Abstract
Background
Cardiac computed tomography (CT) is a well-established tool for the detection of cardiovascular calcium. Coronary artery calcification (CAC) is highly sensitive for the detection of coronary artery disease (CAD) as well as predictive of future cardiovascular (CV) events. Descending thoracic aortic calcification (DTAC) is common in the elderly and its presence is also associated with increased risk of CV events. Previous studies demonstrate that DTAC is associated with obstructive CAD and coronary risk factors. However, no prior studies have examined the association of CAC and DTAC as detected by cardiac CT in a large population-based cohort.
Methods
In the Multi-Ethnic Study of Atherosclerosis, the study population included a population based sample of four ethnic groups (Chinese, White, Hispanic and African-American) of 6814 women and men ages 45−84 years old. Participants underwent non-enhanced cardiac CT and both CAC and DTAC were quantified. DTAC was measured from the lower edge of the pulmonary artery bifurcation to the cardiac apex. Multivariable relative risk regression was used to evaluate relationships between CAC, DTAC and measured cardiovascular risk factors.
Results
Overall 3030 (44%) did not demonstrate any detectable CAC or DTAC. A total of 1930 (28%) had only CAC, 386 (6%) had isolated DTAC, and 1464 (22%) participants were found to have both CAC and DTAC. CAC had a higher prevalence than DTAC in men (58% vs. 45%). Participants with DTAC were older than those with CAC (mean age was 71 and 66 years old, respectively). Participants with DTAC had increased risk for the presence of CAC independent of cardiovascular risk factors (prevalence ratio [PR]; 1.17 95% CI 1.07−1.28). Severity of DTAC was a stronger predictor of the presence of CAC in women as compared to men (PR; 1.04 95% CI 1.02 −1.06, and PR; 0.99 95% CI 0.98− 1.01, respectively).
Conclusions
DTAC was found to be a strong predictor of CAC independent of CV risk factors. Ongoing follow-up of this cohort will evaluate whether DTAC is an independent marker of risk for CV events.
Introduction
Several studies on aortic atheroma using transesophageal echocardiography (TEE) have shown that aortic atherosclerosis was associated with coronary artery disease (CAD) 1,2,3,4,5. TEE, however, is invasive and difficult to apply to a large population based study. Non-enhanced computed tomography (CT) can measure vascular calcification and is a non-invasive modality with a low burden on subjects. Coronary artery calcification detected by CT documents the presence of coronary atheroma and has been shown to add to the prediction of hard cardiovascular (CV) events beyond traditional CV risk factors in both men, women and four major ethnic groups in the U.S.6,7,8,9,10 . The patients with calcification in the descending thoracic aorta have 3.8 times the relative risk for obstructive coronary artery disease (CAD) independent of age.11 Although the prevalence of descending thoracic aortic calcification (DTAC) is relatively low in people under age 60, it increases significantly with age12.
No previous study has examined the relationship of coronary artery and thoracic aortic calcium as detected by computed tomography (CT) in a large population based cohort. The aim of this paper is to determine the relationships between CAC and DTAC with traditional CV risk factors including gender and age.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) was initiated in July 2000 to investigate the prevalence, correlates and progression of subclinical cardiovascular disease in individuals without known cardiovascular disease13. This prospective cohort study includes 6814 women and men ages 45−84 years old recruited from six U.S. communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN) who identified themselves as white, African-American, Hispanic, and Chinese.
Medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002). Information about age, gender, ethnicity, and medical history were obtained by questionnaires. Current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes was defined as a fasting glucose ≥ 126 mg/dl or on hypoglycemic medication. Use of antihypertensive and other medications were based on clinic staff entry of prescribed medications.
Resting blood pressure was measured three times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida) and the average of the last two readings was recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of medication prescribed for hypertension. Body mass index was calculated from the equation weight (kg)/ height (m2).
Total and HDL cholesterol were measured from blood samples obtained after a 12-hour fast. LDL cholesterol was calculated with the Friedewald equation(11). CRP was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Analytical intra-assay CVs ranged from 2.3 − 4.4% and inter-assay CVs ranged from 2.1 − 5.7%. IL-6 was measured by ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN). The laboratory analytical CV for this assay was 6.3%. Fibrinogen was measured using a BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc., Deerfield, IL) with intra-assay and inter-assay analytical CVs of 2.7% and 2.6%, respectively. Factor VIII levels were determined by measuring the clot time of a sample in factor VIII deficient plasma in the presence of activators utilizing the Sta-R analyzer (STA-Deficient VIII; Diagnostica Stago, Parsippany, NJ).
All participants underwent two CT scans at the same time for evaluation of CAC, after signing informed consent. An ancillary study, supported by the National Institutes of Health, was performed to measure aortic and valvular calcification on the scans obtained for the MESA study. This study was approved by the Institutional Review Board of our institution. Three sites used an Imatron C-150XL CT scanner (GE-Imatron, San Francisco, CA), and three sites used a multidetector CT scanner (four slice). The method has been reported previously14. Image slices were obtained with the participant supine, with no couch angulation. A minimum of 35 contiguous images with a 2.5- or 3-mm slice thickness was obtained, starting above the left main coronary artery to the bottom of both ventricles. Each scan was obtained in a single breath hold. Section thickness of 3 mm, field of view of 35 cm, and matrix of 512 × 512 were used to reconstruct raw image data. The nominal section thickness was 3.0 mm for electron beam CT and 2.5 mm for four-detector row CT. Spatial resolution can be described by the smallest volume element, or voxel, for the protocol for each system: 1.15 mm3 for four-detector row CT (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 for electron beam CT (0.68 × 0.68 × 3.00 mm). Descending thoracic aortic calcification (DTAC) was measured on images starting at the lower edge of the pulmonary artery bifurcation inferiorly to the cardiac apex. This region of the thoracic aorta was included on the images on every study of coronary calcium and was quantified by using the same lesion definition(threshold and minimum lesion size) as was used for coronary calcification. The absence of CAC and DTAC was assigned a score of 0.
Statistical Methods
CAC and DTAC were dichotomized as present (Agatston score > 0) or absent (Agatston score =0). Distributions of demographics and cardiovascular risk factors were compared across these groups. Differences in characteristics were compared using ANOVA for continuous variables and χ2 tests for categorical variables. Because the prevalence of CAC in our cohort is greater than 10%, odds ratios (ORs) overestimate the prevalence ratios (PR). Therefore, PR estimates are presented from the regression model y=exp(XTβ). The exponentiated parameters β are interpreted as PR. That is, the probability of detectable CAC was modeled as a function of covariates using a generalized linear model with log link and binomial error distribution. In some cases the model failed to converge with the binomial error, hence we assumed Gaussian error and used robust standard error estimates. Using this method we assessed the relationship between DTAC and the presence of CAC. A simple approach to separate the qualitative difference between absence and presence of DTAC from the quantitative effect of DTAC was used. DTAC was modeled as a dichotomous variable (DTAC=0 versus DTAC>0) and continuous variable (Ln(DTAC+1)) in the same model adjusting for potential confounders. Covariates were entered into the regression model adjusting for demographics (age, gender, and race), cardiovascular risk factors (BMI, HDL, LDL, lipid lowering medication, smoking, hypertension, diabetes mellitus and family history of heart attack) and markers of inflammation (IL-6, fibrinogen and factor VIII). Two-way interactions between DTAC and age, gender and race with CAC as the outcome were examined. We also used the same regression method to look at risk factors separately for CAC and DTAC as dichotomous outcomes. Statistical analyses were performed with SPSS 15.0 software for Windows (SPSS Inc, Chicago, Ill) and STATA 10.0 for Windows (Stata Co, College Station, TX).
Results
A. Participant characteristics
The study population (6814 individuals, 49% men and mean age: 63±10 years) was split according to distribution and frequency of presence of CAC and DTAC. Overall 3030 (45%) did not demonstrate any detectable CAC or DTAC. A total of 1930 (28%) had only CAC, 386 (6%) had isolated DTAC, and 1464 (22%) participants were found to have both CAC and DTAC. One participant had uninterpretable data for DTAC. Three participants were excluded due to the presence of renal failure based on the potential for altered calcium metabolism with renal failure to influence the measurement of DTAC.
Table 1 demonstrates the baseline characteristics of the study population between DTAC and CAC. The overall prevalence of DTAC was 27% and of CAC was 50%. The ethnic makeup of the MESA cohort for prevalence of DTAC and CAC were similar. The prevalence of DTAC by ethnicity was 45% White, 14% Chinese, 21% African-American, and 20% Hispanic and the prevalence of CAC ethnicity was 44%, 12%, 24%, and 20%, respectively. CAC had a higher prevalence in men (58% CAC versus 45% DTAC). Participants with DTAC were significantly older than participants with CAC (mean age was 71 and 66 years old, respectively), had more hypertension (65% DTAC versus 55% CAC), and higher levels of IL-6 (median of 1.42 for DTAC and 1.31 for CAC).
Table 1.
Characteristics of MESA absence/presence of Descending Thoracic calcification and coronary calcium.
| Variables** | DTAC=0 | DTAC>0 | p-value | CAC=0 | CAC>0 | p-value |
|---|---|---|---|---|---|---|
| N (%) | 4960 (73%) | 1850 (27%) | 3416 (50%) | 3393 (50%) | ||
| Age | 59 (9) | 71 (8) | <0.001 | 58 (9) | 66 (10) | <0.001 |
| Men | 2381 (48%) | 829 (45%) | 0.019 | 1249 (37%) | 1961 (58%) | <0.001 |
| Race | ||||||
| White | 1788 (36%) | 835 (45%) | <0.001† | 1127 (33%) | 1496 (44%) | <0.001† |
| Chinese | 544 (11%) | 259 (14%) | 0.001† | 399 (12%) | 404 (12%) | 0.772† |
| African-Americans | 1497 (30%) | 396 (21%) | <0.001† | 1073 (31%) | 820 (24%) | <0.001† |
| Hispanic | 1131 (23%) | 360 (20%) | 0.003† | 817 (24%) | 674 (20%) | <0.001† |
| Smoking | ||||||
| Former | 1755 (36%) | 729 (40%) | 0.002 | 1045 (31%) | 1439 (43%) | <0.001 |
| Current | 671 (14%) | 216 (12%) | 0.041 | 451 (13%) | 436 (13%) | 0.613 |
| BMI (Kg/m2) | 29 (6) | 28 (5) | <0.001 | 28 (6) | 28 (5) | 0.821 |
| Systolic BP (mmHg) | 123 (20) | 136 (23) | <0.001 | 122 (20) | 131 (22) | <0.001 |
| Diastolic BP (mmHg) | 72 (10) | 72 (10) | 0.082 | 71 (10) | 73 (10) | <0.001 |
| anti-hypertensive medication | 1568 (32%) | 964 (52%) | <0.001 | 984 (29%) | 1548 (46%) | <0.001 |
| Hypertension | 1856 (37%) | 1198 (65%) | <0.001 | 1200 (35%) | 1854 (55%) | <0.001 |
| Diabetes | 620 (13%) | 348 (19%) | <0.001 | 364 (11%) | 604 (18%) | <0.001 |
| Family history of heart attack | 1918 (41%) | 813 (48%) | <0.001 | 1203 (37%) | 1528 (48%) | <0.001 |
| Total cholesterol | 194 (35) | 195 (36) | 0.204 | 194 (35) | 195 (36) | 0.305 |
| LDL (mg/dL) | 117 (32) | 117 (31) | 0.826 | 116 (31) | 118 (32) | 0.002 |
| HDL (mg/dL) | 51 (15) | 51 (14) | 0.693 | 53 (15) | 49 (15) | <0.001 |
| Triglycerides * (mg/dL) | 109 [76, 159] | 117 [83, 164] | <0.001 | 106 [75,155] | 117 [82,166] | <0.001 |
| Lipid lowering meds | 652 (13%) | 446 (24%) | <0.001 | 360 (11%) | 738 (22%) | <0.001 |
| CRP* (mg/dL) | 1.87 [0.81, 4.27] | 1.99 [0.92, 4.20] | 0.038 | 1.93 [0.83,4.31] | 1.91 [0.85,4.19] | 0.750 |
| Iinterleukin-6* (pg/mL) | 1.12 [0.72, 1.79] | 1.42 [0.93, 2.14] | <0.001 | 1.10 [0.70,1.76] | 1.31 [0.87,2.02] | <0.001 |
| Fibrinogen (mg/dL) | 342 (73) | 360 (75) | <0.001 | 340 (71) | 354 (76) | <0.001 |
| Factor VIII (%) | 160 (67) | 174 (67) | <0.001 | 157 (65) | 170 (69) | <0.001 |
median[IQR]
represented as Mean ± SD or N (%)
BMI = body mass index; BP = blood pressure; CAC = coronary artery calcium; CRP = C reactive protein; DTAC = descending thoracic aortic calcification; HDL = high density lipoprotein; LDL = low density lipoprotein; SD = standard deviation
= p value reflects uneven distribution of DTAC and CAC by ethnicity. Only Chinese participants had equal proportions of CAC 0 and CAC >0 (399 vs 404, p=ns).
DTAC status by absence/presence of CAC is shown in table 2. Participants with no CAC had an 11% prevalence of DTAC compared to a prevalence of 43% for participants with CAC>0 (p<0.001). Similarly by defining quantity of DTAC as Ln(DTAC+1), participants with no CAC had a significantly lower mean score compared to those with CAC > 0 (mean =0.53, SD=1.87 and mean = 2.45, SD=3.02, respectively, p<0.001).
Table 2.
Descending thoracic calcification status by absence/presence of coronary calcium.
|
CAC=0 (n=3416) |
CAC>0 (n=3394) |
|||
|---|---|---|---|---|
| Characteristic | N (%) | Mean (SD) | N (%) | Mean (SD) |
| DTAC status | ||||
| DTAC=0 | 3030 (89) | 1930 (57) | ||
| DTAC>0 | 386 (11) | 1464 (43) | ||
| Continuous | ||||
| DTAC | ||||
| Ln(DTAC+1) | 0.53 (1.59) | 2.45 (3.02) | ||
CAC=coronary artery calcium; DTAC = descending thoracic aortic calcification
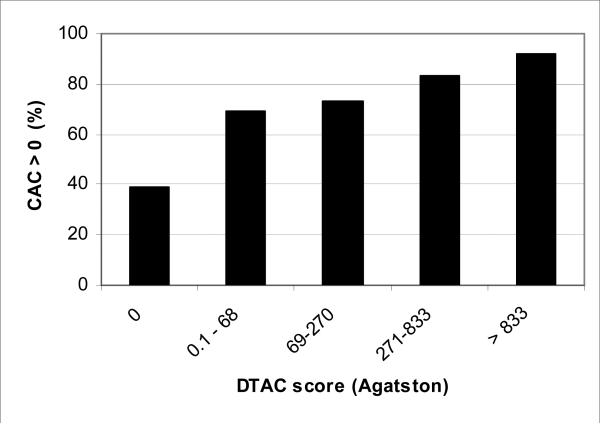
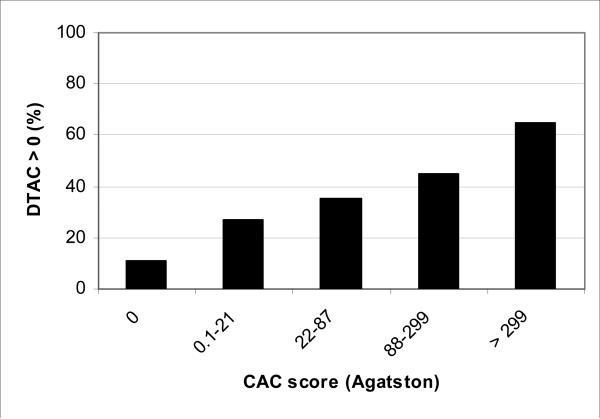
Figure 1 shows the prevalence CAC by presence/absence of DTAC plus quartiles of DTAC for participants with DTAC>0. Similarly, figure 2 shows the prevalence of DTAC by CAC quartiles. Severity of CAC and DTAC increased with prevalence of DTAC and CAC respectively. The prevalence of DTAC went from 6% without CAC (score of zero) to over 60% with CAC scores > 299. The prevalence of CAC went from 39% for those without DTAC to 92% for those with DTAC > 833.
Figure 1. Prevalence of coronary calcium score > 0 by descending aortic calcium.
Categories were selected by 0 and quartile of those positive
Figure 2.
Prevalence of descending aortic calcium > 0 by coronary calcium score.
B. Association of DTAC and CAC with risk factors
Multivariable prevalence ratio estimates for risk factors associated with the presence of CAC and DTAC are shown in table 3. DTAC was more strongly association with age than CAC. A 10-year age increase was associated with a 99% risk increase in the prevalence of DTAC compared to a 37% increase in CAC. The risk of prevalent DTAC was lower among men than women (PR 0.87, 95% CI 0.82− 0.94) while the prevalence of CAC was higher in men (PR 1.38, 95% CI 1.32 and 1.44). Among noted risk factors, hypertension and current smoking status were most strongly associated with the presence of DTAC (PR 1.44, 95% CI 1.30−1.60 and PR 1.35, 95% CI 1.26−1.46, respectively) while multiple risk factors were significantly associated with the prevalence of CAC. The most strongly associated was current smoking, with a PR of 1.24 (95% CI 1.16− 1.32).
Table 3.
Multivariable risk factor analysis for presence of Descending Thoracic calcification and Coronary Calcium.
| DTAC | CAC | |||||
|---|---|---|---|---|---|---|
| Variable | PR | 95% CI | p-value | PR | 95% CI | p-value |
| Age (per 10 yr) | 1.99 | 1.92, 2.06 | <0.001 | 1.37 | 1.34, 1.40 | <0.001 |
| Gender (Female vs Male) | 0.87 | 0.82, 0.94 | <0.001 | 1.38 | 1.32, 1.44 | <0.001 |
| Race | ||||||
| White | 1.00 (ref) | -- | -- | 1.00 (ref) | -- | -- |
| Chinese | 1.05 | 0.96, 1.16 | 0.272 | 0.93 | 0.86, 1.00 | 0.049 |
| African-American | 0.65 | 0.59, 0.74 | <0.001 | 0.76 | 0.72, 0.80 | <0.001 |
| Hispanic | 0.83 | 0.77, 0.91 | <0.001 | 0.84 | 0.80, 0.89 | <0.001 |
| Former smoker | 1.10 | 1.03, 1.17 | 0.003 | 1.11 | 1.06, 1.16 | <0.001 |
| Current smoker | 1.44 | 1.30, 1.60 | <0.001 | 1.24 | 1.16, 1.32 | <0.001 |
| BMI (per SD=5.5) | 0.96 | 0.93, 1.00 | 0.062 | 1.04 | 1.02, 1.07 | <0.001 |
| Hypertension | 1.35 | 1.26, 1.46 | <0.001 | 1.14 | 1.09, 1.19 | <0.001 |
| Diabetes | 1.09 | 1.00, 1.18 | 0.048 | 1.11 | 1.05, 1.17 | <0.001 |
| Family history of heart attack | 1.10 | 1.03, 1.17 | 0.003 | 1.16 | 1.11, 1.21 | <0.001 |
| LDL (per SD=31) | 1.06 | 1.03, 1.10 | <0.001 | 1.07 | 1.05, 1.09 | <0.001 |
| HDL (per SD=15) | 0.95 | 0.91, 0.94 | 0.005 | 0.96 | 0.94, 0.99 | 0.004 |
| Lipid lowering meds | 1.15 | 1.07, 1.23 | <0.001 | 1.18 | 1.12, 1.23 | <0.001 |
| CRP (per SD=5.89) | 1.00 | 0.97, 1.03 | 0.986 | 1.00 | 0.97, 1.02 | 0.683 |
| Interleukin-6 (per SD=1.22) | 1.03 | 1.00, 1.06 | 0.067 | 1.02 | 0.99, 1.04 | 0.074 |
| Fibrinogen (per SD=74) | 1.04 | 1.01, 1.08 | 0.023 | 1.03 | 1.01, 1.05 | 0.007 |
| Factor VIII (per SD=67) | 1.00 | 0.97, 1.03 | 0.909 | 1.03 | 1.01, 1.05 | 0.002 |
(all variables were entered into the model simultaneously)
BMI = body mass index; CAC = coronary artery calcium; CRP = C reactive protein; DTAC = descending thoracic aortic calcification; HDL = high density lipoprotein; LDL = low density lipoprotein; SD = standard deviation
Ethnic differences were also observed. Overall African-Americans and Hispanics were less likely to have any CAC and DTAC compared to Whites. After multivariable adjustment, African-Americans had the lowest PR of DTAC (0.65 95% CI 0.59− 0.74) as well as CAC (PR 0.76 95% CI 0.72 − 0.80) as compared with whites. Chinese were not statistically different to whites for both DTAC and CAC (PR 1.05, 95% CI 0.96−1.16 and PR 0.93, 95% CI 0.86− 1.00, respectively).
C. Association of DTAC with CAC
Table 4 shows the association of CAC with the prevalence and severity of DTAC. After adjustment for risk factors the presence and severity of DTAC were significantly associated with CAC (PR 1.17, 95% CI 1.07−1.28 and 1.02, 95% CI 1.01−1.03 respectively). .A significant interaction was found between severity of DTAC and gender (p-value <0.001 in the fully adjusted model). Table 5 shows the association of DTAC and CAC stratified by gender. In both men and women the presence of DTAC was independently and similarly associated with CAC in unadjusted models (PR 1.42 95% CI 1.28 − 1.57, and PR 1.43 95% CI 1.22 −1.67, respectively). However, in women the severity of DTAC was a stronger predictor of CAC presence than men and remained significant after multivariate adjustment (PR 1.04, 95% CI 1.02−1.06 and PR 0.99, 95% CI 0.98−1.01 respectively).
Table 4.
Association of presence/absence of Coronary Calcium with Descending thoracic calcification in relative risk regression.
| Unadjusted Prevalence Ratio (95% CI) | Fully Adjusted* Prevalence Ratio (95% CI) | |
|---|---|---|
| DTAC status | ||
| DTAC=0 | 1.00 (ref) | 1.00 (ref) |
| DTAC>0 | 1.39 (1.26, 1.53)† | 1.17 (1.07, 1.28)† |
| Continuous DTAC | ||
| Ln(DTAC+1) | 1.07 (1.06, 1.08)† | 1.02 (1.01, 1.03) † |
adjusted for age, gender, race, smoking, hypertension, Diabetes, Family History of Heart Disease, LDL, HDL, lipid lowering medications, interleuken-6, fibrinogen, Factor VIII, body mass index. DTAC = descending thoracic aortic calcification
p< 0.05
Table 5.
Association of Descending Thoracic calcification and coronary calcium stratified by gender.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Prevalence Ratio | 95% CI | p-value | Prevalence Ratio | 95% CI | p-value | |
| Unadjusted | ||||||
| DTAC=0 | 1.00 (ref) | -- | -- | 1.00 (ref) | -- | -- |
| DTAC>0 | 1.43 | 1.22, 1.67 | <0.001 | 1.42 | 1.28, 1.57 | <0.001 |
| Ln(DTAC+1) | 1.11 | 1.09, 1.14 | <0.001 | 1.03 | 1.02, 1.05 | <0.001 |
| Fully adjusted* | ||||||
| DTAC=0 | 1.00 (ref) | -- | -- | 1.00 (ref) | -- | -- |
| DTAC>0 | 1.26 | 1.08, 1.47 | 0.003 | 1.19 | 1.07, 1.32 | 0.001 |
| Ln(DTAC+1) | 1.04 | 1.02, 1.06 | <0.001 | 0.99 | 0.98, 1.01 | 0.430 |
adjusted for age, gender, race, smoking, hypertension, Diabetes, Family History of Heart Disease, LDL, HDL, lipid lowering medications, interleuken-6, fibrinogen, Factor VIII, body mass index. PR=prevalence Ratio; DTAC = descending thoracic aortic calcification
Discussion
This study found a fairly strong relationship between CAC and DTAC. The implications are significant, as DTAC can be obtained and potentially quantified on non-gated CT scans of the lung (diagnostic lung CT or cancer screening). If DTAC is present, additional risk stratification of patients for cardiovascular disease may be warranted. Large cohort studies to investigate the association between DTAC and CAC using CT are limited. In a relatively large population based study, however, Wong et al have reported thoracic aortic calcification and CAC by cardiac CT15 in 2,740 asymptomatic patients. The prevalence of DTAC and CAC in that study was higher than the MESA cohort because the population consisted of patients with multiple cardiovascular risk factors and MESA cohort included large numbers of African-American and Hispanic patients who demonstrated lower prevalences of DTAC than Whites. In both this study and the study by Wong, DTAC demonstrated greater prevalence in women than men. One association that may predispose women to higher levels of DTAC is osteoporosis, as there is emerging data that atherosclerosis and osteoporosis are inter-related.16,17,18,19,20 Furthermore, reports on atherosclerotic plaques have shown that arterial calcification has osteoblastic-like mechanisms, as many bone-forming proteins are expressed in arterial calcification.21,22,23,24,25,26. In addition, oxidized lipids and growth factors, such as TGF-β, can paradoxically inhibit bone formation, yet stimulate atherosclerotic calcification27. These reports suggest that similar factors may promote both osteoporosis and aortic calcification, thereby accounting for the greater prevalences of both osteoporosis and aortic calcification in women as compared to men. Allison et al has demonstrated that the calcifications in all vascular beds increased with age.12 Wong et al also demonstrated that TAC increased with age more than CAC independently of noted risk factors21 (Odds per 10 years; 4.35 and 3.15, respectively). This study also showed that the trend of both DTAC and CAC increased with age (PR per 10 years; 1.99 and 1.37, respectively). The prevalence of DTAC by presence and extent of CAC in this study (Figure 2) are consistent with the results of Wong et al15. However, almost half of patients had DTAC of zero in participants with severe CAC (Agatston score > 299).
Regarding ethnicity, White and Chinese participants demonstrated increased prevalence of DTAC and CAC in comparison with African-Americans and Hispanics. Bild et al previously reported CAC in the MESA cohort and demonstrated that African-Americans had lower prevalence than Whites.28 Ethnic trends were similar for DTAC and CAC, with greater differences for DTAC between African-Americans and Whites than CAC (Table 3). The severity of DTAC and CAC paralleled each other more closely in the Chinese cohort than the other ethnic/racial groups. Whether DTAC in Chinese are more closely related to outcomes will be determined as long term follow up of this cohort continues.
Risk factors for presence of DTAC and CAC were overall similar. However, current smoking and hypertension were stronger independent risk factors of DTAC than CAC. Interestingly, there was a slightly higher prevalence of current smoking (13.5% vs. 11.7%, p=0.04) in the DTAC group with zero calcification, than DTAC>0. Neovascularization of atherosclerotic plaques by nicotine contributes to progression of atherosclerosis29, and release of endogenous epinephrine by nicotine influences cardiac function and blood pressure30, which may place more direct stress on the aorta than the coronary arteries. Smoking cessation was associated with decreased atherosclerotic risk in both the aorta and coronary arteries (PR for current smoker; 1.44 and 1.24, and PR for former smokers; 1.10 and 1.11, respectively). Hypertension more strongly influenced the aorta as compared to the coronary artery. Aortic stiffness closely associated with aortic calcification is directly related to pulse pressure31, and elevates systolic blood pressure.32 These results demonstrate that hypertension was more related to the prevalence of DTAC than CAC. High LDL, low HDL and lipid lowering medication use independently increased both DTAC and CAC. Mean values of LDL and HDL between two groups according to the presence of DTAC were exactly the same and the values were modified by lipid lowering meds. Despite them, high LDL and low HDL increased the prevalence of DTAC after adjustment of risk factors. Goff et al reported on dyslipidemia prevalence, treatment and control of the MESA cohort,33 with an overall prevalence of dyslipidemia of 29.3%, only 53.9% were treated. Nonetheless, lipid lowering medication utilization was associated with the presence of DTAC and CAC independent of other risk factors. In a subsequent study of 4468 patients by Wong et al, total cholesterol values and taking lipid lowering medications significantly increased TAC independently of noted risk factors34. Pre-treatment lipid profiles may explain the predilection of calcification in statin users, but these were generally not available, as statins were instituted prior to the start of MESA in many participants. Following development of both DTAC and CAC in MESA with repeat CT studies should shed insight into the relationship between statin use and calcification.
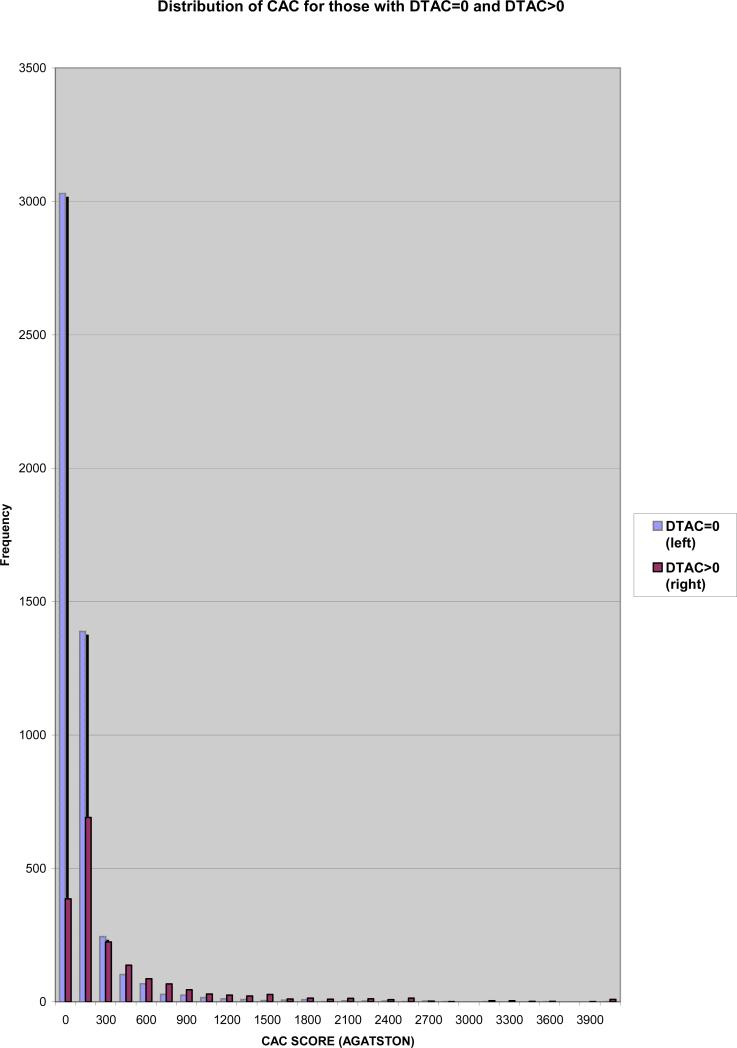
Since both vascular beds (aorta and coronary arteries) are exposed to the same risk factors for atherosclerosis, it is no surprise that DTAC parallels CAC (figure 3). As extra-coronary calcifications including DTAC is detectable with CAC scanning, evaluating and possibly quantifying these calcifications may give additional information about CV risk and may predicting CV events.
Figure 3.
Distribution of Coronary Artery Calcium for those with Descending Aortic Calcium.
The present study based on the MESA cohort has several limitations which have been described previously.28 The participation rate was approximately 30% of those contacted, which is low enough that bias could have been introduced, although similar recruitment methods were used in all ethnic groups, and the participation among those screened (for whom ethnicity was collected) was 70% of whites, 61% of blacks, 59% of Hispanics, and 48% of Chinese. A scoring method for DTAC was used which was identical to the Agatston score for CAC, however there is no histologic or other validation to validate this DTAC scoring method. Also, two types of CT scanners were used for this study, and previous studies have demonstrated that the concordance for presence of calcium between duplicate scans was high and similar for both electron-beam and multi– detector row CT (96%, p=0.92). This previous analysis demonstrated that electron-beam and multi– detector row CT scanners have equivalent reproducibility for measuring coronary artery calcium.35 We have also demonstrated that the reproducibility of TAC is not significantly different between scanner types.36
Previous reports from the MESA cohort verified and considered some characteristics of aortic calcification related to noted risk factors, ethnicity and inflammatory markers37,38,39,40. The prevalence of ascending thoracic aortic calcification, however, was very low and did not influence the prevalence and the severity of the total thoracic aortic calcification (ascending and descending thoracic aortic calcification). For these reasons, we excluded ascending aortic calcification and only used DTAC. The MESA cohort at baseline excluded persons with symptomatic CAD who would develop atherosclerotic involvements. We could not investigate an association between DTAC and obstructive CAD, as participants neither routinely underwent coronary CT angiography or cardiac catheterization. These potential biases could lead to underestimate the prevalence and the severity of DTAC and CAC compared to general population, and may lower the prevalent ratio for risk factors.
DTAC demonstrated a low prevalence in all age groups compared to CAC, but abruptly increased in the elderly. In subgroups according to gender and ethnicity, DTAC had close relations to CAC in middle aged women and 3 ethnic groups (Chinese, African-American and Hispanic). As for noted risk factors, lipid profiles exhibited a similar risk for both DTAC and CAC, and current smoking and hypertension were stronger risk factors of DTAC while diabetes was a stronger risk of CAC. In the light of these results, MESA participants are being followed in regards to an association between DTAC and cardiovascular events to evaluate if this method provides incremental information to the clinician. Given the strong relations between DTAC and CAC, and the association to risk factors, it is possible that the DTAC measures that are available on non-gated lung CT scans could be quantified and used as a marker of atherosclerotic risk. Given the millions of these scans done annually, greater attention should be turned to the incidental finding of DTAC.
Acknowledgement
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fazio GP, Redberg RF, Winslow T, Schiller NB. Transesophageal echocardiographically detected atherosclerotic aortic plaque is a marker for coronary artery disease. J Am Coll Cardiol. 1993;21:144–50. doi: 10.1016/0735-1097(93)90729-k. [DOI] [PubMed] [Google Scholar]
- 2.Nihoyannopoulos P, Joshi J, Athanasopoulos G, Oakley CM. Detection of atherosclerotic lesions in the aorta by transesophageal echocardiography. Am J Cardiol. 1993;71:1208–12. doi: 10.1016/0002-9149(93)90647-u. [DOI] [PubMed] [Google Scholar]
- 3.Parthenakis F, Skalidis E, Simantirakis E, Kounali D, Vardas P, Nihoyannopoulos P. Absence of atherosclerotic lesions in the thoracic aorta indicates absence of significant coronary artery disease. Am J Cardiol. 1996;77:1118–21. doi: 10.1016/s0002-9149(96)00146-4. [DOI] [PubMed] [Google Scholar]
- 4.Khoury Z, Schwartz R, Gottlieb S, Chenzbraun A, Stern S, Keren A. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am J Cardiol. 1997;80:1429–1433. doi: 10.1016/s0002-9149(97)00701-7. [DOI] [PubMed] [Google Scholar]
- 5.Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Petterson TM, O'Fallon WM, Whisnant JP, Wiebers DO, Seward JB. Relation of coronary artery disease and cerebrovascular disease with atherosclerosis of the thoracic aorta in the general population. Am J Cardiol. 2002;89:262–267. doi: 10.1016/s0002-9149(01)02225-1. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JAC, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of Coronary Artery Disease by Cardiac Computed Tomography, A Scientific Statement From the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Raggi P, Berman D, Arad Y, Guerci AD, Callister TQ, Diamond GA. Continuous Probabilistic Prediction of Angiographically Significant Coronary Artery Disease Using Electron Beam Tomography. Circulation. 2002 Apr 16;105(15):1791–6. doi: 10.1161/01.cir.0000014483.43921.8c. [DOI] [PubMed] [Google Scholar]
- 8.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 9.Nasir N, Shaw LJ, Liu ST, Weinstein SR, Mosler TR, Tseng PR, Flores FR, Raggi P, Berman DS, Blumenthal RS, Budoff MJ. Ethnic Differences in the Prognostic Value of Coronary Artery Calcification for All-Cause Mortality. J Am Coll Cardiol. 2007;50:953–60. doi: 10.1016/j.jacc.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 10.Nallamothu BK, Saint S, Bielak LF, Sonnad SS, Peyser PA, Rubenfire M, Fendrick AM. Electron-beam computed tomography in the diagnosis of coronary artery disease: a meta-analysis. Arch Intern Med. 2001;161:833–8. doi: 10.1001/archinte.161.6.833. [DOI] [PubMed] [Google Scholar]
- 11.Takasu J, Mao S, Budoff MJ. Aortic atherosclerosis detected with electron-beam CT as a predictor of obstructive coronary artery disease. Acad Radiol. 2003;10:631–637. doi: 10.1016/s1076-6332(03)80081-8. [DOI] [PubMed] [Google Scholar]
- 12.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 15.Wong ND, Sciammarella M, Arad Y, Miranda-Peats R, Polk D, Hachamovich R, Friedman J, Hayes S, Daniell A, Berman DS. Relation of thoracic aortic and aortic valve calcium to coronary artery calcium and risk assessment. Am J Cardiol. 2003;92:951–5. doi: 10.1016/s0002-9149(03)00976-7. [DOI] [PubMed] [Google Scholar]
- 16.Ouchi Y, Akashita M, De Souza AC, Nakamura T, Orimo H. Age-related loss of bone mass and aortic/aortic valve calcification: reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- 17.Boukhris R, Becker KL. Calcification of the aorta and osteoporosis. JAMA. 1972;219:1307–1311. [PubMed] [Google Scholar]
- 18.Banks LM, Lees B, Macsweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: links between osteoporosis and cardiovascular disease? Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 19.Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazieres B. Comparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic disease. Clin Rheumatol. 1994;13:611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Register TC, Hsu FC, Lohman K, Lenchik L, Bowden DW, Langefeld CD, Xu J, Rich SS, Wagenknecht LE, Freedman BI. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: The diabetes heart study. Bone. 2008;42(1):43–52. doi: 10.1016/j.bone.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993;92:2814–20. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirota S, Imakita M, Kohri K, Ito A, Morii E, Adachi S, Kim HM, Kitamura Y, Yutani C, Nomura S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993;143:1003–1008. [PMC free article] [PubMed] [Google Scholar]
- 25.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 27.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: a possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 28.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 29.Cooke JP. Angiogenesis and the role of the endothelial nicotinic acetylcholine receptor. Life Sc. 2007;80:2347–51. doi: 10.1016/j.lfs.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther. 1984 Sep;36(3):389–95. doi: 10.1038/clpt.1984.193. [DOI] [PubMed] [Google Scholar]
- 31.Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, Vasan RS, Levy D. Predictors of new-onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation. 2005;111:1121–7. doi: 10.1161/01.CIR.0000157159.39889.EC. [DOI] [PubMed] [Google Scholar]
- 32.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–8. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 33.Goff DC, Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, Psaty BM. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–56. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 34.Wong ND, Gransar H, Shaw LJ, Polk D, Berman DS. Comparison of atherosclerotic calcification burden in persons with the cardiometabolic syndrome and diabetes. J Cardiometab Syndr. 2006;1:90–4. doi: 10.1111/j.1559-4564.2006.05618.x. [DOI] [PubMed] [Google Scholar]
- 35.Detrano RC, Anderson M, Nelson J, et al. Coronary calcium measurement : effect of CT scanner type and calcium measure on the rescan reproducibility-MESA study. Radiology. 2005;236:477–84. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 36.Budoff MJ, Katz R, Wong ND, Nasir K, Mao S, Takasu J, Kronmal R, Detrano RC, Carr JJ. Effect Of Scanner Type On The Reproducibility Of Extra-Coronary Measures Of Calcification: The Multi-Ethnic Study Of Atherosclerosis. Acad Radiol. 2007 Sep;14(9):1043–9. doi: 10.1016/j.acra.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Takasu J, Katz R, Nasir K, Carr JJ, Wong N, Detrano R, Budoff MJ. Relationships of aortic wall calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008 Jan 11; doi: 10.1016/j.ahj.2007.11.019. (E pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O'brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT Measurements of Aortic Valve Calcification, Mitral Annulus Calcification, and Aortic Wall Calcification in the Multi-Ethnic Study of Atherosclerosis. Acad Radiol. 2006;13(2):166–72. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 39.Nasir K, Katz R, Takasu J, Shavelle DM, Detrano R, Lima JA, Blumenthal RS, O'Brien K, Budoff MJ. Ethnic differences between extra-coronary measures on cardiac computed tomography: Multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2007 Oct 19; doi: 10.1016/j.atherosclerosis.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takasu J, Katz R, Shavelle DM, O'Brien K, Mao S, Carr JJ, Cushman M, Budoff MJ. Inflammation and descending thoracic aortic calcification as detected by computed tomography: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008 Jan 1; doi: 10.1016/j.atherosclerosis.2007.11.005. [DOI] [PubMed] [Google Scholar]