Abstract
Joint manipulation has long been used for pain relief. However, the underlying mechanisms for manipulation-related pain relief remain largely unexplored. The purpose of the current study was to determine which spinal neurotransmitter receptors mediate manipulation-induced antihyperalgesia. Rats were injected with capsaicin (50 μl, 0.2%) into one ankle joint and mechanical withdrawal threshold measured before and after injection. The mechanical withdrawal threshold decreases 2 h after capsaicin injection. Two hours after capsaicin injection, the following drugs were administered intrathecally: bicuculline, blocks γ-aminobutyric acid (GABAA) receptors; naloxone, blocks opioid receptors; yohimbine blocks, α2-adrenergic receptors; and methysergide, blocks 5-HT1/2 receptors. In addition, NAN-190, ketanserin, and MDL-72222 were administered to selectively block 5-HT1A, 5-HT2A, and 5-HT3 receptors, respectively. Knee joint manipulation was performed 15 min after administration of drug. The knee joint was flexed and extended to end range of extension while the tibia was simultaneously translated in an anterior to posterior direction. The treatment group received three applications of manipulation, each 3 min in duration separated by 1 min of rest. Knee joint manipulation after capsaicin injection into the ankle joint significantly increases the mechanical withdrawal threshold for 45 min after treatment. Spinal blockade of 5-HT1/2 receptors with methysergide prevented, while blockade of α2-adrenergic receptors attenuated, the manipulation-induced antihyperalgesia. NAN-190 also blocked manipulation-induced antihyperalgesia suggesting that effects of methysergide are mediated by 5-HT1A receptor blockade. However, spinal blockade of opioid or GABAA receptors had no effect on manipulation induced-antihyperalgesia. Thus, the antihyperalgesia produced by joint manipulation appears to involve descending inhibitory mechanisms that utilize serotonin and noradrenaline.
Keywords: Joint manipulation, Serotonin, Noradrenaline, Pain, Capsaicin, Spinal cord
1. Introduction
Joint manipulation has long been used as a modality for pain relief. The use of this form of manual therapy has evolved from the traditions of bonesetting to orthodox practice in a number of health care disciplines. Modern manipulative therapy can range from slow oscillating glides to high velocity, low amplitude techniques (Haldeman and Hooper, 1999). Manipulation-induced analgesia has been demonstrated in a number of studies in human subjects (Vernon et al., 1990; Vicenzino et al., 1996, 1998; Zusman et al., 1989). Also, recent meta-analyses of the clinical literature, focusing on spinal manipulation, suggest that manipulative therapy is effective for the treatment of acute and chronic musculoskeletal pain (Bronfort, 1999; van Tulder et al., 1997). However, the underlying physiological mechanisms for joint manipulation-related pain relief remain largely unexplored.
It has been suggested that manipulation-induced analgesia may be a multifactorial effect resulting from beneficial influences on the chemical environment of peripheral joints, facilitation of tissue repair processes, segmental inhibitory processes within the central nervous system and activation of descending inhibitory pathways projecting from the brain to spinal cord (Wright, 1995; Wright and Vicenzino, 1995). Recently, we showed that knee joint manipulation decreases secondary mechanical hyperalgesia in the paw induced by injection of capsaicin into the ankle joint in rat (Sluka and Wright, 2001). Since the manipulation is proximal to the injured joint, these data suggest that central neural mechanisms mediate the reduction in hyperalgesia.
Blockade of spinal receptors can elucidate potential mechanisms for the antihyperalgesia produced by joint manipulation. A number of different receptors found in the dorsal horn of the spinal cord may be involved. Presynaptic inhibition can occur through activation of spinal γ-aminobutyric acid (GABA) receptors on primary afferent fibers, which depolarize the terminal thus inhibiting neurotransmitter release from primary afferents and the consequent incoming afferent activity (see Eccles et al., 1962; Sluka et al., 1995). GABA receptors are also located post-synaptically and when activated hyperpolarize the neuron thus decreasing the effectiveness of excitatory input (Malcangio and Bowery, 1996).
Opioids are involved in both segmental inhibition and descending inhibition (Fields and Basbaum, 1999). However, systemic blockade of opioid receptors with naloxone has no effect on the analgesia produced by manipulation in humans (Vicenzino et al., 2000; Zusman et al., 1989). Descending inhibitory pathways from the rostral ventral medulla (RVM) utilize serotonin as a neurotransmitter and those from dorsolateral pons utilize noradrenline (Fields and Basbaum, 1999). The purpose of this study is to determine, using behavioral pharmacology techniques, which dorsal horn neurotransmitter receptors mediate this manipulation-induced antihyperalgesic effect.
2. Methods
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the National Institutes of Health and the International Association for the Study of Pain policies on use of laboratory animals.
2.1. Joint inflammation
Male Sprague-Dawley rats (250–350 g, Harlan, Indianapolis, IN; n = 113) were anesthetized briefly with halothane (2–5% v/v) and injected with 50 μl of 0.2% w/v capsaicin (Sigma, St Louis, MO) into the left ankle joint. This produces a robust secondary mechanical hyperalgesia of the paw, which is fully developed in 2 h after injection (Sluka, 2002).
2.2. Drug administration
Intrathecal catheters (32 G, polyurethane; 10.5 cm; Recathco, Allison Park, PA) were placed acutely immediately prior to drug administration (Storkson et al., 1996). Prior to insertion, a 32-gauge polyurethane catheter was glued to PE 10 tubing (0.28 mm ID, polyethylene; 5 cm; Becton Dickson and Company, Sparks, MD). Animals were anesthetized with halothane (2–5% v/v) and a 23-gauge hypodermic needle was inserted through the skin into the L5—L6 intervertebral space until a tail flick was noted. The 32-gauge polyurethane catheter was inserted into the intrathecal space through the needle and advanced cranially until 3.5–4.0 cm was under the skin. After placement, the needle was withdrawn. Drugs were then given intrathecally, through the catheter, while the animal remained anesthetized, and the catheter removed after drug administration. Acute catheter placement was chosen instead of direct spinal injection in order to insure that the drug was targeted specifically to the lumbar enlargement and the effects of dilution and diffusion on drug concentration at the target site would be minimized. It was not possible, therefore, to verify catheter placement in these experiments with acute catheter placement since the catheter is removed after drug administration. However, the experimenters placing the catheters have performed hundreds of chronic catheter placements with approximate accuracy of 99% (Skyba et al., 2002; Sluka, 2002). Further, the presence of a tail flick upon needle insertion is a good indication that the needle has access to the intrathecal space.
Bicuculline methiodide (0.3 μg/10 μl; n = 11), naloxone hydrochloride dihydrate (10 μg/10 μl; n = 12), methysergide maleate (30 μg/10 μl; n = 12), or yohimbine hydrochloride (30 μg/10 μl; n = 10) were administered spinally to block γ-aminobutyric acid (GABAA) receptors (Kaneko and Hammond, 1997), opioid receptors (Dickenson et al., 1981; Woolf, 1980), serotonergic (5-HT1 and 5-HT2) receptors (Calejesan et al., 1998), or α2-adrenergic receptors (Sawynok and Reed, 1991), respectively. In addition, 1-(2-methoxyphenyl)-4-(4-[2-phthalimido]butyl)piperazine hydrobromide (NAN-190, 15 μg/10 μl; n = 12), ketanserin tartrate (30 μg/10 μl; n = 14), or 3-tropanyl-3,5-dichlorobenzoate (MDL-72222, 12 μg/10 μl; n = 12) were administered spinally to block 5-HT1A (Mjellem et al., 1993), 5-HT2A (Sasaki et al., 2001), or 5-HT3 receptors (Sasaki et al., 2001), respectively. All doses were chosen based on previously published studies showing selectivity for the specific receptor type/subtype(s), e.g. blocking the analgesic effects of the corresponding receptor agonist. All drugs were purchased from Sigma Chemical Co. (St Louis, MO, USA). Isotonic, sterile saline adjusted to pH 7.2 served as a vehicle control (n = 12). Drugs were given 15 min prior to the joint manipulation while the animal was anesthetized with halothane (1–2% v/v). All animals received only one administration of antagonist or vehicle. None of the drugs administered intrathecally had any effect on gross motor function, i.e. gait or withdrawal to noxious stimuli.
In our laboratory, we previously tested yohimbine hydrochloride, methysergide maleate, NAN-190, ketanserin, and MDL-72222 and showed that the doses used in the current study blocked the analgesic effects of appropriate receptor agonists (Radhakrishnan et al., 2003). Also, in the current study, we confirmed that the doses of naloxone hydrochloride dihydrate and bicuculline methiodide blocked the analgesic effects of their agonists. Two hours after intra-articular capsaicin injection, separate groups of animals were pretreated with bicuculline methiodide (0.3 μg/10 μl; n = 4), naloxone hydrochloride dihydrate (10 μg/10 μl; n = 5), or vehicle (2 groups; n = 9). After 15 min, animals in the naloxone group and one of the vehicle groups received i.t. injections of morphine sulfate (1 μg/μl; Sigma, St Louis, MO), while animals in the bicuculline group and the other vehicle group received i.t. injections of muscimol (0.1 μg/10 μl; Sigma, St Louis, MO).
2.3. Joint manipulation
After drug administration, anesthesia was maintained with 1–2% v/v halothane for approximately 30 min. The knee joint manipulation was performed under anesthesia 15 min after drug administration. The femur ipsilateral to the injection site was stabilized, and manipulation was performed by moving the tibia on the femur. The knee joint was flexed and extended to the end range of extension while the tibia was simultaneously translated in an anterior to posterior direction. The treatment group received three applications of manipulation, each 3 min in duration separated by 1 min of rest. Our group has previously shown this time frame to be optimal for producing antihyperalgesia in this model (Sluka and Wright, 2001). Three control groups were utilized: (1) vehicle was given with manipulation; (2) vehicle was given with anesthesia only; and (3) drugs were given with anesthesia only. Previously, we demonstrated that anesthesia with hand contact (sham) has no effect on the hyperalgesia and is similar to anesthesia alone (Sluka and Wright, 2001).
2.4. Behavioral measurements
Animals were tested for withdrawal thresholds to mechanical stimuli (von Frey filaments) applied to the plantar aspect of the hindpaw (Gopalkrishnan and Sluka, 2000). Von Frey filaments with bending forces from 9 to 418 mN were applied in a progressively increasing manner until the hindpaw was withdrawn or 418 mN was reached. Each filament was applied twice. The filament of lowest bending force from which the animal withdrew was considered the mechanical withdrawal threshold of the hindpaw. After a response, the filaments above and below were tested to confirm the withdrawal threshold. The test—retest reliability of this method was previously established (r2 = 0.7; p = 0.007) (Gopalkrishnan and Sluka, 2000). Tests were performed before and 2 h after capsaicin injection, and 15, 30, 45 min and 1 h after joint manipulation.
2.5. Statistical analysis
Differences in mechanical withdrawal thresholds between groups were assessed with a Kruskal—Wallis analysis of variance test and a Mann—Whitney U post-hoc test. Values were considered significant at P ≤ 0.05. All data are presented as the median with the 25th and 75th percentiles.
3. Results
3.1. Joint manipulation reverses mechanical hyperalgesia
Two hours after injection of capsaicin into the ankle joint, there was a decrease in the mechanical withdrawal threshold of the ipsilateral paw for all groups (P = 0.0001, signed rank test). Manipulation of the knee joint significantly increased the mechanical withdrawal threshold of the ipsilateral paw 15 min (P = 0.03, signed rank test), 30 min (P = 0.05, signed rank test), and 45 min (P = 0.03, signed rank test) after application (Fig. 1). The decrease in withdrawal threshold returned by 60 min.
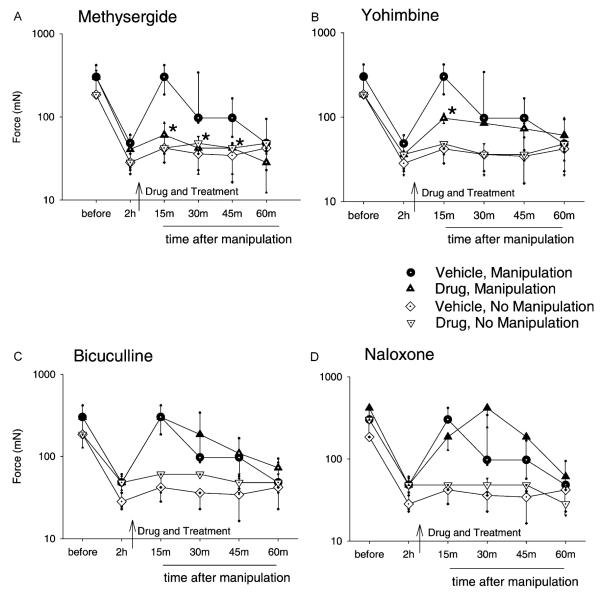
Fig. 1.
Graphs represent the mechanical withdrawal threshold for groups of animals that received drug or vehicle and subsequently knee joint manipulation or no treatment. Separate groups received intrathecal (i.t.) injection of methysergide (A), yohimbine (B), bicuculline (C), or naloxone (D) 2 h after 0.2% intra-articular capsaicin and 15 min prior to joint manipulation. Spinal injection of methysergide (30 μg/10 μl) significantly prevented the manipulation-induced increase in mechanical withdrawal threshold at 15, 30, and 45 min compared with the ‘vehicle, manipulation’ control group. Yohimbine (30 μg/10 μl) had a partial effect, decreasing the mechanical withdrawal threshold 15 min post-manipulation. Neither i.t. bicuculline (0.3 μg/10 μl) nor naloxone (10 μg/10 μl) significantly affected the increase in mechanical withdrawal threshold resulting from knee joint manipulation. Data are presented as medians with 25th and 75th percentiles. *Significantly less than manipulation with vehicle control (P < 0.5).
Analysis of all groups shows a significant group effect for mechanical withdrawal threshold 15 min (χ2 = 68, P = 0.0001), 30 min (χ2 = 52, P = 0.0001), and 45 min (χ2 = 34, P = 0.003) after joint manipulation. The following results are organized by drug treatment.
3.2. Methysergide prevents manipulation-induced antihyperalgesia
Spinal administration of methysergide maleate (30 μg/10 μl), 15 min before manipulation of the knee joint, prevented the increase in mechanical withdrawal thresholds resulting from the treatment. Withdrawal threshold values for the group pretreated with methysergide were significantly less when compared to the manipulation and vehicle group 15 min (P = 0.009), 30 min (P = 0.041), and 45 min (P = 0.026) after manipulation (Fig. 1A). Methysergide without joint manipulation had no effect on the decreased mechanical withdrawal threshold, and thresholds were similar to intrathecal injection of vehicle without joint manipulation.
3.3. Yohimbine attenuates manipulation-induced antihyperalgesia
Intrathecal administration of yohimbine hydrochloride (30 μg/10 μl) 15 min prior to manipulation of the knee joint attenuated the increase in withdrawal threshold, which results from knee joint manipulation. The withdrawal threshold for the group pretreated with yohimbine was significantly less 15 min (P = 0.041) after manipulation when compared to the group that received vehicle prior to manipulation (Fig. 1B). Yohimbine without joint manipulation had no effect on the decreased mechanical withdrawal threshold, and thresholds were similar to intrathecal injection of vehicle under anesthesia without joint manipulation (Fig. 1B).
3.4. Bicuculline or naloxone does not affect manipulation-induced antihyperalgesia
Intrathecal administration of bicuculline methiodide (0.3 μg/10 μl) or naloxone hydrochloride dihydrate (10 μg/10 μl), prior to manipulation of the knee joint, had no effect on the resultant increase in mechanical withdrawal thresholds when compared to vehicle control (Fig. 1C and D). Further, neither drug without joint manipulation affected the decreased mechanical withdrawal thresholds produced by capsaicin, and thresholds were similar to vehicle without joint manipulation (Fig. 1C and D). This suggests that the changes in withdrawal thresholds noted in treatment groups result from joint manipulation and not from administration of the receptor antagonists.
While the doses of bicuculline and naloxone were chosen based on previously published work (Dickenson et al., 1981; Kaneko and Hammond, 1997; Woolf, 1980), we tested them against their agonists in this model to confirm that they were adequate to block spinal GABAA and opioid receptors, respectively. Administration of either morphine or muscimol in vehicle groups increased mechanical withdrawal thresholds 15 and 30 min after drug injection (Table 1). However, pretreatment with naloxone completely blocked the effect of morphine, and pretreatment with bicuculline completely blocked the effect of muscimol on mechanical withdrawal thresholds (Table 1). Thus, the inability of spinal naloxone and bicuculline to prevent or attenuate manipulation-induced changes in mechanical withdrawal thresholds is not due to ineffective blockade of spinal opioid or GABAA receptors.
Table 1.
Data are presented as the median with 25th and 75th percentiles (mN)
| Morphine (1 μg/10 μl) |
Muscimol (0.1 μg/10 μl) |
|||
|---|---|---|---|---|
| 15 min | 30 min | 15 min | 30 min | |
| Vehicle (10 μl) | 302 (185, 418) | 185 (110, 418) | 302 (185, 418) | 185 (160, 244) |
| Naloxone (10 μg/10 μl) | 36 (21, 39)* | 48 (48, 48)* | NA | NA |
| Bicuculline (0.3 μg/10 μl) | NA | NA | 54 (45, 61)* | 61 (55, 61)* |
Significantly different from vehicle (P < 0.05).
3.5. Effects of selective 5-HT receptor antagonists
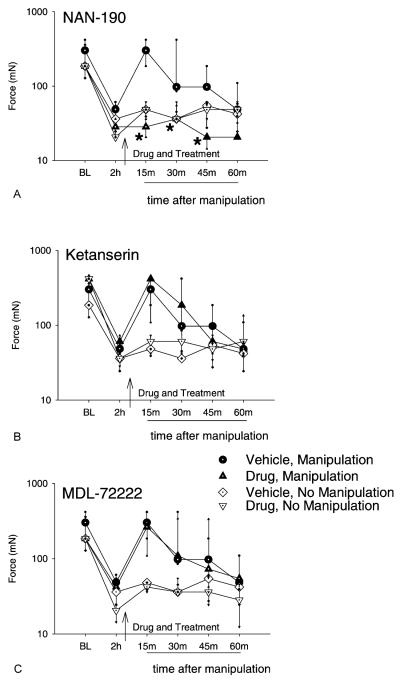
Spinal administration of NAN-190 (15 μg/10 μl, 5-HT1A) 15 min before knee joint manipulation prevented the increase in mechanical withdrawal threshold resulting from the manipulation. Withdrawal threshold values for the ipsilateral side were significantly less than those for the manipulation and vehicle group 15 min (P = 0.002 ), 30 min (P = .0 015), and 45 min (P = 0.002) after manipulation (Fig. 2A). NAN-190 without joint manipulation had no effect on the decreased mechanical withdrawal threshold, and thresholds were similar to intrathecal injection of vehicle without joint manipulation.
Fig. 2.
Graphs represent the mechanical withdrawal thresholds for groups of animals that received intrathecal (i.t.) injection of the 5-HT1A antagonist, NAN-190 (A), the 5-HT2A antagonist, ketanserin (B), or the 5-HT3 antagonist, MDL-72222 (C) 2 h after 0.2% intra-articular capsaicin and 15 min prior to joint manipulation. Spinal administration of NAN-190 (30 μg/10 μl, 5-HT1A) significantly prevented the manipulation-induced increase in mechanical withdrawal threshold at 15 min, 30 min, 45 min compared with the ‘vehicle, manipulation’ control group. Neither i.t. ketanserin (30 μg/10 μl, 5-HT2A) nor MDL-72222 (12 μg/10 μl, 5-HT3) significantly affected the increase in mechanical withdrawal threshold resulting from knee joint manipulation. Data are presented as medians with 25th and 75th percentiles. *Significantly less than manipulation with vehicle control (P < 0.05).
In contrast, spinal administration of ketanserin (30 μg/10 μl, 5-HT2A) or MDL-72222 (12 μg/10 μl, 5-HT3) prior to knee joint manipulation had no effect on the increase in mechanical withdrawal threshold resulting from the treatment (Fig. 2B and C). Ketanserin or MDL-72222 also had no effect on the decreased mechanical withdrawal thresholds resulting from intra-articular capsaicin injection, and thresholds were similar to intrathecal injection of vehicle without joint manipulation.
4. Discussion
Blockade of spinal cord serotonin receptors prevents the antihyperalgesia resulting from knee joint manipulation. Also, blockade of α2-adrenergic receptors in the spinal cord attenuates the antihyperalgesia produced by knee joint manipulation. In contrast, spinal administration of GABAA or opioid receptor antagonists does not affect manipulation-induced antihyperalgesia in this animal model. Further, selective serotonin receptor antagonists were administered to determine the serotonin receptor subtype (s) involved in this antihyperalgesia. Blockade of spinal 5-HT1A receptors, but not 5-HT2A or 5-HT3 receptors, prevents manipulation-induced antihyperalgesia. These data suggest that knee joint manipulation activates descending inhibitory pathways that utilize serotonin and noradrenaline, which inhibit transmission of nociceptive information by acting on 5-HT1A and α2-adrenergic receptors in spinal cord of rats.
It should be noted that this study was designed so that an uninjured joint, proximal to the site of injury was manipulated. This manipulation is within the normal working range of the joint that activates large diameter joint afferents in the uninjured knee joint (see Schaible and Grubb, 1993). Thus, manipulation of the uninjured knee is expected to activate large diameter afferent fibers to reduce hyperalgesia.
4.1. Descending monoaminergic inhibitory pathways
Serotonergic fibers in the spinal cord originate supraspinally in the RVM whereas noradrenergic fibers in the spinal cord originate supraspinally in the dorsolateral pons (Clark and Proudfit, 1991; Fields and Basbaum, 1999; Westlund et al., 1983). Agonists to serotonergic and α2-adrenergic receptors delivered spinally produce analgesia, reduce hyperalgesia, and reduce dorsal horn neuron activity (Ali et al., 1994; Barasi and Clatworthy, 1987; Bardin et al., 2000, 2001; Eide, 1992; Fairbanks and Wilcox, 1999; Oyama et al., 1996; Sawynok and Reed, 1991; Yang et al., 1994; Zhao and Duggan, 1988).
The periaquaductal gray (PAG) sends projections to both RVM and dorsolateral pons (A6/A7 cell groups) (Beitz, 1982; Cameron et al., 1995). From there neurons in both RVM and dorsolateral pons project to the spinal cord (Clark and Proudfit, 1991; Fields and Basbaum, 1999; Skagerberg and Bjorklund, 1985; Sluka and Westlund, 1992; Westlund et al., 1983). There is also evidence that activation of neurons in the RVM, some of which contain substance P, produces spinal antinociception indirectly through connections with descending noradrenergic fibers in the A7 cell group (Nuseir et al, 1999; Yeomans and Proudfit, 1990, 1992). Noradrenaline is the primary neurotransmitter of neurons in the dorsolateral pons and serotonin of neurons in the RVM (Clark and Proudfit, 1991; Fields and Basbaum, 1999; Skagerberg and Bjorklund, 1985). In fact, stimulation of PAG or RVM increases release of 5-HT and noradrenaline spinally (Bowker and Abhold, 1990; Cui et al., 1999; Hammond et al., 1985; Sorkin et al., 1993). Electrical stimulation of any of these descending inhibitory pathways exerts inhibitory effects on dorsal horn neurons (Fields et al., 1977) and produces antinociception (Aimone et al., 1987; Basbaum et al., 1976; Fields and Basbaum, 1999; Jones and Gebhart, 1986; Mayer et al., 1971; Reynolds, 1969; Yeomans et al., 1992). In the current study, spinal administration of methysergide or yohimbine prevents or decreases joint manipulation-induced antihyperalgesia. Since serotonin and noradrenaline in spinal cord are released from descending bulbospinal neurons, our data strongly support the hypothesis that joint manipulation-induced antihyperalgesia involves activation of descending inhibitory pathways. However, since naloxone has no effect on joint manipulation-induced antihyperalgesia, this suggests activation of non-opioid descending inhibitory systems. Further, blockade of GABA receptors in the spinal cord has no effect on joint manipulation-induced antihyperalgesia, suggesting that presynaptic or local inhibitory pathways are likely not involved in joint manipulation antihyperalgesia.
The role of this descending analgesia system in modulating pain-related behavior may depend on duration and type of noxious stimuli involved. Descending serotonergic neurons do not appear to tonically inhibit spinal transmission of noxious mechanical or thermal input in normal animals (Bardin et al., 2000; Zhuo and Gebhart, 1991). In contrast, spinal administration of noradrenergic receptor antagonists decreases hotplate and tail flick latencies in rat, indicating that descending noradrenergic neurons are involved in tonic inhibition of spinal transmission of noxious heat (Proudfit and Hammond, 1981; Sagen and Proudfit, 1984; Zhuo and Gebhart, 1991). There is also pharmacological evidence suggesting both descending serotonergic and noradrenergic pathways are activated in models of chemogenic and neuropathic pain. Intrathecal pretreatment with methysergide or yohimbine increases licking and biting during both phases of the formalin test and post-treatment increases pain-related behaviors in the second phase (Omote et al., 1998). Spinal administration of methysergide or yohimbine also further reduces paw withdrawal latencies to heat after chronic constriction injury (Satoh and Omote, 1996). Further, dorsal horn serotonin and noradrenaline concentrations increase bilaterally after formalin injection and after chronic constriction injury (Omote et al., 1998; Satoh and Omote, 1996). In the current study, spinal administration of serotonin or noradrenaline receptor antagonists does not further reduce mechanical withdrawal thresholds subsequent to intra-articular capsaicin injection. Thus, descending monoaminergic neurons do not appear to inhibit spinal transmission of mechanical stimuli (mechanical hyperalgesia) after capsaicin injection.
4.2. Selective serotonin receptor antagonists
In this study, spinal administration of methysergide prevents joint manipulation-induced antihyperalgesia. The effectiveness of this non-selective serotonin receptor antagonist prompted evaluation of more selective serotonin receptor antagonists. The 5-HT1, 5-HT2, and 5-HT3 types of serotonin receptors are found in the spinal dorsal horn (Kia et al., 1995; Ridet et al., 1994; Thor et al., 1993, see Coggeshall and Carlton, 1997 for review). However, there is some controversy regarding the role of serotonin receptors in spinal antinociception. For instance, spinal administration of 5-HT1A receptor agonists facilitates nociceptive transmission within the spinal cord (Alhaider and Wilcox, 1993; Ali et al., 1994; Zhang et al., 2001b), inhibits spinal nociceptive transmission (Bardin et al., 2001; Mjellem et al., 1992; Oyama et al., 1996), or has no effect (Bardin et al., 2000; Millan, 1994; Sasaki et al., 2001). In the present study, spinal administration of methysergide or NAN-190 prevents antihyperalgesia induced by knee joint manipulation. This suggests that peripheral joint manipulation increases physiological levels of serotonin that predominantly act on spinal 5-HT1A receptors to produce antihyperalgesia.
The central neural mechanisms by which peripheral nonpharmacological manipulations like transcutaneous electrical nerve stimulation (TENS) or manipulative therapy relieve pain are pharmacologically heterogeneous. For instance both low and high frequency TENS decrease secondary hyperalgesia produced by injection of 3% kaolin/carrageenan into the knee joint (Sluka et al., 1998, 1999). However, the mechanisms for TENS-antihyperalgesia differ depending upon frequency of stimulation. Antihyperalgesia produced by low (4 Hz) and high (100 Hz) frequency TENS is dependent upon spinal μ- and δ-opioid receptors, respectively (Sluka et al., 1999), while data from the current study show that antihyperalgesia induced by peripheral knee joint manipulation, administered at a frequency of 0.5–1 cycles per second, does not depend on spinal opioid receptors. Further, blockade of spinal 5-HT2 or 5-HT3 receptors prevents antihyperalgesia induced by low, but not high, frequency TENS, while blockade of spinal α2-adrenergic receptors with yohimbine has no effect (Radhakrishnan et al., 2003). In contrast, our data show that spinal blockade of 5-HT1A and α2-adrenergic receptors prevents or attenuates antihyperalgesia resultant from knee joint manipulation. These findings should be tempered by the fact that the pain model employed may affect serotonin receptor expression. For instance, carrageenan inflammation is associated with upregulation of 5-HT2A mRNA and increases in extracellular 5-HT in the dorsal horn of the spinal cord (Zhang et al., 2000, 2001a).
4.3. Manipulation-induced antihyperalgesia
Manipulation of cervical or thoracic spine produces an immediate, localized hypoalgesic effect and reduces pain in human subjects (Terrett and Vernon, 1984; Vernon et al., 1990; Vicenzino et al., 1996; Zusman et al., 1989). Further, cervical manipulation produces hypoalgesia in, people with lateral epicondylitis or cervical spine pain, that is accompanied by sympathoexcitation: evidenced by changes in skin conductance, blood flow and/or skin temperature (Sterling et al., 2001; Vicenzino et al., 1998). In addition, the initial hypoalgesic effect of spinal manipulation is not reversed by systemic administration of naloxone (Vicenzino et al., 2000; Zusman et al., 1989). Wright and colleagues hypothesize that this coordinated hypoalgesic and autonomic response may be the result of activation of descending inhibitory pathways in the central nervous system. Specifically they suggest that joint manipulation activates the lateral periaqueductal gray since glutamate excitation of the lateral PAG produces a similar response, namely that of non-opioid analgesia, sympathetic excitation, and motor facilitation (Bandler and Shipley, 1994; Cannon et al., 1982; Lovick, 1991). These data from human subjects are consistent with the current findings in this animal model. Taken together, we hypothesize that joint manipulation produces a non-opioid form of analgesia, mediated by spinal serotonergic and noradrenergic receptors utilizing descending inhibitory pathways from the RVM and dorsolateral pons.
Although manipulation in clinical settings is often performed at the site of injury or the spine, a segmentally related but non-involved joint was chosen in order to minimize local peripheral effects and focus on those effects mediated by the central nervous system. Further studies are necessary to more directly investigate supraspinal involvement and evaluate other pain models. As the time/force profiles of different forms of manipulation vary, other forms of joint manipulation should also be evaluated.
Acknowledgements
This study was funded by NIH grants R21 AT 001130-02 and KO2 AR 02201.
References
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaquaductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–36. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Alhaider AA, Wilcox GL. Differential roles of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor subtypes in modulating spinal nociceptive transmission in mice. J Pharmacol Exp Ther. 1993;265(1):378–85. [PubMed] [Google Scholar]
- Ali Z, Wu G, Kozlov A, Barasi S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: results from behavioral and electrophysiological studies. Brain Res. 1994;661(1–2):83–90. doi: 10.1016/0006-8993(94)91184-3. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–89. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barasi S, Clatworthy A. The effects of intrathecally applied noradrenaline and 5-hydorxytryptamine on spinal nocifensive reflexes in the rostral transmission of noxious information to the thalamus in the rat. Neurosci Lett. 1987;78:328–32. doi: 10.1016/0304-3940(87)90382-x. [DOI] [PubMed] [Google Scholar]
- Bardin L, Lavarenne J, Eschalier A. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain. 2000;86(1–2):11–18. doi: 10.1016/s0304-3959(99)00307-3. [DOI] [PubMed] [Google Scholar]
- Bardin L, Tarayre JP, Koek W, Colpaert FC. In the formalin model of tonic nociceptive pain, 8-OH-DPAT produces 5-HT1A receptor-mediated, behaviorally specific analgesia. Eur J Pharmacol. 2001;42:109–14. doi: 10.1016/s0014-2999(01)01029-9. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci USA. 1976;73:4685–8. doi: 10.1073/pnas.73.12.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz AJ. The sites of origin of brainstem neurotensin and serotonin projections to the rodent nucleus raphe magnus. J Neurosci. 1982;2:829–42. doi: 10.1523/JNEUROSCI.02-07-00829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowker RM, Abhold RH. Evoked changes in 5-hydroxytryptamine and norepinephrine release: in vivo dialysis of the rat dorsal horn. Eur J Pharmacol. 1990;175:101–6. doi: 10.1016/0014-2999(90)90159-4. [DOI] [PubMed] [Google Scholar]
- Bronfort G. Spinal manipulation: current state of research and its indications. Neurol Clin. 1999;17:91–111. doi: 10.1016/s0733-8619(05)70116-x. [DOI] [PubMed] [Google Scholar]
- Calejesan AA, Ch’ang MHC, Zhuo M. Spinal serotonergic receptors mediate facilitation of a nociceptive reflex by subcutaneous formalin injection into the hindpaw in rats. Brain Res. 1998;798:46–54. doi: 10.1016/s0006-8993(98)00394-1. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseoleus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol. 1995;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982;243:315–21. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;538:231–45. doi: 10.1016/0006-8993(91)90435-x. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Rev. 1997;24(1):28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- Cui M, Feng Y, McAdoo DJ, Willis WD. Periaqueductal gray stimulation-induced inhibition of nociceptive dorsal horn neurons in rats is associated with the release of norepinephrine, serotonin, and amino acids. J Pharmacol Exp Ther. 1999;289:868–76. [PubMed] [Google Scholar]
- Dickenson AH, Le Bars D, Besson J-M. Endogenous opiates and nociception: a possible functional role in both pain inhibition and detection as revealed by intrathecal naloxone. Neurosci Lett. 1981;24:161–4. doi: 10.1016/0304-3940(81)90241-x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG, Schmidt RF. Central pathways responsible for depolarization of primary afferent fibres. J Physiol. 1962;161:237–57. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide PK. Stimulation of 5-HT1 receptors in the spinal cord changes substance P-induced behaviour. Neuropharmacol. 1992;31:541–5. doi: 10.1016/0028-3908(92)90185-r. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Moxonidien, a selective α2-adrenergic and imidazoline receptor agonist, produces spinal antinociception in mice. J Pharmacol Exp Ther. 1999;290:403–12. [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; New York: 1999. pp. 243–57. [Google Scholar]
- Fields HL, Basbaum AI, Clanton CH, Anderson SD. Nucleus raphe magnus inhibition of spinal cord dorsal horn neurons. Brain Res. 1977;126:441–53. doi: 10.1016/0006-8993(77)90596-0. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity and pulse duration of TENS on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–90. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- Haldeman S, Hooper PD. Mobilization, manipulation, massage and exercise for the relief of musculoskeletal pain. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; St Louis: 1999. pp. 1399–418. [Google Scholar]
- Hammond DL, Tyce GM, Yaksh TL. Efflux of 5-hydroxytryptamine and noradrenaline into spinal cord superfusates during stimulation of the rat medulla. J Physiol (London) 1985;359:151–62. doi: 10.1113/jphysiol.1985.sp015579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Quantitative characterization of ceruleospinal inhibition of nociceptive transmission in the rat. J Neurophys. 1986;56:1397–410. doi: 10.1152/jn.1986.56.5.1397. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hammond DL. Role of spinal γ-aminobutyric acidA receptors in formalin induced nociception in the rat. J Pharmacol Exp Ther. 1997;282:928–38. [PubMed] [Google Scholar]
- Kia HK, Miquel MC, McKernan RM, Laporte AM, Lombard MC, Bourgoin S, Hamon M, Verge D. Localization of 5-HT3 receptors in the rat spinal cord: immunohistochemistry and in situ hybridization. Neuroreport. 1995;6:257–61. doi: 10.1097/00001756-199501000-00008. [DOI] [PubMed] [Google Scholar]
- Lovick TA. Interactions between descending pathways from the dorsal and ventrolateral periaqueductal gray matter in the rat. In: Depaulis A, Bandlier R, editors. The midbrain periaqueductal gray matter: functional, anatomical, and neurochemical organization. Plenum Press; New York: 1991. pp. 101–20. [Google Scholar]
- Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci. 1996;17:457–62. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Wolfe TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–4. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin and pain: evidence that activation of 5-HT1A receptors does not elicit antinociception against noxious thermal, mechanical and chemical stimuli in mice. Pain. 1994;58(1):45–61. doi: 10.1016/0304-3959(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Mjellem N, Lund A, Eide PK, Storkson R, Tjolsen A. The role of 5-HT1A and 5-HT1B receptors in spinal nociceptive transmission and in the modulation of NMDA induced behaviour. Neuroreport. 1992;3(12):1061–4. doi: 10.1097/00001756-199212000-00007. [DOI] [PubMed] [Google Scholar]
- Mjellem N, Lund A, Hole K. Different functions of spinal 5-HT1A and 5-HT2 receptor subtypes in modulating behaviour induced by excitatory amino acid receptor agonists in mice. Brain Res. 1993;626(12):78–82. doi: 10.1016/0006-8993(93)90565-5. [DOI] [PubMed] [Google Scholar]
- Nuseir K, Heidenreich BA, Proudfit HK. The antinociception produced by microinjection of a cholinergic agonist in the ventromedial medulla is mediated by noradrenergic neurons in the A7 catecholamine cell body. Brain Res. 1999;822:1–7. doi: 10.1016/s0006-8993(98)01195-0. [DOI] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced nociception activates a monoaminergic descending inhibitory system. Brain Res. 1998;814:194–8. doi: 10.1016/s0006-8993(98)01086-5. [DOI] [PubMed] [Google Scholar]
- Oyama T, Ueda M, Kuraishi Y, Akaike A, Satoh M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci Res. 1996;25(2):129–35. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- Proudfit HK, Hammond DL. Alterations in nociceptive threshold and morphine-induced analgesia produced by intrathecally administered amine antagonists. Brain Res. 1981;218:393–9. doi: 10.1016/0006-8993(81)91318-4. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, King EW, Dickman JK, Herold CA, Johnston NF, Spurgin ML, Sluka KA. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003;105:205–13. doi: 10.1016/s0304-3959(03)00207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–5. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Tamir H, Privat A. Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: a light and electron microscopic study using an anti-id-iotypic antiserum. J Neurosci Res. 1994;38:109–21. doi: 10.1002/jnr.490380114. [DOI] [PubMed] [Google Scholar]
- Sagen J, Proudfit HK. Effect of intrathecally administered noradrenergic antagonists on nociception in the rat. Brain Res. 1984;310:295–301. doi: 10.1016/0006-8993(84)90152-5. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ishizaki K, Obata H, Goto F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur J Pharmacol. 2001;424:45–52. doi: 10.1016/s0014-2999(01)01117-7. [DOI] [PubMed] [Google Scholar]
- Satoh O, Omote K. Roles of monoaminergic, glycinergic and GABAergic inhibitory systems in the spinal cord in rats with peripheral mononeuropathy. Brain Res. 1996;728:27–36. [PubMed] [Google Scholar]
- Sawynok J, Reed A. Noradrenergic and purinergic involvement in spinal antinociception of 5-HT and 2-methyl-serotonin. Eur J Pharmacol. 1991;204:301–9. doi: 10.1016/0014-2999(91)90856-l. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55(1):5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–80. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci. 2002;22(13):5687–93. doi: 10.1523/JNEUROSCI.22-13-05687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77:97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–6. [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Spinal projections of the locus coeruleus and the nucleus subcoeruleus in the Harlan and the Sasco Sprague—Dawley rat. Brain Res. 1992;579:67–73. doi: 10.1016/0006-8993(92)90742-r. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Willis WD, Westlund KN. The role of dorsal root reflexes in neurogenic inflammation. Pain Forum. 1995;4:141–9. [Google Scholar]
- Sluka KA, Wright A. Knee joint mobilization reduces secondary mechanical hyperalgesia induced by capsaicin injection into the ankle joint. Eur J Pain. 2001;5:81–7. doi: 10.1053/eujp.2000.0223. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, McAdoo DJ, Willis WD. Raphe magnus stimulation induced antinociception in the cat is associated with release of amino acids as well as serotonin in the lumbar dorsal horn. Brain Res. 1993;618:95–108. doi: 10.1016/0006-8993(93)90433-n. [DOI] [PubMed] [Google Scholar]
- Sterling M, Jull G, Wright A. Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity. Man Ther. 2001;6:72–81. doi: 10.1054/math.2000.0378. [DOI] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–72. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Terrett AC, Vernon H. Manipulation and pain tolerance. A controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. Am J Phys Med. 1984;63:217–25. [PubMed] [Google Scholar]
- Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B, and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience. 1993;55:235–52. doi: 10.1016/0306-4522(93)90469-v. [DOI] [PubMed] [Google Scholar]
- van Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic nonspecific low back pain. A systematic review of randomized controlled trials of the most common interventions. Spine. 1997;22:2128–56. doi: 10.1097/00007632-199709150-00012. [DOI] [PubMed] [Google Scholar]
- Vernon HT, Aker P, Burns S, Viljakaanen S, Short L. Pressure pain threshold evaluation of the effect of spinal manipulation in the treatment of chronic neck pain: a pilot study. J Manipulative Physiol Ther. 1990;13:13–16. [PubMed] [Google Scholar]
- Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther. 1998;21:448–53. [PubMed] [Google Scholar]
- Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74. doi: 10.1016/S0304-3959(96)03221-6. [DOI] [PubMed] [Google Scholar]
- Vicenzino B, O’Callahan J, Kermode F, Wright A. No influence of naloxone on the initial hypoalgesic effect of spinal manual therapy. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Proceedings from the Ninth World Congress on Pain. IASP Press; Seattle: 2000. pp. 1039–44. [Google Scholar]
- Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Descending noradrenergic projections and their spinal terminations. Prog Brain Res. 1983;57:219–38. doi: 10.1016/S0079-6123(08)64131-X. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Analgesia and hyperalgesia produced in the rat by intrathecal naloxone. Brain Res. 1980;189:593–7. doi: 10.1016/0006-8993(80)90375-3. [DOI] [PubMed] [Google Scholar]
- Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther. 1995;1:11–16. doi: 10.1054/math.1995.0244. [DOI] [PubMed] [Google Scholar]
- Wright A, Vicenzino B. Cervical mobilisation techniques, sympathetic nervous system effects and their relationship to analgesia. In: Schacklock M, editor. Moving in on pain. Butterworth Heinneman; Adelaide: 1995. pp. 164–73. [Google Scholar]
- Yang SW, Zhang ZH, Wang R, Xie YF, Qiao JT, Dafny N. Norepinephrine and serotonin induced antinociception are blocked by naloxone with different dosages. Brain Res Bull. 1994;35:113–7. doi: 10.1016/0361-9230(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Clark FM, Paice JA, Proudfit HK. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain. 1992;48:449–61. doi: 10.1016/0304-3959(92)90098-V. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Projections of substance P-immunoreactive neurons located in the ventromedial medulla to the A7 noradrenergic nucleus of the rat demonstrated using retrograde tracing combined with immunocytochemistry. Brain Res. 1990;532(1–2):329–32. doi: 10.1016/0006-8993(90)91777-e. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience. 1992;49(3):681–91. doi: 10.1016/0306-4522(92)90236-u. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Gao X, Ji GC, Wu GC. Expression of 5-HT2A receptor mRNA in rat spinal dorsal horn and some nuclei of brainstem after peripheral inflammation. Brain Res. 2001a;900(1):146–51. doi: 10.1016/s0006-8993(01)02283-1. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Gao X, Zhang LM, Wu GC. The release of serotonin in rat spinal dorsal horn and periaqueductal gray following carrageenan inflammation. Neuroreport. 2000;11(16):3539–43. doi: 10.1097/00001756-200011090-00027. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Yang ZL, Gao X, Wu GC. The role of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptors in modulating spinal nociceptive transmission in normal and carrageenan-injected rats. Pain. 2001b;92:201–11. doi: 10.1016/s0304-3959(01)00259-7. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Duggan AW. Idazoxan blocks the action of noradrenaline but not spinal inhibition from electrical stimulation of the locus coeruleus and nucleus Kolliker-fuse of the cat. Neuroscience. 1988;25:997–1005. doi: 10.1016/0306-4522(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1991;46:211–22. doi: 10.1016/0304-3959(91)90078-C. [DOI] [PubMed] [Google Scholar]
- Zusman M, Edwards BC, Donaghy A. Investigation of a proposed mechanism for the relief of spinal pain with passive joint movement. J Man Med. 1989;4:58–61. [Google Scholar]