sensitization, as evident in augmented locomotion scores across days. ANOVA of these data revealed a significant effect of METH treatment on locomotion (F(1,28) = 208.6, p < 0.0001) as well as a significant effect of day (F(9,252) = 23.57, p < 0.0001). While there was no significant overall effect of lead treatment, there was a significant interaction between the factors of lead treatment, METH treatment, and day (F(9,252) = 2.964, p < 0.002). Subsequent contrasts indicated that lead exposed rats showed greater locomotion following METH administration than did control rats on days 3, 4 and 5 and suggest that the METH treated groups converged on day 6 and were comparable thereafter.
Figure 1.
Mean group changes in total distance traveled scores (cm) over a 45 min period for control and lead-pretreated rats injected daily with either vehicle (VEH) or 1.0 mg/kg METH (n=8 per group) on Days 1–10. The bar above each symbol reflects the standard error of the mean for that value. Significant differences (p < 0.05) between control and lead-pretreated groups are denoted by a *.
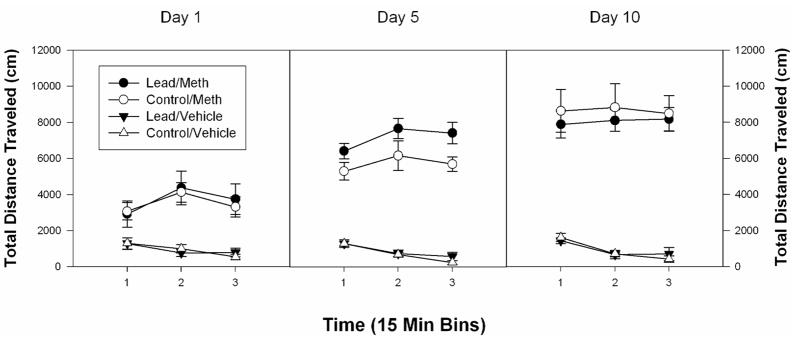
To further explore these data, the total distance traveled scores from Days 1, 5 and 10 were further analysed, as a function of 15 min time bin (see Figure 2). On Day 1, there was noted a significant effect of drug (F(1,28) = 34.2, p < 0.002) but no effect of group nor any interaction between group and drug or group, drug and time. On Day 5, the analyses indicated a significant effect of drug (F(1,28) = 269.3, p < 0.0001) and of group (F(1,28) = 5.2, p < 0.0001) but no interaction between group and drug or group, drug and time. By Day 10, the effect of drug was significant (F(1,28) = 134.6, p < 0.0001) whereas the effect of group was not.
Figure 2.
Mean group changes in total distance traveled scores (cm) over three successive 15 min periods for control and lead-pretreated rats injected daily with either vehicle (VEH) or 1.0 mg/kg METH (n=8 per group) on Days 1, 5, and 10. The bar above each symbol reflects the standard error of the mean for that value.
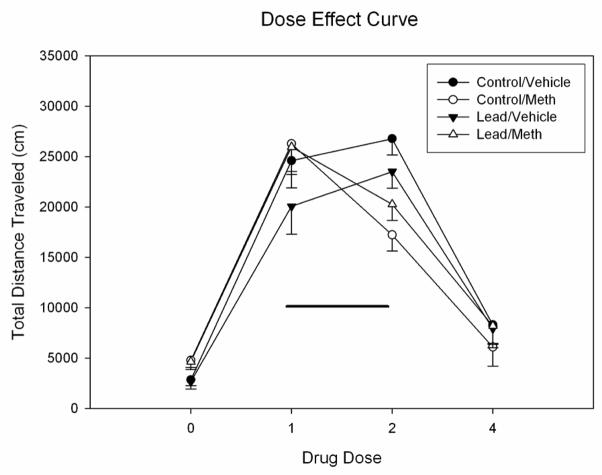
On Day 11 (see Figure 3: 0.0 mg/kg drug dose), all rats were treated with vehicle prior to the 45 min test period. Rats previously treated with METH showed significantly elevated locomotion scores that were similar for both control and lead groups. ANOVA of Day 11 locomotion data revealed a significant stimulatory effect of METH pretreatment on locomotion after vehicle treatment (F(1,28) = 15.47, p < 0.001), but no effect of lead group nor an interaction between METH pretreatment and lead group. This difference on Day 11 may reflect a degree of conditioned reactivity to METH, but it is unlikely due to a drug carryover effect, given the rapid clearance of METH in the rat (i.e. a half-life of approximately 1 hr: cf. Rivière et al., 1999; Segal and Kuczenski, 2006).
Figure 3.
Mean group total distance traveled scores (cm) for rats treated with 0, 1.0, 2.0 and 4.0 mg/kg METH starting on Day 11. The control and lead-pretreated groups (n=8 per group) in this panel were treated on Days 1–10 with either vehicle or 1.0 mg/kg METH. The bar above each symbol reflects the standard error of the mean for that value. Significant differences (p < 0.05) between rats pretreated with VEH and rats pretreated with METH are denoted by a *.
Methamphetamine Dose Effect Tests
Injection of control and lead-pretreated rats with an ascending dose series of 1.0, 2.0 and 4.0 mg/kg METH produced an inverted-U pattern in locomotion (Figure 3). Because rats formerly treated with METH showed greater locomotion after vehicle, the locomotion data from Day 11 were used as a covariate for the dose-effect data for Days 12, 13 and 14. This split-plot ANOVA revealed a significant effect of METH dose which reflected a linear decrease in locomotion as METH dose increased from 1.0 to 4.0 mg/kg (F(1,27) = 13.306, p < 0.001). There was a significant interaction between METH pretreatment and dose (F(2,54) = 10.225, p < 0.0001) but no effects of lead exposure, METH group, nor a significant interaction between METH group and lead exposure. As expected, rats pretreated with 1.0 mg/kg METH for 10 days exhibited greater locomotion after injection of 1.0 mg/kg METH on Day 12 than did rats pretreated with vehicle. At 2.0 mg/kg METH, rats pretreated with vehicle on Days 1–10 showed a significant increase in locomotion, whereas rats pretreated with METH displayed a decrease in locomotion (relative to their scores after 1.0 mg/kg METH). At 4.0 mg/kg METH, all groups decreased their locomotion from that evident after 2.0 mg/kg METH.
Discussion
In the present study, rats exposed to lead during gestation and lactation showed an increased rate of development of locomotor sensitization during the first five days of METH administration. On Day 6 and thereafter control and lead-pretreated rats reached a similar plateau of locomotion in response to repeated daily METH treatments. The locomotor results are generally consistent with the augmented behavioral sensitization induced by perinatal lead in male rats exposed to morphine (Nation et al., 2000) and to cocaine (Nation et al., 2000). In the present study, this augmentation was short-lived and only apparent during the initial days of METH exposure. The short-lived duration of the difference between lead and control rats may reflect our choice of the 1.0 mg/kg METH dose, which induced rapid sensitization such that the control rats caught up with the lead rats by Day 6. As noted above, this dose is sufficiently high to induce sensitization, but not stereotypy (Hall et al., 2008). Of interest here is the fact that perinatal lead exerts a consistent pattern of augmentation of behavioral reactivity in spite of the widely diverging mechanisms of action of cocaine (a dopamine transporter antagonist), methamphetamine (a transporter substrate drug that releases dopamine, norepinephrine, and serotonin) and morphine (an indirect dopamine agonist via inactivation of GABA cells that innervate nucleus accumbens neurons).
With regard to the impact of perinatal lead exposure on intravenous self-administration, the profile becomes complex. A portion of the complexity reflects an interaction between developmental age and lead exposure. We reported that the enhanced sensitivity to cocaine following developmental lead exposure is opposite that noted with adult exposure, i.e., in adults lead exposure decreases cocaine sensitivity (Nation et al., 1996). Additionally, there appear to be major differences between different drugs of abuse, especially cocaine and METH with regard to the impact of perinatal lead exposure on drug self-administration in adulthood. As noted above, perinatal lead exposure augments self-administration of cocaine (Nation et al., 2004; Rocha et al., 2005; Valles et al., 2005) and increases the chances for relapse in a reinstatement paradigm (Nation et al., 2003). In contrast, Rocha et al. (2008a) reported that perinatal lead exposure retards acquisition of a METH self-administration response and decreases relapse potential. Elsewhere, Rocha et al. (2008b) found that early lead exposure results in a downward shift in the METH self-administration dose-effect curve, and it also decreased progressive ratio responding for METH. These data point to lead-induced antagonism of METH action and in that regard are compatible with an earlier study of heroin self-administration (Rocha et al., 2004).
The present study indicates that perinatal lead augments METH locomotor sensitization as does cocaine, but does not indicate the locus or mechanism of action at which this effect occurs. The present study also does not indicate why this effect is different than that of METH self-administration (Rocha et al., 2008a, 2008b). Although there is a sizable literature regarding the effects of postweaning lead exposure on relevant drug-related neural systems (refer to Cory-Slechta, 1995), our understanding of the effects of preweaning lead exposure on neural mechanisms that are central to defining drug reactivity is limited (Devoto et al., 2001). Lead can alter meso-limbic dopamine function by reducing presynaptic autoreceptors or dopamine transporters and thus augment DA release, (Cory-Slechta, 1997; Pokora et al., 1996; Zuch et al., 1998). Postnatal lead, however, also alters neurochemical systems that in turn interact with mesolimbic dopamine. Lasley and colleagues have shown, for example, that lead has the capacity to diminish hippocampal glutamate function as well as GABA release (Lasley and Gilbert, 1996). The multiple sites of action of lead in brain have the potential to complicate the analyses of changes in drug action (White et al., 2007). Even more importantly, almost no information exists regarding the potential enduring mechanistic changes caused by early lead exposure in instances where the lead exposure regimen has been discontinued, as was the case in the present study. Studies are underway to examine the impact of perinatal lead exposure on brain dopamine function.
Acknowledgments
This research was supported by United States Public Health Grants DA13188 and MH65728 to the late J.R.N. (who died on May 27, 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. History of the methamphetamine problem. J Psychoactive Drugs. 2000;32:137–41. doi: 10.1080/02791072.2000.10400221. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Neurobehavioral assessment in studies of exposure to neurotoxicants. Neurotoxicity and developmental disabilities. Int Rev Res Ment Retard. 2006;30:263–300. [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. New Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21(1):21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Ann Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- Crèvecoeur D, Rutkowski B, Rawson RA. The rise in treatment admissions for methamphetamine use in Los Angeles County from 2001 through 2005. J Psychoactive Drugs. 2007 Nov;(Suppl 4):383–92. doi: 10.1080/02791072.2007.10399899. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Ibba A, Fratta W, Pani L. Lead intoxication during intrauterine life and lactation but not during adulthood reduces nucleus accumbens dopamine release as studied by brain microdialysis. Toxicol Lett. 2001;121:199–206. doi: 10.1016/s0378-4274(01)00336-8. [DOI] [PubMed] [Google Scholar]
- Ensminger ME, Anthony JC, McCord J. The inner city and drug use: initial findings from an epidemiological study. Drug Alcohol Depend. 1997 Dec 15;48(3):175–84. doi: 10.1016/s0376-8716(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Avila HM, Gulley JM. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: Evidence for qualitative differences in behavior. Psychopharmacology. 2008;195:469–78. doi: 10.1007/s00213-007-0923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Hubbs-Tait L, Nation JR, Krebs N, Bellinger DC. Neurotoxicants, micronutrients, and social environments: individual and combined effects on children’s development. Psychol Sci in the Pub Interest. 2005;5:57–121. doi: 10.1111/j.1529-1006.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- Iritani BJ, Hallfors DD, Bauer DJ. Crystal methamphetamine use among young adults in the USA. Addiction. 2007;102(7):1102–13. doi: 10.1111/j.1360-0443.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- Kemp FW, Neti PV, Howell RW, Wenger P, Louria DB, Bogden JD. Elevated blood lead concentrations and vitamin D deficiency in winter and summer in young urban children. Environ Health Perspect. 2007;15:630–5. doi: 10.1289/ehp.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Presynaptic glutamatergic function in dentate gyrus in vivo is diminished by chronic exposure to inorganic lead. Brain Res. 1996 Oct 14;736(1–2):125–34. doi: 10.1016/0006-8993(96)00666-x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Uhl GR. Dopamine transporter mutants with cocaine resistance and normal dopamine uptake provide targets for cocaine antagonism. Mol Pharmacol. 2002;61:885–91. doi: 10.1124/mol.61.4.885. [DOI] [PubMed] [Google Scholar]
- Mielke HW. Lead in the inner cities. Am Sci. 1999;87:62–73. [Google Scholar]
- Mielke HW, Gonzales C, Powell E, Mielke PW., Jr Urban soil-lead (Pb) footprint: retrospective comparison of public and private properties in New Orleans. Environ Geochem Health. 2008 Jun;30(3):231–42. doi: 10.1007/s10653-007-9111-3. [DOI] [PubMed] [Google Scholar]
- Nation JR, Cardon AL, Heard HM, Valles R, Bratton GR. Perinatal lead exposure and relapse to drug-seeking behavior in the rat: A cocaine reinstatement study. Psychopharmacology. 2003;168:236–43. doi: 10.1007/s00213-003-1405-2. [DOI] [PubMed] [Google Scholar]
- Nation JR, Livermore CL, Burkey RT. Chronic lead exposure attenuates sensitization to the locomotor-stimulating effects of cocaine. Drug Alcohol Depend. 1996;41:143–9. doi: 10.1016/0376-8716(96)01237-9. [DOI] [PubMed] [Google Scholar]
- Nation JR, Miller DK, Bratton GR. Developmental lead exposure alters the stimulatory properties of cocaine at PND 30 and PND 90 in the rat. Neuropsychopharmacology. 2000;24:444–54. doi: 10.1016/S0893-133X(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Nation JR, Smith KR, Bratton GR. Early developmental lead exposure increases sensitivity to cocaine in a self-administration paradigm. Pharmacol Biochem Behav. 2004;77:127–35. doi: 10.1016/j.pbb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991–1994. Environ Health Perspect. 1998;11:745–50. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokora MJ, Richfield EK, Cory-Slechta DA. Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: time course of effects and interactions with chronic dopamine agonist treatments. J Neurochem. 1996 Oct;67(4):1540–50. doi: 10.1046/j.1471-4159.1996.67041540.x. [DOI] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28:755–60. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Condon TP. Why do we need an Addiction supplement focused on methamphetamine? Addiction. 2007;102:1–4. doi: 10.1111/j.1360-0443.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- Rivière GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999 Dec;291(3):1220–6. [PubMed] [Google Scholar]
- Rocha A, Valles R, Bratton GR, Nation JR. Developmental lead exposure alters methamphetamine self-administration in the male rat: acquisition and reinstatement. Drug and Alcohol Depend. 2008a;95:23–9. doi: 10.1016/j.drugalcdep.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Self-administration of heroin in rats: effects of low-level lead exposure during gestation and lactation. Psychopharmacology. 2004;174:203–210. doi: 10.1007/s00213-003-1742-1. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Enhanced acquisition of cocaine self-administration in rats developmentally exposed to lead. Neuropsychopharmacology. 2005;30:2058–64. doi: 10.1038/sj.npp.1300729. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Hart N, Bratton GR, Nation JR. Developmental lead exposure attenuates methamphetamine dose-effect self-administration performance and progressive ratio responding in the male rat. Pharmacol Biochem Behav. 2008b;89:508–14. doi: 10.1016/j.pbb.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;479:23. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sandberg PR, Zoloty SA, Willis R, Ticarich CD, Rhoads K, Nagy RP, et al. Digiscan activity: automated measurement of thigmotactic and stereotypic behavior in rats. Pharmacol Biochem Behav. 1987;27:569–72. doi: 10.1016/0091-3057(87)90369-8. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Human methamphetamine pharmacokinetics simulated in the rat: single daily intravenous administration reveals elements of sensitization and tolerance. Neuropsychopharmacology. 2006;31:941–55. doi: 10.1038/sj.npp.1300865. [DOI] [PubMed] [Google Scholar]
- Valles R, Rocha A, Cardon AL, Bratton GR, Nation JR. The effects of the GABAA antagonist bicuculline on cocaine self-administration in rats exposed to lead during gestation/lactation. Pharmacol Biochemistry Behav. 2005;80:611–19. doi: 10.1016/j.pbb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davis KW, Clifford PS, Rothman RB, Blough BE. Changes in feeding and locomotion induced by amphetamine analogs in rats. Drug Alcohol Depend. 2009 Mar 1;100(3):234–9. doi: 10.1016/j.drugalcdep.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuch CL, O’Mara DJ, Cory-Slechta DA. Low-level lead exposure selectively enhances dopamine overflow in nucleus accumbens: an in vivo electrochemistry time course assessment. Toxicol Appl Pharmacol. 1998;150(1):174–85. doi: 10.1006/taap.1998.8396. [DOI] [PubMed] [Google Scholar]