1. Introduction
Corticotropin-releasing factor (CRF), a highly conserved 41-amino acid peptide, is well known for its critical role in the regulation of the hypothalamic-pituitary-adrenal/interrenal (HPA/I) axis, the major neuroendocrine component of the vertebrate stress response (Vale et al., 1981). In addition to its key role as a regulator of the HPA/I, CRF also acts at multiple sites throughout the central nervous system (CNS) to induce rapid autonomic, neuroendocrine, and behavioral responses to a stressor (Dunn and Berridge, 1990; Smagin et al., 2001; Henrichs and Koob, 2004). In both mammalian and non-mammalian vertebrates, stress-induced release or central administration of CRF elicits a variety of ‘anxiety-like’ behaviors, including enhanced locomotion, an effect that is maintained in hypophysectomized animals and blocked by pre-treatment with centrally administered CRF receptor (CRFR) antagonists (Sutton et al., 1982; Moore et al., 1984; Britton et al., 1986; Lowry and Moore, 1991; Clements et al., 2002; Crespi and Denver, 2004). Therefore, in addition to regulation of the HPA/I, CRF is also viewed as a neuromodulator/neurotransmitter subserving diverse functions within the vertebrate CNS, which prepare an animal to respond effectively and rapidly to an acute stressor.
CRF, like other neuropeptides, exerts its neurophysiological actions and subsequent behavioral effects within the CNS by binding to G protein-coupled receptors (GPCR; Bale and Vale, 2004). To date, two CRF GPCR subtypes have been identified and cloned in both mammalian and non-mammalian vertebrates: CRF1 and CRF2 (Dautzenberg et al., 1997; Pohl et al., 2001; Bale and Vale, 2004). Recent evidence suggests that the former receptor subtype is largely responsible for the initiation of CRF-induced enhancement of locomotion, as well as other stress-related adaptive behaviors (Müller et al., 2003; Nguyen et al., 2006). On binding, the ligand-receptor complex is internalized into cells rapidly (within 5-10 min), in a time-dependent manner, and predominantly via a clathrin-mediated endocytotic pathway (Hauger et al., 2000; Rasmussen et al., 2004; Perry et al., 2008; Reyes et al., 2008). Following repeated agonist exposure, this internalization process is capable of modulating a cell's responsiveness by causing a loss of CRFR at the cell's surface, thereby attenuating the cell's sensitivity to continued ligand challenge (Dautzenberg et al., 2001; Hauger et al., 2003; Gainetdinov et al., 2004).
Despite abundant evidence implicating CRF in the enhancement of locomotion, the CRF target neurons that underlie the peptides' locomotor stimulating effects remain largely unknown. Indeed, previous research has demonstrated CRF-immunoreactive (ir) neurons and CRFR binding sites distributed widely throughout the vertebrate brain, including regions implicated in locomotor control, such as the medullary brainstem (Merchenthaler, 1984; De Souza et al., 1985; Sakanaka et al., 1987; Potter et al., 1994; Chalmers et al., 1995). Although compelling, the presence of CRF-ir and/or CRFR expressing neurons is not sufficient evidence that the receptors are functional and/or responsible for initiating CRF's locomotor enhancing effects. Thus, the purpose of the present study was to develop a novel fluorescent conjugate of CRF and validate its efficacy as a tool for identification of neuronal targets for CRF's actions in vivo. Toward this end, we developed a fluorescent conjugate of CRF by linking it to a rhodamine based fluorescent dye containing a seven-atom aminohexanoyl spacer, 5(6)-TAMRA-X (TAMRA), to produce the conjugate CRF-TAMRA 1. The CRF-TAMRA 1 conjugate was then used to track CRF internalization into target neurons in locomotion-controlling regions of the medullary brainstem in a well-studied behavioral model: CRF facilitation of locomotion in the roughskin newt (Taricha granulosa; Lowry et al, 1990; Lowry and Moore, 1991; Lowry et al., 1996; Lowry and Moore, 2006). Here, we describe the conjugate's synthesis, purification, and mass spectral analysis, as well as the time course, specificity, and dose-responsiveness of its neuronal internalization. Lastly, we verified the functional efficacy of CRF-TAMRA 1 by showing that the conjugate retains its typical behavior-activating effects in vivo. This combination of in vivo neurohistological and behavioral testing of the conjugate's functional properties was similar to that used in our recent development of a fluorescent conjugate of the peptide arginine vasotocin (AVT-OG 1; Lewis et al., 2004; Lewis et al., 2005).
2. Material and methods
2.1 Conjugation of 5(6)-TAMRA-X, SE to corticotropin-releasing factor to produce CRF-TAMRA 1
All reagents and solvents were obtained from commercial sources without further purification unless otherwise stated. Distilled deionized water was obtained from a Millipore Nanopure system. Synthesis of the CRF-TAMRA 1 mixture (Fig. 1) was accomplished by adding 100 μL of 0.1 M phosphate buffer (pH 8.5) to a manufacturer's vial containing 0.1 mg of corticotropin-releasing factor (CRFr/h) (2.1019 × 10-5 mmoles, Sigma-Aldrich, C-3042, MW 4757.52) and a micromagnetic stirring bar. The vial was recapped and stirred for 5 min at ambient room temperature. To this solution was added 6.0 μL (4.6825 × 10-5 mmoles) of 5(6)-TAMRA-X N-hydroxysuccinimide active ester (Invitrogen, T6105, MW 640.69) in dimethylformamide (DMF; stock solution was prepared from 0.5 mg of active ester in 100 μL of DMF). An additional 6.0 μL of DMF was used to complete the transfer. The vial was recapped, covered with foil to exclude light and the mixture was stirred for 18 h at ambient room temperature.
Fig. 1.
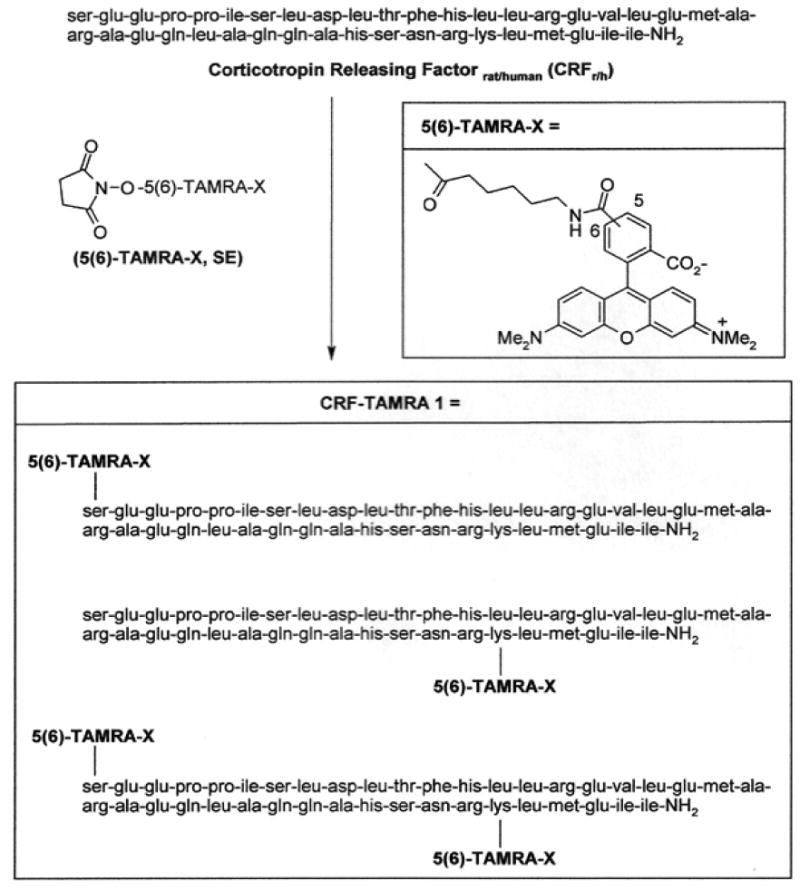
Synthesis of the 5(6)-TAMRA-X-Corticotropin-Releasing Factor Conjugate (CRF-TAMRA 1) mixture.
Preparative C18 RP-HPLC was conducted using a Phenomenex Jupiter 10 μm, 300A 250 × 21.20 mm column while monitoring at both 210 and 543 nm, with a flow rate of 4.0 mL/min. The product was a complex mixture of two regioisomers at the dye linker center in addition to the two possible conjugation sites within the CRF peptide (Fig. 1). The mixture was eluted from the preparative column with retention times of 53.4 and 54.9 min. Re-analysis of the material to confirm purity was accomplished using analytical C18 RP-HPLC (flow rate 1.0 mL/min; retention times of 33.70 and 36.64 min) (Fig. S1). No attempt was made to separate the mixture and this preparative HPLC process assured that no unmodified CRF was present in the sample. Mass spectral analysis was conducted by MALDI-TOF and afforded a product mass of 5306 at m/z 5330 [M+Na]+ corresponding to the mono-conjugated TAMRA-CRF (CRF-TAMRA 1 mixture) as the predominant product (Fig. S2). In addition, determination of the solubility of the TAMRA-CRF conjugate mixture in 100% H2O was accomplished by fluorescence spectroscopy with a calibration curve constructed using TAMRA dye in 100% H2O. Fluorescence spectroscopy revealed solubility of CRF-TAMRA 1 conjugate was 0.356 μg of conjugate per 100 μL of H2O or 3.56 ng/μL. This translates into 4.0579 × 1011 molecules per 1.0 μL of H2O.
2.2 Animals and surgical procedures
Adult, male roughskin newts (N = 43) were collected from Benton County, Oregon, USA. Newts were housed in a community tank supplied with a continuous flow of aerated well water (13-14 °C), fed chopped beef heart and maintained on a simulated natural light/dark cycle. All procedures were previously approved by The University of Wyoming Animal Care and Use Committee, conducted in accordance with international standards on animal welfare, and compliant with The European Communities Council Directive of 24 November 1986 and The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical procedures were similar to those routinely used in this laboratory (Lowry et al., 1996; Lewis et al., 2004). Following anesthetization by immersion in 0.1% MS-222 (tricaine methanesulfonate, Sigma-Aldrich, E10521), cellulose tissue soaked in anesthetic was draped over the newt's body to maintain anesthesia and facilitate in transcutaneous respiration during surgery. All surgical procedures, with the exception of those used for testing histological specificity of conjugate internalization, involved implantation of a cannula guide for remote intracerebroventricular (icv) delivery of test substances or vehicle to the right lateral ventricle in awake animals. The cannula guide, a 4.5 mm length of polyethylene tubing (PE 50), was implanted directly over the right lateral ventricle and anchored by dental cement to a stainless steel screw in the left frontal skull bone. Following surgery, the newt was allowed to recover in 13-14 °C, aerated well water. Approximately 2 hr into recovery and while still anesthetized, the newt was placed in a circular glass container (22 cm diameter) filled with 2.5 cm of cold, aerated well water and marked on the bottom with 8 equally spaced radial lines. A 26 ga stainless steel cannula (5 mm in length, Becton Dickinson) was connected to a microsyringe, via a length of polyethylene tubing (PE 50), and inserted into the cannula guide for icv delivery of hormone/s or an associated vehicle control to the right lateral ventricle without further handling of the newt during the experiment. Vehicle consisted of amphibian Ringer's solution (9.6 g/L Tyrode's Salts, Sigma-Aldrich, T2145) supplemented with 1 g/L NaHCO3.
For tests of the specificity of CRF-TAMRA 1 conjugate internalization by medullary neurons, newts were anaesthetized as described above. The head was stabilized with a support system attached to the skull and the dorsal aspect of the hindbrain was exposed for direct application of hormone, test compounds, and/or associated vehicle controls to the surface of the medulla. After surgical preparation, the newt was removed from anesthetic, immersed except for the head in cold, aerated well water, and allowed to recover for 2-4 h. Prior to recovery from anesthesia, each newt was also injected with the myoneural blocking agent gallamine triethioiodide (0.2-0.6 mL, ip; 2% solution in amphibian Ringer's). Full recovery from anesthesia is necessary for restoration of normal brain activity in order to assess neuronal internalization of the conjugate. The procedure of being awake and immobilized in this way has been verified to not be stressful to the newt (Rose et al., 1993; Rose et al., 1995).
2.3 Assessment of the time course for neuronal internalization and dose-effectiveness of CRF-TAMRA 1
Time course for CRF-TAMRA 1 internalization into medullary neurons was assessed with three groups of newts in which each newt was administered 14.24 ng of CRF-TAMRA 1 (in 4 μL Ringer's) to the right lateral ventricle. The intervals between conjugate infusion and sacrifice of the newt were 5 min (n = 5), 30 min (n = 5), and 60 min (n = 5). At the end of the CRF-TAMRA 1 exposure period, newts were killed by rapid decapitation and brains were immediately rinsed with an ice-cold phosphate-buffered saline (PBS) solution followed by fixation in a 30% sucrose solution in 10% formalin. These periods of exposure to the conjugate were based on their correspondence to the onset and peak of this peptide's reported neurobehavioral effects (Lowry et al., 1990; Lowry et al., 1996).
To determine the degree to which neuronal internalization of CRF-TAMRA 1 was dose-dependent, 9 additional newts received intraventricular infusion of 8 μL Ringer's containing one of three doses of CRF-TAMRA 1: 3.56 ng (n = 3), 14.24 ng (n = 3), or 28.48 ng (n = 3). The middle and high doses of the conjugate were selected based on previous findings demonstrating significant locomotor enhancing effects of the native peptide at equimolar doses (12.5 ng and 25 ng CRF in 2 μL amphibian Ringer's; Lowry et al., 1990) whereas, the lowest dose was selected based on limitations of CRF-TAMRA 1 solubility in water (3.56 ng/μL). In addition, locomotor behavior was recorded continuously throughout each experiment on 8 mm videotapes with a Sony video (DCR-TRV480) camera. Locomotion was quantified by counting the number of lines crossed by each newt during the 5 min period prior to and the 30 min period following icv administration of the low (3.56 ng/8 μL), middle (14.24 ng/8 μL), or high (28.48 ng/8 μL) dose of the conjugate. Upon termination of each experiment, the newt was immediately sacrificed by rapid decapitation; the brain was rinsed in ice-cold PBS and processed using the neurohistological procedures described below.
2.4 Assessment of the specificity and neurobehavioral efficacy of CRF-TAMRA 1
To determine the degree of specificity for neuronal internalization of CRF-TAMRA 1, the medulla was pre-treated with 2 μL of Ringer's containing one of the following: unlabeled CRF (1500 ng/2 μL; n = 3), a cocktail containing the non-specific CRF receptor antagonists, alpha-helical CRF(9-41) (αhCRF(9-41); Sigma-Aldrich, C246; 1500 ng/μL) and D-Phe-CRF(12-41) ([D-Phe12,Nle21,38,α-Me Leu37]-CRF(12–41); Bachem, H-3266; 1500 ng/μL; n = 3), or a vehicle (VEH; 2 μL Ringer's; n = 4) alone. This treatment was followed 5 min later by topical application of 14.24 ng/4 μL CRF-TAMRA 1 (3.56 ng/μL) to the surface of the medulla. After 30 min of exposure to the CRF-TAMRA 1 conjugate, the medulla was immediately rinsed with ice-cold PBS and the newt was decapitated. The brain was then processed using standard neurohistological procedures (see below).
In addition to histological verification of CRF-TAMRA 1 specificity, we also tested the specificity of CRF-TAMRA 1 at physiologically relevant doses on locomotor behavior in awake, freely behaving newts. Locomotor behavior was monitored and recorded as described in the previous section. Specificity of the CRF-TAMRA 1 conjugate on locomotor behavior was tested by icv administration of 1 μL of Ringer's containing either 500 ng/μL of αhCRF(9-41) (αhCRF + CRF-TAMRA 1; n = 3) or VEH alone followed 5 min later by infusion of 4 μL Ringer's containing 14.24 ng of CRF-TAMRA 1 (VEH + CRF-TAMRA 1; n = 3). As a further control, we tested the possible effect of the unconjugated fluorescent dye, TAMRA, on locomotion by icv administration of 1 μL VEH followed 5 min later by infusion of 1.72 ng/4 μL of TAMRA (VEH + TAMRA; n = 3). This dosage of TAMRA was equimolar to the concentration of TAMRA (1.72 ng/4 μL) in a solution containing 14.24 ng CRF-TAMRA 1 in 4 μL Ringer's.
2.5 Quantification of CRF-TAMRA 1 internalization
After each test procedure, the newt was immediately decapitated, the exposed brain rinsed twice in ice-cold PBS, and fixed in situ for 24 h in a 30% sucrose solution in 10% formalin. The brain was then removed, rinsed and fixed for an additional 24-48 h (30% sucrose in 10% formalin). Brains were embedded in O.C.T. (Tissue Tek) and transversely sectioned at 20 μm with a cryostat. Serial sections were thaw-mounted onto glass slides and dried for at least 8 h prior to coverslipping using Vectashield with DAPI (VECTOR) to fluorescently stain cell nuclei.
Digital images of brain sections were acquired with an epifluorescence microscope (Olympus Bx51) and a SPOT RT-KE® digital video imaging system with accompanying software. Appropriate filter cube sets were used for TAMRA (Chroma 31004; DM 595 nm, BP 520-600 nm) and DAPI (Olympus 31009; DM 400 nm, BP 320-400 nm) visualization. To quantify CRF-TAMRA 1 labeling, monochromatic grey scale images were taken of every fifth section (100 μm intervals) extending from the most caudal portion of the optic tectum to the closed medulla at 200 × magnification. Parameters of signal gain, gamma, and exposure time for image acquisition were identical across all animals. Image J (NIH) was used to count the number of neurons with DAPI-stained nuclei that showed CRF-TAMRA 1 labeling. A neuron was counted as CRF-TAMRA 1 positive if at least 10 fluorescent endosomal units were visible within the DAPI-stained nucleated neuron. An endosome was defined as a spot of fluorescence greater than 0.1 μm but less than 0.5 μm in diameter (Lutz, et al., 1991). These values are consistent with other studies in which endosomes have been counted for quantification purposes (Lutz et al., 1991; Tsao et al., 2001; Lewis et al., 2004). Cell counting was accomplished by overlaying each image with a 300 × 300 μm grid directly over the midline of the tissue section using Adobe Photoshop (v. 6.0). Only cells that contained a visible DAPI-labeled nucleus that fell within this grid were counted. Mean number of CRF-TAMRA 1 labeled neurons were compared across treatment conditions with one-way analysis of variance (ANOVA) and post-hoc tests using Tukey's honestly significant difference (HSD).
Additionally, a Bio-Rad laser-scanning confocal microscope with accompanying Bio-Rad Laser 2100 software supplied by The University of Wyoming Microscopy Core Facility was used to provide high-resolution images of neurons labeled with the CRF-TAMRA 1 conjugate. Images were scanned using appropriate laser settings corresponding to the excitation wavelengths for TAMRA (green He/Ne laser ex: 658 nm) and DAPI (Blue Diode laser; ex: 488 nm) with an optical thickness of 2 μm.
3. Results
3.1 Neuronal internalization of CRF-TAMRA 1
Visualization of topical or icv administered CRF-TAMRA 1 internalization by epifluorescent and confocal microscopy revealed distinct patterns of fluorescence in a morphologically diverse neuronal population throughout the medullary brainstem. Most notable was labeling of very large cells (≥ 30 μm in diameter) in the medial and paramedial regions of the medulla (Fig. 2, A-D). Overall, neuronal CRF-TAMRA 1 internalization appeared punctate (Fig. 2, C and D), with clusters of fluorescent endosomal-like foci distributed throughout the soma, often surrounding the nucleus. Endosome-like fluorescence was also seen in proximal and secondary dendritic projections and was largely absent in the nucleus (Fig. 2, B and C). However, in a small subset of cells, the presence of diffuse nuclear fluorescence was also evident at longer brain exposure times to the conjugate (30 min and 60 min; Fig. 3).
Fig. 2.
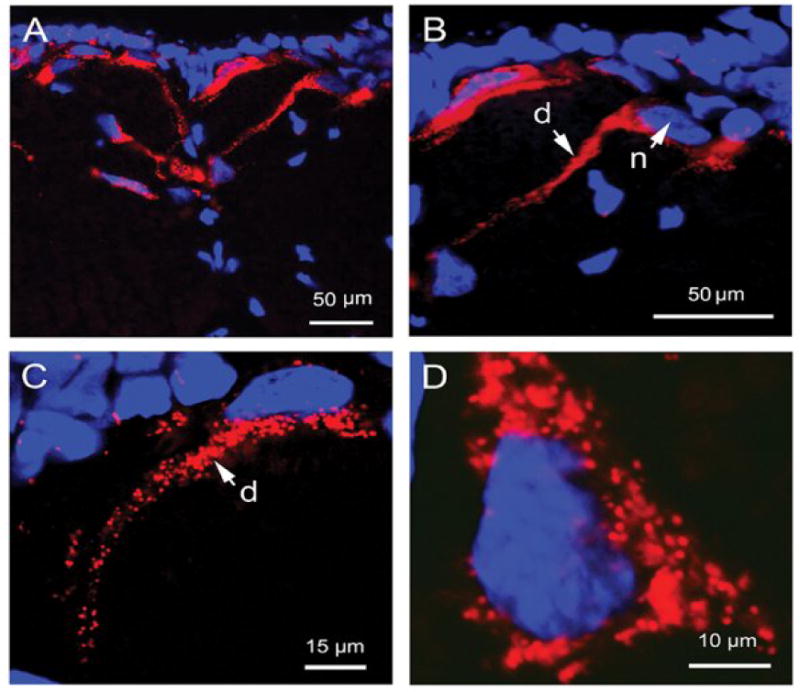
Confocal and epifluorescent microscopic images (A-D) of medullary neurons showing DAPI-labeled cell nuclei (blue) and CRF-TAMRA 1 (red) internalization. (A) Epifluorescent image illustrating the distribution of CRF-TAMRA 1 labeled neurons located in the paramedial and medial regions of the medulla. (B) Higher resolution confocal image taken of the same section shown in (A) illustrating neuronal internalization of fluorescent CRF-TAMRA 1 conjugate in the cell body adjacent to the nucleus (n) and a long dendritic process (d). (C) and (D) Single confocal optical scans (2 μm) illustrating the punctate or endosome-like appearance of CRF-TAMRA 1 internalization into a DAPI-labeled neuron located in the medial region of the medulla (C) and at higher resolution (D, zoomed in 5 times), respectively.
Fig. 3.

Confocal greyscale (A) and differential interference contrast (B) images (zoomed in 5 times) of a medullary neuron showing diffuse nuclear (n) labeling 30 min after intraventricular CRF-TAMRA 1 (14.24 ng/4 μL) infusion.
Neuronal internalization of fluorescence after intraventricular administration of CRF-TAMRA 1 (14.24 ng/4 μL) was extremely rapid, a finding that fits well with CRF's known rapid locomotor-stimulating actions (Lowry et al., 1990; Lowry et al., 1996). Within 5 min of icv CRF-TAMRA 1 administration, labeling of fine dendritic processes was visible, as was the appearance of punctate fluorescence. At 30 min, diffuse fluorescence was also visible, along with the presence of larger aggregations of fluorescent endosome-like foci distributed throughout the cytoplasmic and perinuclear regions of labeled cells. At 60 min, fluorescence due to CRF-TAMRA 1 internalization appeared more diffuse with some punctate labeling still present, but to a lesser degree than that seen at 5 and 30 min of exposure. One-way ANOVA revealed a significant time-dependent effect on CRF-TAMRA 1 neuronal internalization across the three exposure times [F (2, 14) = 6.71; P = 0.01; Fig. 4A]. Labeled neurons were present in significantly higher numbers at 30 min than at 5 min (Tukey's HSD; P = 0.05) or 60 min (Tukey's HSD; P = 0.01; Fig. 4A).
Fig. 4.

(A) Mean and standard errors (SEM) for the number of labeled neurons in each group of newts (5/group) as a function of time after intraventricular CRF-TAMRA 1 administration. Labeled neurons were present at all time periods examined, with the mean number labeled at 5 (mean = 110.4, SEM = 16.7) and 60 min (mean = 83.6, SEM = 15.9) significantly lower than that observed at 30 min (mean = 202.2, SEM = 34.6). (B) Mean numbers of labeled medullary neurons as a function of low (3.56 ng/8 μL; n = 3), middle (14.24 ng/8 μL; n = 3), or high (28.48 ng/8 μL; n = 3) doses of the CRF-TAMRA 1 conjugate. There were no significant differences in mean number of labeled neurons across the low (mean = 63.7, SEM = 15.3), middle (mean = 89, SEM = 5.5) or high dose (mean = 96.7, SEM = 10.7) groups. (C) Mean (SEM) number of line crossings for each group of newts as a function of low (3.56 ng/8 μL; n = 3), middle (14.24 ng/8 μL; n = 3), or high (28.48 ng/8 μL; n = 3) CRF-TAMRA 1 dose. Newts receiving intraventricular administration of the middle dose (mean = 218.7, SEM = 35.7) showed a significant enhancement in locomotor activity (number of line crossings) compared to groups of newts that received low (mean = 20.7, SEM = 2.8) or high conjugate doses (mean = 32.7, SEM = 26.4). (D) Reduced CRF-TAMRA 1 neuronal labeling due to CRF or CRF receptor antagonist pre-treatment. Unlabeled CRF (CRF + CRF-TAMRA 1; mean = 81.7, SEM = 18.1; n = 3) pre-treatment or a combination of the non-specific, CRF receptor antagonists, αhCRF(9-41) and D-Phe-CRF(12-41) (αhCRF + D-Phe-CRF + CRF-TAMRA 1; mean = 72.7, SEM = 15.4; n = 3), administered 5 min prior to CRF-TAMRA 1, significantly decreased the mean number of labeled cells compared to VEH pre-treatment followed by CRF-TAMRA 1 (VEH + CRF-TAMRA 1; mean = 175.3, SEM = 16.5; n = 4) administration.
With the CRF-TAMRA 1 doses used in this study (3.56, 14.24, and 28.48 ng/8 μL), there were no significant (one-way ANOVA) dose-dependent effects in the mean number of CRF-TAMRA 1 labeled cells (Fig. 4B). However, there was a significant dose-related effect on locomotor behavior following icv administered CRF-TAMRA 1 [one-way ANOVA; F (2, 8) = 32.77, P = 0.001], with the highest level of locomotion occurring after infusion of the middle dose (Tukey's HSD; P = 0.001; Fig. 4C). Conversely, intraventricular infusion of high doses of CRF-TAMRA 1 (28.8 ng/8 μL) resulted in a dramatic reduction in locomotor activity compared to administration of the middle dose (14.24 ng/8 μL), but not low doses (3.56 ng/8 μL; Fig. 4C) of the conjugate, producing an inverted U-shaped function.
3.2 CRF-TAMRA 1 internalization and neurobehavioral effects were specific and attenuated by CRF receptor ligands
CRF-TAMRA 1 internalization was attenuated by pre-treatment with CRF and CRFR antagonists, αhCRF(9-41) and D-Phe-CRF(12-41) (Fig. 4D and 5). One-way ANOVA showed a significant difference in the mean number of CRF-TAMRA 1 labeled neurons across the three treatment conditions [F (2, 9) = 12.18; P = 0.005; Fig. 4D]. Pre-treatment with either unlabeled CRF (1500 ng/2 μL) or the CRF receptor antagonists, αhCRF(9-41) (1500 ng/μl) and D-Phe-CRF(12-41) (1500 ng/μl), 5 min prior to CRF-TAMRA 1 (14.24 ng/4 μL) treatment, significantly reduced the mean number of CRF-TAMRA 1 labeled neurons compared to VEH (2 μL Ringer's) pre-treatment (Tukey's HSD P = 0.01, P = 0.008, respectively, Fig. 4D).
Fig. 5.

Epifluorescent microscopic images (A-I) taken from transverse sections through the rostromedial medulla illustrating DAPI (left panels; A, D, and G), CRF-TAMRA 1 (middle panels; B, E, and H), and the overlay of the corresponding DAPI and CRF-TAMRA 1 images (right panels; C, F, and I) across the three different treatment conditions: (A-C) VEH pre-treatment followed 5 min later by CRF-TAMRA 1 (VEH + CRF-TAMRA 1) administration, (D-F) unlabeled CRF pre-treatment 5 min prior to CRF-TAMRA 1 (CRF + CRF-TAMRA 1) administration, and (G-I) pre-treatment with αhCRF(9-41) and D-Phe-CRF(12-41) 5 min prior to CRF-TAMRA 1 (αhCRF + D-Phe-CRF + CRF-TAMRA 1) administration. Pre-treatment with unlabeled CRF (CRF + CRF-TAMRA 1) or the CRF antagonists, αhCRF and D-Phe-CRF (αhCRF + D-Phe-CRF + CRF-TAMRA 1), 5 min prior to CRF-TAMRA 1 administration, significantly reduced the number of CRF-TAMRA 1 labeled neurons compared to conditions in which a VEH pre-treatment was given (VEH + CRF-TAMRA 1). Insert is a schematic transverse section through the rostral medulla with dashed lines designating the region shown in images A-I (4v corresponds to the fourth ventricle of the medulla).
CRF-TAMRA 1 infusion was also associated with specific CRF-like behavioral effects as shown by the significant enhancement in locomotion following intracerebral administration of the conjugate (VEH + CRF-TAMRA 1) and the significant decrease in locomotor activity following pre-treatment with αhCRF(9-41) (αhCRF + CRF-TAMRA 1; Fig. 6). Repeated measures ANOVA conducted on the number of line crossings 5 min prior to treatment (baseline) and across 6 consecutive 5 min time intervals post-conjugate treatment revealed a significant difference in the mean number of lines crossed for the three conditions [F (2, 6) = 13.01; P = 0.007; Fig. 6]. Post-hoc tests showed that treatment with VEH + CRF-TAMRA 1 significantly increased the mean number of line crossings at 5 min (P < 0.05), 10 min (P < 0.05), 15 min (P < 0.05), 25 min (P ≤ 0.01) and 30 min (P ≤ 0.05) compared to conditions wherein the unconjugated TAMRA dye (VEH + TAMRA) or an antagonist (αhCRF + CRF-TAMRA 1) pre-treatment was given (Fig. 6).
Fig. 6.

CRF-TAMRA 1 induced locomotor activation. Means and standard errors (SEM) for the number of line crossings in each group of newts after intraventricular infusion of VEH followed 5 min later by icv administration of CRF-TAMRA 1 (VEH + CRF-TAMRA 1; n = 3), pre-treatment with the CRF receptor antagonist, αhCRF(9-41), followed 5 min later by CRF-TAMRA 1 (αhCRF + CRF-TAMRA 1; n = 3) or VEH pre-treatment followed 5 min later by infusion of the TAMRA dye alone (VEH + TAMRA; n = 3). VEH + CRF-TAMRA 1 administration produced a rapid (within 5 min) and significant enhancement in locomotor activity at 5, 10, 15, 25, and 30 min following CRF-TAMRA 1 intraventricular infusion, compared to αhCRF + CRF-TAMRA 1 or VEH + TAMRA treatment groups.
4. Discussion
We have described the synthesis and functional characteristics of the fluorescent conjugate, CRF-TAMRA 1, produced by covalent attachment of the rhodamine dye, TAMRA, to the CRF peptide. We have also demonstrated the receptor specificity and CRF-like functional effects of CRF-TAMRA 1 through examination of the conjugate's internalization by neurons, as well as its subsequent potent and rapid activation of locomotor behavior. Thus, this conjugate affords, for the first time, a means of identifying potential target neurons mediating the behavioral or other neuronally mediated effects of CRF. In addition, the conjugate provides a unique opportunity for the real time visualization and tracking the peptide's internalization into target neurons while concurrently monitoring the peptide's neurophysiological actions in vivo.
Neuronal internalization of CRF-TAMRA 1 after intraventricular or topical administration revealed a distinct pattern of punctate, endosome-like fluorescence, consistent with previous neurohistological and immunofluorescence studies demonstrating internalization of radioactively tagged ligands, fluorescent retrograde tracers, and fluorescently labeled CRFR antibodies (Wessendorf 1991; Rasmussen et al., 2004; Perry et al., 2008). Our findings also corroborate prior reports of granular-like fluorescence in rat septohippocampal cholinergic neurons 24 h following injection of a fluorochromated antibody directed against the p75 neurotrophin receptor, Cy3-tagged anti-p75 (Kacza et al., 2000; Wu et al. 2000). Moreover, a similar endosomal-like patterning of fluorescence was observed by Lewis et al. following topical application of the fluorescent conjugated peptide, AVT-OG 1, to the surface of the medulla in Taricha, at identical conjugate exposure periods (i.e., 5, 30 and 60 min) employed in the current study (Lewis et al., 2004; Lewis et al., 2005).
Internalization of the fluorescent CRF-TAMRA 1 conjugate into medullary neurons within the caudal brainstem of the newt was extremely rapid and time-dependent. Internalization of fluorescence occurred within 5 min of administration of the conjugate, peaked at 30 min of exposure and declined by 60 min to the level seen at 5 min exposure periods. Within 5 min of infusion to the lateral ventricle, endosomal uptake of CRF-TAMRA 1 fluorescence was principally seen localized to the perimeter of the soma and primary dendrites of medullary neurons. By 30 and 60 min, many of these endosomal units were found aggregated into larger clusters near the perinuclear space of the cell body, possibly reflecting the sequestering of ligand-receptor complexes into late endosomes and lysosomes for degradation and subsequent recycling (Innamorati et al., 2001; Maxfield and McGraw, 2004). In addition, we also observed diffuse nuclear labeling in a small population of medullary neurons at both 30 and 60 min exposure periods to the CRF-TAMRA 1 conjugate. Although this finding was relatively rare and not formally quantified, we cannot exclude the possibility that the diffuse nuclear labeling observed here resulted from the presence of cytoplasmic fluorescence above or below the plane of sectioning, thus giving the false appearance of fluorescence contained within the nucleus.
Although there was no significant dose-dependency for conjugate internalization across the three doses examined (3.56 ng/8 μL, 14.24 ng/8 μL, and 28.8 ng/8 μL), icv CRF-TAMRA 1 administration did stimulate locomotor behavior in a dose-dependent fashion, a finding that parallels CRF's reported actions in this same species of newt, albeit at much higher doses of the native peptide (Lowry et al., 1990; Lowry et al., 1996). Specifically, we found that icv infusion of 14.24 ng/8 μL CRF-TAMRA 1 significantly enhanced locomotion compared to low (3.56 ng/8 μL) or high (28.48 ng/8 μL) doses of the conjugate. The lack of a significant dose-dependent effect for the number of labeled cells across the three doses examined, could have resulted for several reasons. First, the conjugate dose range used in this study may have been too narrow, particularly on the low dose end. Second, the comparatively small sample size (n = 3) for each group of newts may have reduced overall statistical power. Regardless of the lack of dose-responsivity for conjugate internalization, a robust dose-dependent effect for locomotor behavior at equimolar doses of CRF used in previous investigations (Lowry et al., 1990) demonstrated that the CRF-TAMRA 1 conjugate was acting in a functionally comparable manner to the native CRF peptide.
Our results also verified that neuronal internalization of CRF-TAMRA 1 was substantially specific to the CRF receptor system as shown by the significant decrease in CRF-TAMRA 1 labeled neurons following topical administration of the unconjugated peptide (CRF) or a cocktail containing the competitive CRFR antagonists, αhCRF(9-41) and D-Phe-CRF(12-41), directly to the surface of the medulla. A significant attenuation of CRF-TAMRA 1 internalization was produced by pre-treatment with either unconjugated CRF or a combination of the CRFR antagonists, αhCRF(9-41) and D-Phe-CRF(12-41). Not unexpectedly for an in vivo study, complete blocking was not observed. Rather, pre-treatment with unconjugated CRF or the CRFR antagonists, led to an approximately 40% reduction in CRF-TAMRA 1 internalization compared to VEH pre-treatment 5 min prior to CRF-TAMRA 1 administration. Several factors could account for less than complete blocking of CRF-TAMRA 1 internalization. First, unlike in vitro competition studies where a very wide range of antagonist concentrations can readily be used and bioavailability of active compounds is likely greater (see below), this in vivo study was designed to address the action of CRF-TAMRA 1 from a more realistic functional perspective. Significantly, antagonist administration effectively blocked the behavioral effect of CRF-TAMRA 1. Another factor that could have lessened effective blockade of CRF-TAMRA 1 internalization is high affinity binding to the CRF-binding protein (CRF-BP; Ungless et al., 2003). Similarly, CRF-BP may have sequestered unconjugated CRF applied topically to the medulla during pre-treatment, thereby undermining blockade of CRF-TAMRA 1 internalization. In addition, the affinities of αhCRF(9-41) and D-Phe-CRF(12-41) for the CRF1 and CRF2 receptor have not been characterized in this species, although functional indicators, like attenuation of CRF-induced locomotion here and in work by Lowry and others (Lowry and Moore, 1991) indicate relatively high affinity for αhCRF(9-41). In summary, the blocking we achieved in the control conditions was sufficient to significantly attenuate CRF-TAMRA 1 internalization in medullary neurons of Taricha as well as block CRF-TAMRA 1-induced locomotion, supporting the interpretation that CRF-TAMRA 1 shows selectivity for CRFR in this species.
The specificity and neurobiological efficacy of icv administered CRF-TAMRA 1 was also verified behaviorally, by assays of locomotion, which showed rapid, significant enhancement at similar time courses and doses previously seen for CRF (Lowry et al., 1990; Lowry et al., 1991). Additionally, the CRF-TAMRA 1 enhanced locomotion was significantly attenuated by pre-treatment with the competitive CRF receptor antagonist, αhCRF(9-41), providing further evidence for the specificity of conjugate action in the awake, behaving animal.
In conclusion, our findings show that CRF-TAMRA 1 selectively labels CRF target neurons in the newt hindbrain and retains CRF's typical neurobehavioral effect. Thus, CRF-TAMRA 1 is a promising new tool for tracking CRF internalization into target neurons and relating this process to the neuropeptides neurophysiological and behavioral actions in the intact, behaving animal. If used in combination with optical approaches such as, 2-photon microscopy or calcium imaging, the fluorescent CRF-TAMRA 1 conjugate could provide an opportunity for real time visualization and mapping of CRF neuronal targets in the CNS while neurophysiological processes are monitored, thereby facilitating establishment of a closer causal connection between signaling events in identified neurons and neurophysiological mechanisms mediating CRF's effects in response to stress. The neurons internalizing CRF-TAMRA 1 could be further characterized using immunohistochemical staining methods that would reveal the chemical phenotypes of these target neurons. We have begun preliminary work of this type, showing that serotonin-containing medullary raphé neurons in the newt brainstem are among those internalizing CRF-TAMRA 1 (Hubbard et al., 2007).
Supplementary Material
Acknowledgments
Supported by Grant Number: P20 RR015553 (to J. D. R) and P20 RR15640 (to Dr. Francis W. Flynn) from The National Center for Research Resources (NCRR), a component of The National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Special thanks to Dr. Frank Moore, Samuel Bradford, Justin Jones, Dr. Chris Lowry, Dr. Christine Lewis, Dr. Emma Coddington, Dr. Steve Farmer, and Dr. Zhaojie Zhang for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bale TL, Vale W. CRF and CRF receptors: role in stress responsivity and other behaviors. Ann Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Dana R, Risch SC, Koob GF. Corticotropin-releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat brain. Brain Res. 1986;369:303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements S, Larsen DA, Dickhoff WW, Schreck CB. Central administration of corticotropin-releasing hormone stimulates locomotor activity in juvenile Chinook salmon (Oncorhynchus tshawytscha) Gen Comp Endocrinol. 2002;125:319–327. doi: 10.1006/gcen.2001.7707. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Ontongeny of corticotropin-releasing factor effects on locomotion and foraging in the western spadefoot toad (Spea hammondii) Horm Behav. 2004;46:399–410. doi: 10.1016/j.yhbeh.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Braun S, Hauger RL. GRK3 mediates desensitization of CRF1 receptors: a potential mechanism regulating stress adaptation. Am J Physiol Regul Integr Comp Physiol. 2001;280:R935–R946. doi: 10.1152/ajpregu.2001.280.4.R935. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Dietrich K, Palchaudhuri MR, Spiess J. Identification of two corticotropin-releasing factor receptors from Xenopus laevis. J Neuroendocrinol. 1997;16:279–288. doi: 10.1046/j.1471-4159.1997.69041640.x. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–3201. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RJ, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Braun S, Catt KJ, Dautzenberg FM. Mediation of corticotropin-releasing factor type 1 receptor phosphorylation and desensitization by protein kinase C: a possible role in stress adaptation. J Pharmacol Exp Ther. 2003;306:794–803. doi: 10.1124/jpet.103.050088. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist induced phosphorylation of the human CRF receptor type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Commun. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- Henrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role of activation, arousal, and affective regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Hubbard CS, Dolence EK, Shires JA, Rose JD. Corticotropin-releasing factor is internalized in medullary raphe serotonergic neurons of roughskin newts: a possible mechanism for corticotropin-releasing factor induced enhancement of locomotion. Soc Neurosci Abstr. 2007 QQ1, 730.19. [Google Scholar]
- Innamorati G, Le Gouill C, Balamotis M, Birnbaumer M. The long and short cycle: alternative intracellular routes for trafficking of G protein-coupled receptors. J Bio Chem. 2001;276:13096–13103. doi: 10.1074/jbc.M009780200. [DOI] [PubMed] [Google Scholar]
- Kacza J, Grosche J, Seeger J, Brauer K, Brückner G, Härtig W. Laser scanning and electron microscopic evidence for rapid and specific in vivo labeling of cholinergic neurons in the rat basal forebrain with fluorochromated antibodies. Brain Res. 2000;856:232–238. doi: 10.1016/s0006-8993(00)02239-3. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Dolence EK, Hubbard CS, Rose JD. Identification of roughskin newt medullary vasotocin target neurons with a fluorescent vasotocin conjugate. J Comp Neurol. 2005;491:381–389. doi: 10.1002/cne.20701. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Dolence EK, Zhang Z, Rose JD. Fluorescent vasotocin conjugate for the identification of the target cells for brain actions of vasotocin. Bioconjug Chem. 2004;15:909–914. doi: 10.1021/bc049928x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Deviche P, Moore FL. Effects of corticotropin-releasing factor (CRF) and opiates on amphibian locomotion. Brain Res. 1990;513:94–100. doi: 10.1016/0006-8993(90)91093-v. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Corticotropin-releasing factor (CRF) antagonist suppresses stress-induced locomotor activity in an amphibian. Horm Behav. 1991;25:84–96. doi: 10.1016/0018-506x(91)90041-f. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL. Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinology. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rose JD, Moore FL. Corticotropin-releasing factor enhances locomotion and medullary neuronal firing in an amphibian. Horm Behav. 1996;30:50–59. doi: 10.1006/hbeh.1996.0008. [DOI] [PubMed] [Google Scholar]
- Lutz W, Salisbury JL, Kumar R. Vasopressin receptor-mediated endocytosis: current view. Am J Physiol. 1991;261:F1–F13. doi: 10.1152/ajprenal.1991.261.1.F1. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nature Rev. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin-releasing factor (CRF)-like immunoreactivity in the rat central nervous system: extra-hypothalamic distribution. Peptides. 1984;5:53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Moore FL, Roberts J, Bevers J. Corticotropin-releasing factor (CRF) stimulates locomotor activity in intact and hypophysectomized newts (amphibia) J Exp Zoology. 1984;231:331–333. doi: 10.1002/jez.1402310305. [DOI] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpi P, Droste SK, Kühn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;10:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nguyen NK, Hetzenauer A, Thoeringer CK, Wurst W, Deussing JM, Holsboer F, Müller MB, Singewald N. Conditional CRF receptor 1 knockout mice show altered neuronal activation pattern to mild anxiogenic challenge. Psychopharmacol. 2006;188:374–385. doi: 10.1007/s00213-006-0513-1. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Junger S, Kohout TA, Hoarell SRJ, Struthers RS, Grigoriadis DE, Maki RA. Distinct conformations of the corticotropin-releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2008;280:11560–11568. doi: 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- Pohl S, Darlison MG, Clarke WC, Lederis K, Richter D. Cloning and functional pharmacology of two corticotropin-releasing factor receptors from a teleost fish. Eur J Pharmacol. 2001;430:193–202. doi: 10.1016/s0014-2999(01)01391-7. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TN, Novak I, Nielsen SM. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and beta-arrestin 1 recruitment. Eur J Biochem. 2004;271:4366–4374. doi: 10.1111/j.1432-1033.2004.04371.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JD, Kinnaird JR, Moore FL. Neurophysiological effects of vasotocin and corticosterone on medullary neurons: implications for hormonal control of amphibian courtship behavior. Neuroendocrinol. 1995;62:406–417. doi: 10.1159/000127030. [DOI] [PubMed] [Google Scholar]
- Rose JD, Moore FL, Orchinik M. Rapid neurophysiological effects of corticosterone on medullary neurons: relationship to stress-induced suppression of clasping in an amphibian. Neuroendocrinol. 1993;57:815–824. doi: 10.1159/000126440. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:298–356. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Heinrichs SC, Dunn A. The role of CRF in behavioral responses to stress. Peptides. 2001;22:713–724. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin-releasing factor produces behavioral activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G protein-coupled receptors. Trend Pharmacol Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β—endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- von Zastrow M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytotic pathways. Life Sci. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Wessendorf MW. Flouro-Gold: composition, and mechanism of uptake. Brain Res. 1991;553:135–148. doi: 10.1016/0006-8993(91)90241-m. [DOI] [PubMed] [Google Scholar]
- Wu M, Shanabrough M, Leranth C, Meenakshi A. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


