Abstract
We present a strategy for adoptive immunotherapy using T-lineage committed lymphoid precursor cells generated by Notch1-based culture. We found that allogeneic T-cell precursors can be transferred to irradiated individuals irrespective of major histocompatibility complex (MHC) disparities and give rise to host-MHC restricted and host-tolerant functional allogeneic T cells, improving survival in irradiated recipients as well as enhancing anti-tumor responses. T-cell precursors transduced to express a chimeric receptor targeting hCD19 resulted in significant additional anti-tumor activity, demonstrating the feasibility of genetic engineering of these cells. We conclude that ex vivo generated MHC-disparate T-cell precursors from any donor can be used universally for ‘off-the-shelf’ immunotherapy, and can be further enhanced by genetic engineering for targeted immunotherapy.
T-cell deficiencies can occur in many physiological and pathophysiological settings. Thymic involution during aging represents the most important cause of thymic atrophy resulting in impaired T-cell function1. Various autoimmune disorders, genetic diseases, hematological malignancies and infectious diseases are associated with defective T-cell immunity2–4. Iatrogenic or unintentional exposures to cytostatic or cytotoxic agents, such as chemotherapeutics or gamma-irradiation, frequently cause transient or long-lasting T-cell deficiencies5,6. Hematopoietic stem cell transplantation (HSCT) is associated with a particularly prolonged defect in T-cell function, yet the immunotherapeutic activity of this procedure is critical for its overall anti-tumor effect7. Several recent clinical studies demonstrate that early lymphocyte recovery decreases the risk for malignant relapse and improves overall survival after autologous HSCT for various malignancies8–14. In the allogeneic setting, T cells are primarily responsible for graft-versus-host disease (GVHD), which is caused by an immune response of donor T cells to target organs of host origin, and graft-versus-tumor (GVT) activity, which results from the immune response of donor cells to residual malignant cells. Optimizing GVT activity while minimizing GVHD is one of the major challenges in clinical HSCT.
T cell–based therapies such as donor leukocyte infusion15 or protocols involving ex vivo expansion and manipulation of T cells to generate tumor- or virus-specific cells have been used for many years as strategies to enhance immune reconstitution and anti-tumor activity after HSCT16–21. However, these therapies are associated with a variety of problems, including limited availability of suitable cells (T cells have to be either autologous or MHC-matched allogeneic), contamination with residual malignant T cells when using autologous cells, GVHD when using allogeneic cells, requirement of in vivo cytokine administration and a short life span of adoptively transferred cells. Recent advances include successful tumor immunotherapy with allogeneic T cells in the absence of GVHD in mixed allogeneic bone marrow chimeras22 and the establishment of Notch-based culture systems that can be used for the development of mouse or human hematopoietic stem (HS) cells into various hematopoietic lineages, in particular the T-cell lineage23–26. These systems allow the generation of large numbers of T-cell precursors for adoptive therapy27–29. Upon infusion of donor HS cells into lethally irradiated recipients, these cells engraft in the thymus and enhance T-cell reconstitution and function, resulting in significantly increased antimicrobial resistance and GVT activity27. The progeny of these engrafted precursors did not cause GVHD, indicating that they were subject to effective selection processes resulting in host tolerance27.
The aim of this study was to evaluate if allogeneic T-cell precursors are safe and effective when used for adoptive transfer across MHC barriers in the absence of allogeneic HS cells to improve anti-tumor activity in immunosuppressed recipients.
RESULTS
Allogeneic T-cell precursor reconstitution after syngeneic HSCT
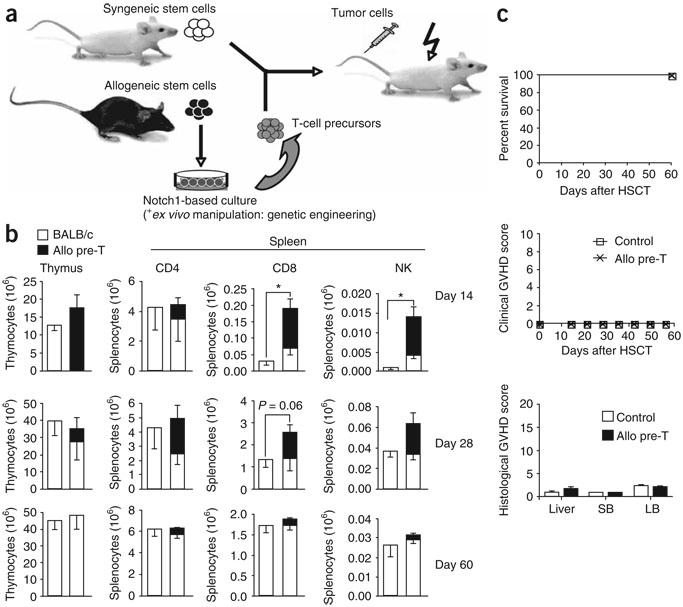
We used OP9 bone marrow stromal cells expressing the Notch ligand DL1 (OP9-DL1), the growth factor Flt3-ligand and the cytokine interleukin-7 for the in vitro generation of T-cell precursors (predominantly with a phenotype comparable to double CD4 and CD8 negative (DN)2 and DN3 thymocytes) from lineage (lin)−Sca-1+c-kithi bone marrow–derived HS cells23,27. To assess the immunotherapeutic activity of adoptively transferred in vitro–generated allogeneic T-cell precursors, we infused lin−Sca-1+c-kithi HS cells derived from BALB/c mice bone marrow with or without in vitro-generated T-cell precursors derived from C57BL/6 mice into lethally irradiated BALB/c hosts, and analyzed their effects on immune reconstitution and GVHD, as well as their anti-tumor activity (Fig. 1a). We found significant increases (P < 0.05) in early (days 14 and 28) T-cell and natural killer (NK)-cell reconstitution due to allogeneic T-cell precursors, but no differences between control and treatment groups at day 60 after HSCT (Fig. 1b). Notably, using syngeneic lin− bone marrow instead of purified HS cells hardly impaired thymic engraftment of adoptively transferred allogeneic T-cell precursors (Supplementary Fig. 1 online), illustrating the robustness of this system. We conclude that adoptive transfer of allogeneic T-cell precursors results in an early wave of allogeneic T and NK cells, but does not impair long-lasting lymphoid reconstitution from autologous HS cells. It is noteworthy that small numbers of myeloid and dendritic cells originated from adoptively transferred precursor cells (up to 10% of splenic CD11b+ and CD11c+ cells), but natural killer T (NKT) cells did not (data not shown).
Figure 1.
Adoptively transferred allogeneic T-cell precursors enhance T and NK cell reconstitution and do not induce GVHD. (a) Lethally irradiated BALB/c mice were transplanted with syngeneic purified HS cells (or lin− bone marrow cells); control mice received HS cells only, the treatment group received additional T-cell precursors generated in OP9-DL1 cocultures. In some cases, transplantation recipients were intravenously challenged with tumor cells to study anti-tumor activity. allo, allogeneic. (b) Lethally irradiated BALB/c recipients were transplanted with BALB/c mouse HS cells (Ly9.1); control mice received HS cells only, the treatment group received additional C57BL/6-derived in vitro–generated T-cell precursors (CD45.1+). At days 14, 28 and 60 after HSCT, animals were killed and thymi and spleens were harvested. Origin of cells, whether from BALB/c or C57BL/6 mice, was determined by total cellularity and multicolor flow cytometric analysis using Ly9.1- and CD45.1-specific antibodies. T and NK cells were analyzed using antibodies to CD3, CD4, and CD8, and DX5, respectively. Combined data of more than three independent experiments are presented. Values represent mean cell numbers and s.e.m. (n = 5–10). *, P < 0.05. (c) Lethally irradiated BALB/c recipients were transplanted with lin− BALB/c mouse bone marrow; control mice received bone marrow only, the treatment group received additional C57BL/6 T-cell precursors. Survival was monitored daily, a clinical GVHD score was monitored weekly. Histopathological analysis of signs of subclinical GVHD in liver, small bowel and large bowel was performed 8 weeks after transplantation. Data are representative of more than three independent experiments. Mean values and s.e.m. are presented (n = 5).
To assess whether our approach to enhancing thymic regeneration has the potential to be effective in aging recipients, we transplanted 1-year-old mice and compared recipients of syngeneic HS cells alone with recipients of adoptively transferred allogeneic T-cell precursors. We found that transferred T-cell precursors can engraft in irradiated thymi of old mice and support reconstitution of mostly CD8+ T cells, as well as NK cells (Supplementary Fig. 2 online). In addition, we previously reported that adoptively transferred T-cell precursors undergo both thymic and extrathymic T-cell development, and that extrathymic T-cell development is enhanced in athymic compared with euthymic recipients. Furthermore, we found that a combination of T-cell precursor and keratinocyte growth factor (KGF) administration can enhance thymic engraftment of T-cell precursors and that thymic engraftment of adoptively transferred T-cell precursors has long-term thymopoietic effects (probably due to lymphostromal interaction)27. Taken together, our data indicate that even with reduced thymic function, T-cell precursor immunotherapy can still be effective.
Allogeneic T-cell precursor transfer was not associated with any clinical or sub-clinical signs of GVHD as determined by mortality, clinical GVHD score30, histopathological analysis of GVHD target organs31 (Fig. 1c) or weight loss (data not shown).
T- cell receptor selection of allogeneic T-cell precursors
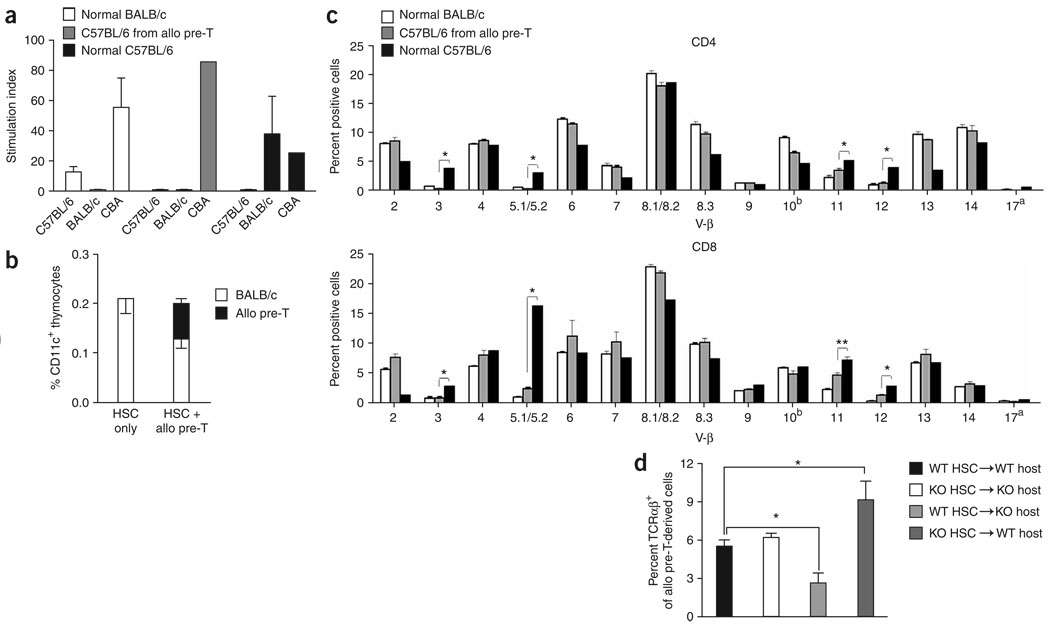
C57BL/6 mice T cells originating from adoptively transferred allogeneic T-cell precursors in syngeneic HSCT recipients (BALB/c mice) displayed tolerance to both host and donor, but were able to mount an immune response to third-party antigens as determined by mixed leukocyte reaction assays (Fig. 2a), indicating that these T cells were subject to negative selection on MHC molecules from both C57BL/6 and BALB/c mice. Analysis of thymic dendritic cells revealed a mixed chimerism (35% allogeneic dendritic cells) in recipients of allogeneic T-cell precursors (Fig. 2b), illustrating that small numbers of allogeneic antigen-presenting cells derived from the T-cell precursors make a relevant contribution to thymic negative selection. To better assess negative selection of the progeny of the adoptively transferred allogeneic T-cell precursors, we determined their T-cell receptor (TCR) repertoire by V-β family analysis. Mouse mammary tumor virus (MMTV) genes encode superantigens that induce apoptosis of developing T cells with a V-β region that binds the MMTV superantigen. Owing to this mechanism, V-β 3, 5.1/5.2, 11 and 12 TCR-bearing T cells are clonally eliminated either completely or partially in BALB/c mice but not in C57BL/6 mice32–36. We therefore transferred C57BL/6-derived T-cell precursors to syngeneic (BALB/c → BALB/c, Fig. 2c) or allogeneic (C57BL/6→ BALB/c, Supplementary Fig. 3 online) HSCT recipients and analyzed the expression of the above-named V-β chains. Normal T cells of BALB/c or C57BL/6 origin were used as additional controls. After syngeneic HSCT, the TCR repertoire of T cells derived from allogeneic T-cell precursors resembled that of T cells of syngeneic HS cell background and differed significantly (P < 0.01) from that of normal T cells of allogeneic background. Using Abb/B2m double targeted mutation mice (characterized by no detectable MHC class II expression and only low levels of MHC class I expression) as hosts or stem cell donors, we analyzed whether hematopoietic or nonhematopoietic cells, of donor or host origin, are responsible for positive and negative selection of adoptively transferred MHC-expressing allogeneic T-cell precursors (Fig. 2d). We found significantly (P < 0.05) less TCR selection in MHC-deficient hosts, and this failure could not be overcome by adding MHC-positive syngeneic HS cells, indicating that positive selection of adoptively transferred allogeneic T-cell precursors depends on interaction with MHC molecules on nonhematopoietic host cells. Furthermore, when we administered allogeneic T-cell precursors to (MHC−/−→WT) chimeras, the percentage of TCRαβ+ thymocytes increased significantly (P < 0.05) compared with (WT→WT) controls. This increase suggests a defect in negative selection resulting from the absence of MHC molecules on hematopoietic donor cells. Finally, we found in T-cell stimulation assays that CD8+ C57BL/6 mice T cells originating from allogeneic T-cell precursors that had been transferred to syngeneic HSCT recipients (BALB/c mice) could recognize antigens only in the context of BALB/c mice MHC but not in the context of C57BL/6 mice MHC (Supplementary Fig. 4 online).
Figure 2.
Adoptively transferred allogeneic T-cell precursors develop into host MHC restricted and donor/host tolerant T cells. (a) Lethally irradiated BALB/c recipients were transplanted with BALB/c mice HS cells and received additional C57BL/6-derived T-cell precursors. Animals were killed at day 55–60 after HSCT and purified splenic T cells of BALB/c (normal BALB/c) and C57BL/6 (C57BL/6 from allo pre-T) origin were used as effector cells in mixed leukocyte reaction cultures. C57BL/6 T-cells from untreated C57BL/6 mice (normal C57BL/6) were used as additional controls. Cells were cultured for 5 d with irradiated C57BL/6 mouse, BALB/c mouse or CBA mouse splenocytes as stimulators. Combined data of three independent experiments are presented as mean ± s.e.m. (n = 8). (b) Lethally irradiated BALB/c recipients weretransplanted with BALB/c mouse HS cells and with or without additional C57CL/6-derived in vitro-generated T-cell precursors. Animals were killed on day 14 after HSCT and thymi were harvested for multicolor flow cytometric analysis of CD11c+ cells of BALB/c and C57BL/6 origin. Mean ± s.e.m. are presented (n = 5). The experiment was repeated at least three times. (c) Lethally irradiated BALB/c recipients were transplanted with BALB/c mouse HS cells and received additional C57CL/6-derived in vitro-generated T-cell precursors. Animals were killed on day 60 after HSCT and splenocytes were obtained for multicolor flow cytometric analysis of the TCR-Vβ families on CD4+ and CD8+ cells of C57BL/6 origin. BALB/c and C57BL/6 splenocytes from untreated BALB/c and C57BL/6 mice (normal BALB/c and C57BL/6) were used as controls. Mean ± s.e.m. are presented (n = 5–8). Combined data from two independent experiments are presented. *, P < 0.01; **, P < 0.05. (d) Lethally irradiated C57BL/6 wild-type recipients or C57BL/6 MHC class I and II–deficient mice were transplanted with syngeneic MHC-positive or MHC-deficient HS cells. All animals received BALB/c-derived MHC-positive T-cell precursors on day 0. Animals were killed on day 14 after HSCT and thymocytes were obtained for multicolor flow cytometric analysis of BALB/c-derived TCRαβ+ cells. Mean ± s.e.m. of combined data from two independent experiments are presented (n = 4–6). *, P < 0.05. allo pre-T, allogeneic T-cell precursors.
Immunotherapy with T-cell precursors after sublethal irradiation
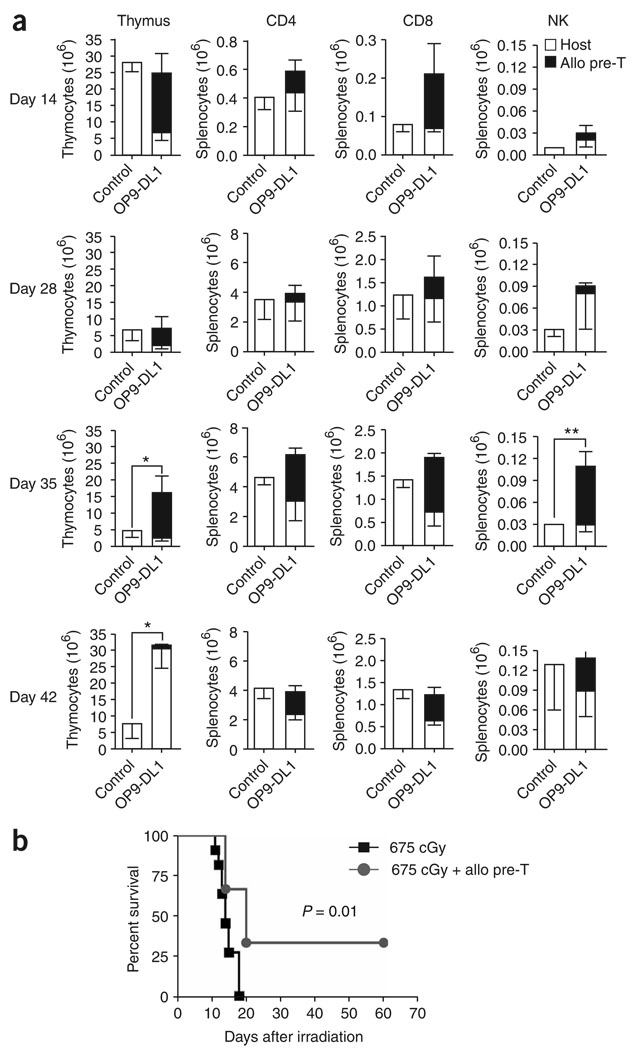
In addition to syngeneic HSCT recipients, we also infused allogeneic (derived from C57BL/6 mice) T-cell precursors into BALB/c mice receiving sublethal or lethal irradiation doses without stem cell rescue. This resulted in enhanced early T- and NK-cell reconstitution (Fig. 3a) and improved survival (Fig. 3b). Consistent with our findings in the allogeneic HSCT setting27, thymopoiesis was significantly (P < 0.05) increased in the treatment group compared with the control group even after the wave of allogeneic cells had left the thymus, suggesting an additional benefit of T-cell precursor administration on thymic reconstitution because of lymphostromal interaction. Previous studies have proposed positive feedback and cross talk between developing thymocytes and thymic stroma37.
Figure 3.
Adoptively transferred allogeneic T-cell precursors enhance T and NK cell reconstitution and improve survival in irradiated hosts. (a) BALB/c recipients were irradiated with a single dose of 650–687 cGy. Control mice received irradiation only; the treatment group received additional C57BL/6-derived in vitro-generated T-cell precursors. At days 14, 28, 35 and 42 after irradiation, animals were killed and thymi and spleens were harvested. BALB/c or C57BL/6 origin of cells was determined by total cellularity and multicolor flow cytometric analysis using Ly9.1- and CD45.1-specific antibodies, respectively. T and NK cells were analyzed using antibodies to CD3, CD4, and CD8, and DX5, respectively. Values represent mean cell numbers ± s.e.m. (n = 5–12). Combined data of two independent experiments are presented. *, P < 0.05; **, P < 0.01. (b) BALB/c recipients were irradiated with a single dose of 675 cGy. Control mice received irradiation only; the treatment group received additional C57BL/6-derived in vitro-generated T-cell precursors. Survival was monitored daily. One of three independent experiments is presented (n = 6–11).
Anti-tumor activity of transferred allogeneic T-cell precursors
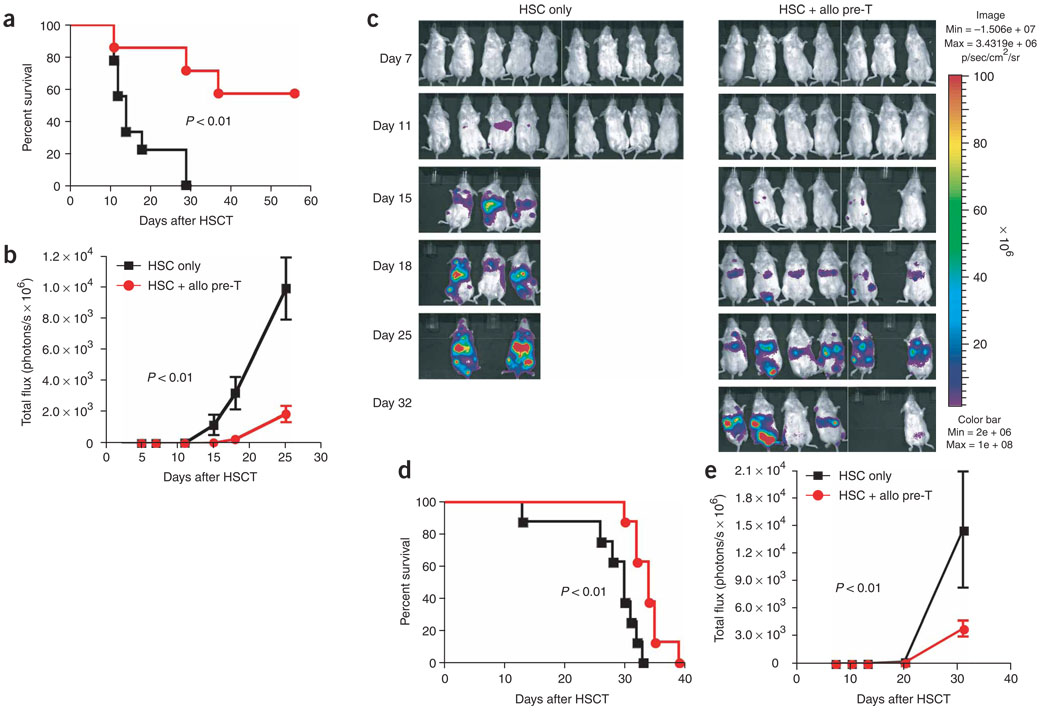
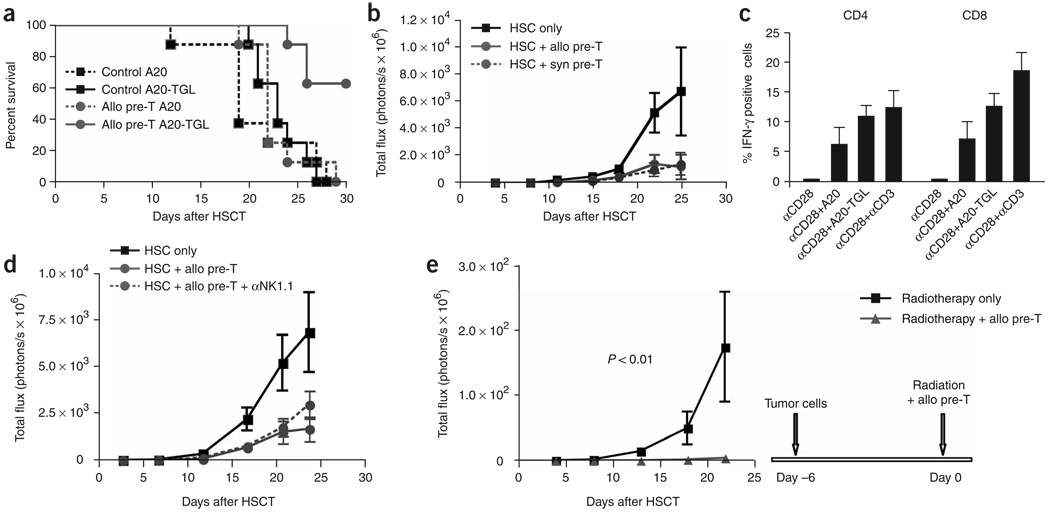
To assess the efficacy of allogeneic T-cell precursors for tumor immunotherapy, we adoptively transferred T-cell precursors derived from C57BL/6 mice, along with BALB/c mice HS cells, into lethally irradiated BALB/c mice that were challenged with bioluminescent tumor cells (A20-TGL lymphoma cells (Fig. 4a–c) or Renca-TGL renal carcinoma cells (Fig. 4d,e)). We found significant (P < 0.01) anti-tumor activity compared with recipients of HS cells only, as determined by survival and in vivo bioluminescence imaging. We also tested this strategy in the absence of syngeneic HS cells and found the same benefit (Supplementary Fig. 5 online).
Figure 4.
Adoptively transferred allogeneic T-cell precursors mediate significant anti-tumor responses. (a–c) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells; control mice received HS cells only, the treatment group received additional C57BL/6-derived in vitro-generated T-cell precursors on day 0. All mice received 2.5 × 105 luciferase-expressing A20-TGL tumor cells intravenously on day 0. Over the course of 32 d after injection, the whole body distribution of transduced tumor cells was monitored using in vivo bioluminescence imaging. Survival is shown in a, the bioluminescent signal intensity for every group at six time points presented as mean ± s.e.m. is shown in b and pseudo-color images superimposed on conventional photographs are shown in c (n = 7–9). (d–e) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells; control mice received HS cells only, the treatment group received additional C57BL/6-derived in vitro-generated T-cell precursors on day 0. All mice received 5 × 104 luciferase-expressing Renca-TGL tumor cells intravenously on day 0. Over the course of 31 d after injection, the whole body distribution of transduced tumor cells was monitored using in vivo bioluminescence imaging. Survival is shown in d and the bioluminescent signal intensity for every group at five time points presented as mean ± s.e.m. is shown in e (n = 7–9). Experiments were performed at least twice.
When we assessed GVT activity of adoptively transferred allogeneic T-cell precursors against A20-TGL cells (very immunogenic, due to expression of herpes simplex thymidine kinase, firefly luciferase and GFP) or less immunogenic A20 cells, we found a survival benefit only in the A20-TGL group (Fig. 5a). These results suggest that the anti-tumor activity of T-cell precursors is related to the expression of immunogenic tumor-associated antigens or neoantigens, such as TGL. We therefore hypothesized that syngeneic T-cell precursors should also be able to mediate anti-tumor activity, provided the targeted tumor was strongly immunogenic. Using the A20-TGL model, we demonstrated that immunotherapy with allogeneic or syngeneic T-cell precursors resulted in the same degree of anti-tumor activity (Fig. 5b), underlining the importance of immunogenicity for a strong T-cell response. Indeed, we found that T cells derived from adoptively transferred allogeneic T-cell precursors responded with more interferon (IFN)-γ secretion upon in vitro stimulation with A20-TGL cells than with A20 cells (Fig. 5c). In addition, our findings presented in Supplementary Figure 4 (weak response of C57BL/6 mice T-cells to EL4-TGL in contrast to a strong response of BALB/c and allogeneic T-cell precursor–derived T cells to A20-TGL) suggest an important role of costimulatory molecules for potent anti-tumor responses, as A20 cells highly express CD86 and lack CD80 whereas EL4 cells show only weak expression of CD86 and lack CD80 (data not shown).
Figure 5.
Response to immunotherapy with T-cell precursors depends on the immunogenicity of the tumor but not on MHC disparity. (a) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells; control mice received HS cells only, the treatment groups received additional C57BL/6-derived in vitro-generated T-cell precursors on day 0. All mice received 2.5 × 105 tumor cells i.v. on day 0, either A20-TGL or A20 cells, and survival was monitored (n = 8). (b) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells; control mice received HS cells only, the treatment groups received additional C57BL/6 or BALB/c-derived in vitro-generated T-cell precursors on day 0. All mice received 2.5 × 105 A20-TGL tumor cells i.v. on day 0. Over the course of 25 d after injection, the whole body distribution of luciferase expressing tumor cells was monitored using in vivo bioluminescence imaging. The bioluminescent signal intensity for every group at seven time points presented as mean ± s.e.m. (n = 6–8). (c) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells, received additional C57BL/6 -derived in vitro-generated T-cell precursors on day 0 and were challenged with 2.5 × 105 A20-TGL tumor cells intravenously on day 0. On days 27 to 32 after HSCT, animals were killed and splenic T cells were cultured overnight in the presence of soluble CD28-specific antibodies ± irradiated A20 cells, A20-TGL cells or immobilized antibodies directed against CD3. Cells were stained for CD45.1, CD4, CD8, INF-γ and rat lgG1-κ (isotypic control) and analyzed for IFN-γ expression on C57BL/6-derived CD4+ and CD8+ T cells. Combined data of three independent experiments are shown. Mean values ± s.e.m. are presented (n = 10–12). Histogram comparisons were performed by overton subtraction. (d) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells; control mice received HS cells only, the treatment groups received additional C57BL/6-derived in vitro-generated T-cell precursors on day 0 ± NK1.1 depleting antibodies (500 µg intraperitoneally days −1 to 6). All groups received 0.25 × 106 A20-TGL tumor cells i.v. on day 0. At the indicated time points, tumor growth was determined by in vivo bioluminescence imaging and is presented as mean ± s.e.m. (n = 6–8). (e) BALB/c recipients were irradiated with 250 cGy and received 2.5 × 105 A20-TGL cells i.v. on day −6. On day 0, all mice received a second radiation dose of 650 cGy and were transplanted with BALB/c HS cells. Control mice received HS cells only, the treatment group received additional C57BL/6-derived in vitro-generated T-cell precursors on day 0. At the indicated time points, tumor growth was determined by in vivo bioluminescence imaging and is presented as mean ± s.e.m. (n = 6–7).
Adoptive transfer of T-cell precursors also results in early reconstitution of NK cells (Fig. 2a). To assess the possible contribution of NK cells to anti-tumor activity against A20-TGL cells, we compared recipients receiving allogeneic precursor cells ± NK cell-depleting antibody treatment, revealing that depletion of NK cells derived from adoptively transferred precursor cells had only a minor effect on antitumor activity (Fig. 5d). We also found that, as expected, with increasing tumor burdens the anti-tumor effect becomes less effective (Supplementary Fig. 6 online). Thus, in general, but particularly in the setting of advanced and poorly immunogenic tumors, T-cell precursors are unlikely to eradicate a tumor completely if they are not combined with additional therapeutic modalities such as radiotherapy, chemotherapy or molecular therapy. To emulate more clinically relevant settings, we therefore challenged mice with A20-TGL cells 6 d before adoptive immunotherapy with allogeneic T-cell precursors combined with radiotherapy (650 cGy) (Fig. 5e). This model allowed us not only to assess the efficacy of this combination treatment, but also to address the important issue of thymic negative selection, as in this setting T-cell precursors expressing TCRs specific for tumor-associated antigens would be more likely subjected to negative selection. However, we found very strong anti-tumor activity that by far exceeded the effect of adoptive immunotherapy in our conventional tumor experiments (20-fold less tumor growth by day 27 compared to recipients of radiation alone), indicating the selection of a functional TCR repertoire and synergistic effects of combined radiotherapy and T-cell precursor immunotherapy.
Genetic engineering of antigen-specific T-cell precursors
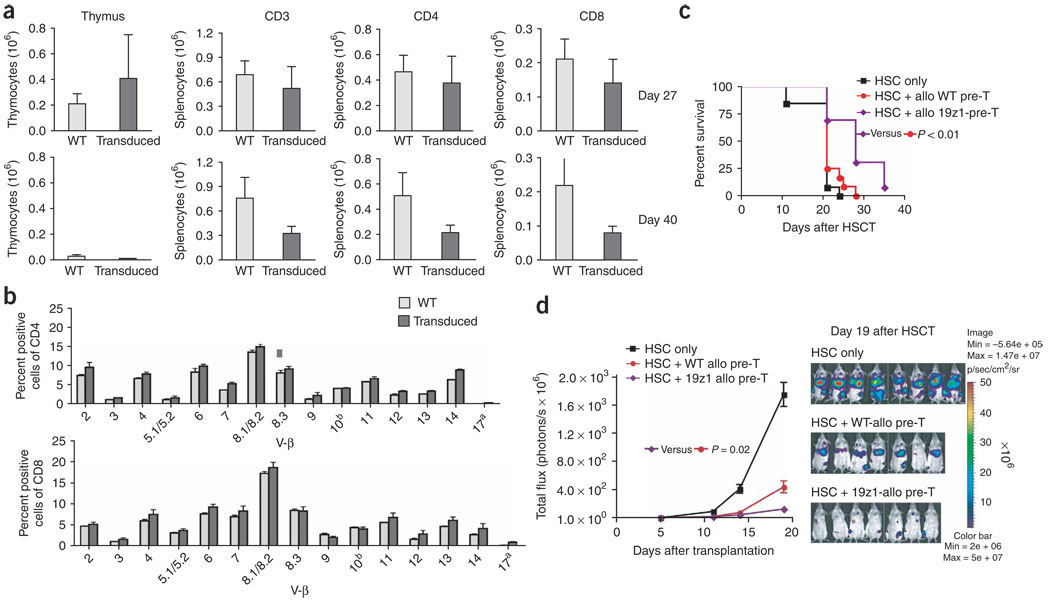
TCR gene or antigen receptor gene transfer has been used as a strategy to engineer antigen-specific T-cell immunity and has shown remarkable success in the treatment of malignancies in preclinical models38–41. Because many malignant tumors in humans are not very immunogenic, it would be highly desirable to generate tumor antigen–specific precursor cells in our culture system42. To allow positive selection of transduced precursor cells, it is important to transfer a gene that does not interfere with endogenous TCR expression and selection. Instead of TCR gene transfer, we therefore engineered T-cell precursors to stably express a chimeric antigen receptor named 19z1, consisting of an extracellular antigen-binding domain targeting human CD19 (derived from an anti-CD19 antibody) and an intracellular signal transduction domain derived from human CD3-ζ, enabling antigen recognition in an MHC-independent manner. This chimeric antigen receptor has previously been used to genetically engineer peripheral blood-derived human T cells, resulting in the eradication of disseminated intramedullary tumors in immunodeficient mice39. However, it should be noted that the clinical usefulness of this technology has yet to be demonstrated and is currently being studied at our center. Transduction efficiencies of the DN2 subset (which is the relevant subset for thymic engraftment) were routinely in the range of 50% and up to 70–80% in DN1 and DN4 cells by day 21 of culture (Supplementary Fig. 7 online). To assess the feasibility of anti-tumor therapy with tumor-specific T-cell precursors, we adoptively transferred 19z1-transduced allogeneic T-cell precursors and analyzed their progeny at days 27 and 40 after transplantation. We found both CD3+CD4+ and CD3+CD8+ 19z1-expressing peripheral T cells in these animals (Fig. 6a), and the percentage of transduced T cells of allogeneic origin corresponded with the percentage of transduced DN2 cells in the cultures that had been used for adoptive transfer, suggesting normal positive and negative selection of transduced T-cell precursors. TCR repertoire analysis revealed no difference between transduced and untransduced cells (Fig. 6b). In vitro stimulation with target T cells expressing hCD19 or an irrelevant antigen (hPSMA) revealed a strong response (expression of IFN-γ as well as increased Lck recruitment) to hCD19 in 19z1-transduced T cells compared to untransduced, wild-type (WT) C57BL/6 T-cells (Supplementary Figs. 8 and 9 online). Next, we determined whether adoptively transferred 19z1-expressing T-cell precursors could enhance GVT activity in HSCT recipients that had been challenged with a 19z1-sensitive tumor (A20 lymphoma transduced to express hCD19). Importantly, expression of hCD19 by A20 did not result in a significant increase in immunogenicity (Supplementary Fig. 5). Indeed, survival was significantly (P < 0.01) improved in recipients of transduced T-cell precursors as opposed to recipients of wild-type T-cell precursors or of HS cells only (Fig. 6c). Moreover, when we challenged mice with A20-TGL-hCD19 to determine tumor growth by in vivo bioluminescence imaging, we found significantly (P = 0.02) increased anti-tumor activity in recipients of 19z1-expressing T-cell precursors compared with recipients of untransduced T-cell precursors (Fig. 6d and Supplementary Fig. 10 online). Our data therefore indicate that engineered T-cell precursors give rise to antigen-specific host-tolerant T cells that can display cytotoxic activity upon stimulation with their specific antigen, migrate to the site of antigen expression in vivo and persist for at least 2 months after transfer (data not shown). We did not observe any adverse effects.
Figure 6.
Genetically engineered antigen-specific T-cell precursors give rise to tumor-responsive CD8+ and CD4+ T cells coexpressing chimeric antigen receptor and endogenous TCR. (a) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells and received additional C57BL/6(CD45.1)-derived in vitro generated T-cell precursors transduced to express 19z1. At days 27 and 40 after HSCT, animals were killed and thymi and spleens were harvested. C57BL/6 origin of cells was determined by total cellularity and multicolor flow cytometric analysis using CD45.1-specific antibodies. T cells were analyzed using antibodies to CD3, CD4, CD8 and 19z1. Values represent mean cell numbers ± s.e.m. (n = 5). One of three independent experiments is presented. (b) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells and received additional C57BL/6-derived in vitro generated T-cell precursors transduced to express 19z1. At day 27 after HSCT, animals were killed and splenocytes were obtained for multicolor flow cytometric analysis of the TCR-Vβ families on CD4+ and CD8+ cells of C57BL/6 origin. Mean ± s.e.m. are presented (n = 3). (c) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells only or received additional, either unmanipulated or genetically engineered 19z1-expressing, C57BL/6 -derived T-cell precursors. All mice received 2.5 × 105 A20-hCD19 cells intravenously on day 0, and survival was monitored daily. Tumor death was confirmed by necropsy and hCD19 expression of lethal tumors was confirmed by flow cytometric analysis. Combined data of two independent experiments are presented (n = 13). (d) Lethally irradiated BALB/c recipients were transplanted with BALB/c HS cells only or received additional C57BL/6-derived T-cell precursors (unmanipulated or genetically engineered to express 19z1) on day 0. All mice received 3.3 × 105 A20-TGL-hCD19 tumor cells intravenously on day 0, and tumor growth was monitored by in vivo bioluminescence imaging. Mean ± s.e.m. of bioluminescent signal intensity of five time points as well as pseudo-color images superimposed on conventional photographs on day 19 after HSCT are presented (n = 7–8).
DISCUSSION
The use of adoptive T-cell therapies is often limited by barriers imposed by MHC disparity, resulting in rejection, alloreactivity and impaired antigen presentation. This study shows for the first time that lymphoid precursor cells from any donor can be transferred to any individual irrespective of MHC disparities. Our data demonstrate that allogeneic T-cell precursors, when adoptively transferred (with or without syngeneic HS cells) to irradiated recipients, develop into functional mature T cells in the absence of a complete donor hematopoietic system. MHC restriction of these cells is determined by host MHC molecules, which are encountered during T-cell development43–45. Tolerance of the resulting T cells is dual (donor and host) as a consequence of antigen-presenting cell chimerism (owing to the dendritic cell lineage potential of T-cell precursors up to the DN2 stage46,47). The major advantage of T cell lineage committed precursor cells with limited dendritic cell capacity is their potential to reconstitute an immunosuppressed host with host MHC restricted allogeneic T cells, while preserving a predominantly host antigen-presenting cell chimerism. Controlling antigen-presenting cell chimerism translates into host tolerant and functional TCR selection, averting GVHD and promoting immunity. Therefore, cell therapy with T-cell precursors allows for universal T-cell immunotherapy, which is currently impossible with ex vivo generated or manipulated mature T cells. We provide evidence for the efficacy of this strategy as tumor immunotherapy in combination with prior cytostatic conditioning, as well as a countermeasure for lymphopenia due to radiation-induced injury. True off-the-shelf therapy requires the use of cryopreserved cells, which is routinely done in the clinical setting with human hematopoietic progenitor cells. Using our mouse model, we found that adoptive transfer of cryopreserved allogeneic T-cell precursors to irradiated recipients resulted in stable thymic engraftment (Supplementary Fig. 11 online).
Genetic engineering is a powerful strategy to design tumor-associated antigen-specific cells. Potential targets for transgenic receptors include immunogenic tumor proteins21,48,49 or viral antigens in virus-associated cancers such as Epstein-Barr virus (EBV)-related lymphoma or lymphoproliferative disease16,19,50. HS cells have been transduced with TCR genes to obtain tumor-specific T cells42,51. However, HS cells are usually quiescent, which makes them more difficult to transduce than rapidly proliferating cells such as early T-cell precursors (in particular DN1). An important issue in engineering mature T cells to express antigen-specific receptors is the possibility of generating potentially harmful novel receptor specificities as a consequence of combining transgenic receptor and endogenous TCR chains52. This scenario is of no concern when engineering early T-cell precursors, because these cells have not yet selected their TCR. The main challenge of this approach is to generate antigen-specific T-cell precursors that can be positively selected and are not subject to negative selection. The former can be accomplished by transferring genes encoding chimeric antigen receptors instead of TCRs, allowing endogenous TCR expression and resulting in intact positive selection of transgenic cells. The risk of clonal deletion can be reduced by targeting antigens that are distinct from self-antigens so that auto-reactivity (if any) displayed by the antigen receptor is below the threshold that induces negative selection. Because the mechanism of negative selection is very sensitive, additional strategies to overcome this barrier should be explored, such as using promoters that allow temporal control of transgene expression (conditional expression, or even better, maturation-dependent expression). Furthermore, suicide switches as a safety feature and costimulatory signals to prevent T-cell anergy and apoptosis could be incorporated to ensure optimal functionality, while avoiding unwanted adverse effects of engineered cells39,52. However, our strategy to transduce T-cell precursors to express a chimeric antigen receptor targeting hCD19 resulted in excellent transduction efficiency and the in vivo generation of high numbers of appropriately selected T cells expressing the chimeric antigen receptor, which were capable of significant anti-tumor activity without any undesirable auto/alloreactivity. Combining tumor-specific T-cell precursors with tumor vaccination strategies could further enhance the efficacy of this approach.
We conclude that adoptively transferred ex vivo generated allogeneic T-cell precursors can develop into host-MHC restricted T cells characterized by dual tolerance and selection of a functional TCR repertoire, even in a fully mismatched thymic epithelial MHC environment. The advantages of using T-cell precursors for immunotherapy are obvious: T-cell precursors do not have to be MHC matched because GVHD is not an issue, and the use of allogeneic precursor cells instead of autologous cells eliminates the risk of contamination with residual malignant patient cells and enables the generation and storage of virtually unlimited quantities of precursor cells for universal use. This procedure has not only substantial logistic and technical advantages, but it facilitates the use of ex vivo manipulation protocols, in particular genetic engineering, to generate antigen-specific or otherwise enhanced designer cells. Adoptive transfer of MHC-mismatched and genetically enhanced T-cell precursors therefore represents a labor-saving and cost-effective strategy for targeted immunotherapy for patients with malignant diseases and/or T-cell deficiencies.
METHODS
Cells and cell lines
Single cell suspensions were prepared from spleen and thymus according to standard protocols. Harvest medium consisted of RPMI-1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine. OP9-DL1, a bone marrow stromal cell line of (C57BL/6 × C3H)F2-op/op origin transduced with DL1, was described previously22. A20, a B-cell lymphoma cell line of BALB/c origin, and Renca, a renal cell carcinoma cell line of BALB/c origin, were kindly provided by A. Houghton (Memorial Sloan Kettering Cancer Center). A20 was either used untransduced or retrovirally transduced, either to express a triple fusion protein consisting of Herpes simplex virus thymidine kinase, enhanced green fluorescent protein (eGFP) and firefly luciferase (TGL)53, or to express human CD19 (transduction with SFG-hCD19 oncoretroviral vectors derived from gpg29 cells)38, or to express both TGL and hCD19. Renca was retrovirally transduced to express TGL, as described above. The construction of EL4-hCD19 and EL4-hPSMA has been described previously38. Cell culture medium consisted of RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine (for A20 and Renca), Dulbecco Modified Eagle’s Medium (DMEM, Life Technologies) supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine (for EL4) and αMEM supplemented with 20% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin (for OP9-DL1). Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Assessment of GVHD, GVT, in vivo BLI
The severity of GVHD was assessed with a clinical GVHD scoring system as previously described29. Briefly, ear-tagged animals in coded cages were individually scored every week for five clinical parameters on a scale from zero to two: weight loss, posture, activity, fur and skin. A clinical GVHD index was generated by summation of the five criteria scores (zero–ten). Survival was monitored daily. Animals were killed after HSCT for histopathological analysis of GVHD target organs (small bowel, large bowel and liver). Organs were harvested, formalin-preserved, paraffin-embedded, sectioned and hematoxylin/eosin-stained. A semiquantitative score consisting of 19 to 22 different parameters associated with GVHD was calculated, as described previously30. In GVT experiments, bioluminescent signal intensity of tumor-bearing mice was determined twice weekly. Fifteen minutes after intra-peritoneal injection of 3 mg/mouse D-Luciferin (Xenogen), mice were anaesthetized and placed into the light tight chamber of anIVIS 200 bioluminescence imaging system (Xenogen). Gray-scale photographic images of the mice were acquired first and then a low-level bioluminescent signal was recorded. Pseudo-color images showing the whole body distribution of bioluminescent signal intensity were superimposed on the gray-scale photographs and total flux (photons/sec) was determined for individual mice. The cause of death was confirmed by necropsy and histopathology.
Retroviral transduction and expansion of T-cell precursors
T cell precursors were developed in vitro from C57BL/6 bone marrow–derived HS cells using the OP9-DL1 culture system, as described above. Lentiviral vectors were produced by tripartite transfection of 293T cells with pRRL-hPGKpr-19z1-WPRE, pCMVΔR8.92 and pUCMD.G54. Vector supernatants were concentrated by ultra-centrifugation and 0.75–1.5 × 108 total TU used to transduce 5 × 105 T-cell precursors (coculture days 4–6) over 2 d in 24-well tissue culture plates coated with 15 µg/ml retronectin and 10 µg/ml DL1ext-IgG. Transduced cells were then expanded for an additional 14–21 d by OP9-DL1 coculture.
Statistics
All results in this manuscript are based on two-sided test statistics. P < 0.05 was considered statistically significant. The Mann-Whitney U-statistic was used to compare flow cytometric data. In vivo data regarding survival, weight changes and photon intensity determined by in vivo BLI were collected in studies assessing GVHD and GVT. For those studies, mice were randomly assigned to the treatment groups and the area under the curve (AUC) was used to summarize the weight and photon trajectory of each mouse under study. Not all the mice were followed for the full length of the study. The primary reason for censoring was death or sacrifice, and ignoring this type of informative censoring may result in a biased treatment comparison. To eliminate this bias, a test statistic was formed using the information up to the minimum follow-up time for each cross-treatment mouse pair. By eliminating the uneven censorship between mouse pairs in different groups, a test statistic can be constructed that has mean zero when the growth rates in the two groups are equal. The statistic is based on the average difference in the censored AUC curves between treatment groups55. The log rank test statistic was used to compare survival curves between groups. For the analyses of the imaging studies, the P values were generated from a permutation test, using the AUC and log-rank test statistics. The application of the permutation procedure was due to the small number of animals in these studies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants HL69929, CA33049 and CA107096 from the National Institutes of Health (NIH), by awards from the Leukemia and Lymphoma Society, the Ryan Gibson Foundation, the Elsa U. Pardee Foundation, the Byrne Fund, the Emerald Foundation and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by William H. Goodwin and Alice Goodwin and the Commonwealth Foundation for Cancer Research (M.R.M.v.d.B.). J.L.Z. is the recipient of a fellowship grant from the Lymphoma Research Foundation, J.C.M. and G.R. are supported by a Cancer Research Institute Pre-Doctoral Fellowship and by NIH MSTP grant GM07739, M.S. and J.C.M are supported by NIH grant CA40350, M.S., I.R. and J.C.M. are supported by NIH grant CA59350, and J.C.Z.-P. is supported by a Canada Research Chair in Developmental Immunology. Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Small-Animal Imaging Research Program (SAIRP) grant R24 CA83084 and NIH Center grant P30 CA08748, are gratefully acknowledged. The authors would like to thank the staff of the Research Animal Resource Center for excellent animal care. A20, a B-cell lymphoma cell line derived from BALB/c mice, and Renca, a renal cell carcinoma cell line derived from BALB/c mice, were kindly provided by A. Houghton (Memorial Sloan Kettering Cancer Center).
Footnotes
Note: Supplementary information is available on the Nature Biotechnology website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Mackall CL, Gress RE. Thymic aging and T-cell regeneration. Immunol. Rev. 1997;160:91–102. doi: 10.1111/j.1600-065x.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 2.Grunebaum E, Sharfe N, Roifman CM. Human T cell immunodeficiency: when signal transduction goes wrong. Immunol. Res. 2006;35:117–126. doi: 10.1385/ir:35:1:117. [DOI] [PubMed] [Google Scholar]
- 3.Fischer A, et al. Naturally occurring primary deficiencies of the immune system. Annu. Rev. Immunol. 1997;15:93–124. doi: 10.1146/annurev.immunol.15.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Chinen J, Finkelman F, Shearer WT. Advances in basic and clinical immunology. J. Allergy Clin. Immunol. 2006;118:489–495. doi: 10.1016/j.jaci.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Lehrnbecher T, Foster C, Vázquez N, Mackall CL, Chanock SJ. Therapy-induced alterations in host defense in children receiving therapy for cancer. J. Pediatr. Hematol. Oncol. 1997;19:399–417. doi: 10.1097/00043426-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Yarilin AA, et al. Late T cell deficiency in victims of the Chernobyl radiation accident: possible mechanisms of induction. Int. J. Radiat. Biol. 1993;63:519–528. doi: 10.1080/09553009314550681. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 8.Joao C, et al. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–871. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 9.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol. Blood Marrow Transplant. 2005;11:181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Gordan LN, et al. Correlation of early lymphocyte recovery and progression-free survival after autologous stem-cell transplant in patients with Hodgkin’s and non-Hodgkin’s Lymphoma. Bone Marrow Transplant. 2003;31:1009–1013. doi: 10.1038/sj.bmt.1704050. [DOI] [PubMed] [Google Scholar]
- 11.Porrata LF, et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–1318. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 12.Porrata LF, et al. Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin’s disease. Br. J. Haematol. 2002;117:629–633. doi: 10.1046/j.1365-2141.2002.03478.x. [DOI] [PubMed] [Google Scholar]
- 13.Porrata LF, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 14.Porrata LF, Ingle JN, Litzow MR, Geyer S, Markovic SN. Prolonged survival associated with early lymphocyte recovery after autologous hematopoietic stem cell transplantation for patients with metastatic breast cancer. Bone Marrow Transplant. 2001;28:865–871. doi: 10.1038/sj.bmt.1703236. [DOI] [PubMed] [Google Scholar]
- 15.Kolb HJ, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 16.Gottschalk S, Heslop HE, Rooney CM. Adoptive immunotherapy for EBV-associated malignancies. Leuk. Lymphoma. 2005;46:1–10. doi: 10.1080/10428190400002202. [DOI] [PubMed] [Google Scholar]
- 17.Gottschalk S, Heslop HE, Rooney CM. Treatment of Epstein-Barr virus-associated malignancies with specific T cells. Adv. Cancer Res. 2002;84:175–201. doi: 10.1016/s0065-230x(02)84006-4. [DOI] [PubMed] [Google Scholar]
- 18.Rooney CM, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 19.Rooney CM, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 20.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N. Engl. J. Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 21.Yotnda P, et al. Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J. Clin. Invest. 1998;101:2290–2296. doi: 10.1172/JCI488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luznik L, et al. Successful therapy of metastatic cancer using tumor vaccines in mixed allogeneic bone marrow chimeras. Blood. 2003;101:1645–1652. doi: 10.1182/blood-2002-07-2233. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by Delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 24.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(−)cord blood cells. J. Clin. Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9–DL1 stromal cell line without thymus microenvironment. Blood Cells Mol. Dis. 2004;33:227–232. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.La Motte-Mohs RN, Herer E, Zúñiga-Pflücker JC. Induction of T cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 27.Zakrzewski JL, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat. Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 28.Dallas MH, Varnum-Finney B, Martin PJ, Bernstein ID. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta 1. Blood. 2007;109:3579–3587. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt TM, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat. Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 30.Cooke KR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 31.Hill GR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 32.Acha-Orbea H, MacDonald HR. Superantigens of mouse mammary tumor virus. Annu. Rev. Immunol. 1995;13:459–486. doi: 10.1146/annurev.iy.13.040195.002331. [DOI] [PubMed] [Google Scholar]
- 33.Abe R, Kanagawa O, Sheard MA, Malissen B, Foo-Phillips M. Characterization of a new minor lymphocyte stimulatory system. I. Cluster of self antigens recognized by “I-E-reactive” V beta s, V beta 5, V beta 11, and V beta 12 T cell receptors for antigen. J. Immunol. 1991;147:739–749. [PubMed] [Google Scholar]
- 34.Woodland D, Happ MP, Bill J, Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990;247:964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- 35.Bill J, Kanagawa O, Woodland DL, Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J. Exp. Med. 1989;169:1405–1419. doi: 10.1084/jem.169.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vacchio MS, Hodes RJ. Decreased expression of specific V beta families is associated with expression of multiple MHC and non-MHC gene products. J. Exp. Med. 1989;170:1335–1346. doi: 10.1084/jem.170.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat. Rev. Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 38.Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA, Schumacher TN. Immunotherapy through TCR gene transfer. Nat. Immunol. 2001;2:957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- 39.Brentjens RJ, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 40.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 41.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67:2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 44.Ernst BB, Surh CD, Sprent J. Bone marrow-derived cells fail to induce positive selection in thymus reaggregation cultures. J. Exp. Med. 1996;183:1235–1240. doi: 10.1084/jem.183.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J. Exp. Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen HQ, et al. T/NK bipotent progenitors in the thymus retain the potential to generate dendritic cells. J. Immunol. 2003;171:3401–3406. doi: 10.4049/jimmunol.171.7.3401. [DOI] [PubMed] [Google Scholar]
- 48.Liggins AP, Guinn BA, Banham AH. Identification of lymphoma-associated antigens using SEREX. Methods Mol. Med. 2005;115:109–128. doi: 10.1385/1-59259-936-2:109. [DOI] [PubMed] [Google Scholar]
- 49.Greiner J, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006;108:4109–4117. doi: 10.1182/blood-2006-01-023127. [DOI] [PubMed] [Google Scholar]
- 50.Cohen JI. Benign and malignant Epstein-Barr virus-associated B-cell lymphoproliferative diseases. Semin. Hematol. 2003;40:116–123. doi: 10.1053/shem.2003.50018. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, Qin XF, Baltimore D, van Parijs L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proc. Natl. Acad. Sci. USA. 2002;99:6204–6209. doi: 10.1073/pnas.092154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.June CH. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terwey TH, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–3330. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May C, et al. Therapeutic haemoglobin synthesis in β-thalassaemic mice expressing lentivirus-encoded human β-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 55.Vardi Y, Ying Z, Zhang CH. Two-sample tests for growth curves under dependent right censoring. Biometrika. 2001;88:949–960. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.