Abstract
Heteromultivalency provides a route to increase binding avidity and to high specificity when compared to monovalent ligands. The enhanced specificity can potentially serve as a unique platform to develop diagnostics and therapeutics. To develop new imaging agents based upon multivalency, we employed heterobivalent constructs of optimized ligands. In this report, we describe synthetic methods we have developed for the preparation of heterobivalent constructs consisting of ligands targeted simultaneously to the melanocortin receptor, hMC4R, and the cholecystokinin receptors, CCK-2R. Modeling data suggest that a linker distance span of 20–50 Å is needed to crosslink these two G-protein coupled receptors (GPCRs). The two ligands were tethered with linkers of varying rigidity and length, and flexible polyethylene glycol based PEGO chain or semi-rigid [poly(Pro-Gly)] linkers were employed for this purpose. The described synthetic strategy provides a modular way to assemble ligands and linkers on solid-phase supports. Examples of heterobivalent ligands are provided to illustrate the increased binding avidity to cells that express the complementary receptors.
Keywords: Solid-phase peptide synthesis, Multivalency, Heterobivalent ligands, Heterodimers, Linkers, Receptor combination approach, Targeted agents
Introduction
Multivalent ligands are characterized by the simultaneous binding of an entity that displays multiple molecular recognition elements to multiple receptors or epitopes borne on another entity such as a cell surface. These interactions could be homomultivalent if the same binding partners are involved (e.g., bivalent antibody IgE), or heteromultivalent if different binding partners are involved (e.g., T-cell immune synapse, DNA-primer annealing). Multivalent interactions are an important feature of molecular recognition in nature, as cells often encounter naturally occurring multivalent arrays, e.g. host-virus interactions (see Ref. Mammen et al. (1998) for a detailed review). A prominent feature of multiple binding is the significant enhancement of the affinity (avidity) of an agent whose constituent ligands may otherwise exhibit weak binding. A representative example is the binding of decavalent immunoglobulin IgM to a DNP-derivatized surface of bacteriophage φX174, which binds with a 106-fold higher association constant compared to its cleaved (peptic digest) monovalent forms (Hornick and Karush 1972). Another important aspect of these interactions is the high degree of specific binding that results from heterovalent binding partners. For example, an 18-mer oligonucleotide primer can selectively differentiate and bind to its complementary DNA base-pair sequence out of billion competing sequences (see Ref. Handl et al. (2004a) for a discussion on this topic).
We postulate that by combining one or more copies of different specific ligands into a heteromultivalent construct, it should be possible to create compounds that will specifically and selectively bind to cells bearing the appropriate mix of complementary cell-surface receptors (Gillies and Hruby 2003; Caplan and Rosca 2005). Thus, individual cell populations can be defined by specific combinations of expressed cell surface molecules rather than by single receptors (pharmacogenomics). In addition, using combinations instead of single receptors increases the number of unique targets on the cell of interest (Morse et al. unpublished). Therefore, using a cell-surface protein combination that is expressed on a cancer cell but not on a normal cell, heteromultivalent constructs displaying cognate binding motifs can be built and targeted specifically to the desired tumor population in vivo.
As a proof-of-concept for agents based on this receptor combination approach, we employed a heterobivalent construct with optimized lead compounds connected by a suitable linker. We had previously demonstrated with homovalent and heterovalent constructs that increased binding affinity could be achieved from avidity effects due to likely simultaneous binding of multiple ligand epitopes (Handl et al. 2007; Sharma et al. 1994; Sharma et al. 1996; Vagner et al. 2004, 2008). Therefore, we sought to design a synthetic strategy that was modular, flexible, easily scalable and practical to the preparation of bivalent peptides, homovalent or heterovalent, with or without optional functional moieties such as fluorescent labels, imaging tags, etc. In this research report, we describe the synthesis of a library of heterobivalent constructs that were composed of ligands complementary to the human melanocortin receptor Type 4 (hMC4R) and the cholecystokinin-2 receptor (CCK-2R), connected by semi-rigid or flexible linkers. Using cells that expressed either or both melanocortin-4 receptors and CCK-2 receptors, we demonstrate that these heterologous receptors could be crosslinked with resultant increases in avidity.
Materials and Methods
Materials
Nα-Fmoc protected amino acids were purchased from Syn-Pep (Dublin, CA) or from Novabiochem (San Diego, CA). Tentagel Rink amide resin was acquired from Rapp Polymere (Tubingen, Germany). HOBt and HOCt (Cl-HOBt) were purchased from IRIS Biotech (Marktredwitz, Germany). The following side chain protecting groups were used for the amino acids: Arg(Ng-Pbf); Glu(O-tBu); His(Nim-Trt); Ser(tBu), Trp(Ni-Boc). Peptide synthesis solvents, reagents, and acetonitrile for HPLC were reagent grade, were acquired from VWR (West Chester, PA) or Sigma-Aldrich (Milwaukee, WI), and were used without further purification unless otherwise noted. All the compounds were manually assembled using 5–50 ml plastic syringe reactors equipped with a frit and Domino manual synthesizer obtained from Torviq (Niles, MI).
Solid-Phase Peptide Synthesis
Heterobivalent ligands were synthesized on Tentagel Rink amide resin (initial loading 0.2 mmol/g) using Nα-Fmoc protecting groups and a standard DIC/HOCt or HBTU/HOBt activation strategy. The resin was swollen in THF for an hour, washed with DMF, and Fmoc protecting group removed with 20% piperidine in DMF (2 + 20 min). The resin was washed with DMF, 1.0 M HOBt in DMF, and finally with DMF and the first amino acid coupled using preactivated 0.3 M HOCt ester in DMF (3 equiv. of Nα-Fmoc amino acid, 3 equiv. of HOCt and 6 equiv. of DIC). An on-resin test using bromophenol blue was used for qualitative and continuous monitoring of reaction progress (Krchnak et al. 1988). A few drops of a 50 μM solution of bromophenol blue in 0.5 M of HOBt/DMF (bright yellow color) was added to the whole batch of resin, stirred for few seconds and the resin washed with DMF. Free amino groups yield blue coloration to beads, which turns to yellow in their absence as the reaction proceeds to completion. The resins were re-coupled with 3 equiv. of amino acid, 3 equiv. of HBTU and 6 equiv. of DIEA in DMF. To avoid deletion sequences for such long peptides, the re-coupling was performed at all steps and a third time coupling performed with symmetric anhydride method (2 equiv. of amino acid and 1 equiv. of DIC in dichloromethane) only wherever beads still tested Kaiser positive. Any unreacted NH2 groups on the resin thereafter were capped using an excess of 50% acetic anhydride in pyridine for 5 min. When the coupling reaction was finished, the resin was washed with DMF, and the same procedure was repeated for the next amino acid until all the amino acids were coupled. Frequently during the synthetic steps, a small amount of peptide was cleaved and analyzed by HPLC to monitor the synthesis and purity of the intermediate. After the final amino acid was incorporated, the Nα-Fmoc groups were deprotected and the free N-termini were capped with acetic anhydride. The resin was washed with DMF, DCM and THF and dried. A cleavage cocktail (10 ml per 1 g of resin) of TFA (91%), water (3%), triisopropylsilane (3%) and thioanisole (3%) was injected into the resin and stirred for 4 h at room temperature. The crude peptides were isolated from the resin by filtration, the filtrate was reduced to low volume by evaporation using a stream of nitrogen, and the peptides were precipitated in ice-cold diethyl ether, centrifuged and washed several times with ether, dried, dissolved in water and lyophilized to give off-white solid powders that were stored at −20°C until used. The yields of the crude peptides were 50–80% based on the resin weight gain, and overall, the purified yields for the syntheses were 5–20% based on the loading of the resin.
For PEGO (short for polyethyleneglycol and glyoxalate units) spacer introduction, the Nα-terminal of peptide was coupled with glycolic acid spacer using 10 equiv. of diglycolic anhydride (5 min; repeat if necessary). The reaction was qualitatively monitored by change in color of bromophenol blue treated resins as described earlier. The resin was then washed with DMF, DCM and finally with anhydrous DMF. This was followed by imidazolide formation with 10 equiv. of carbonyldiimidazole in anhydrous DMF (30 min). The resin was washed with anhydrous DMF and attachment of PEG-diamine performed with 50% of 4,7,10-trioxa-1,13-tridecanediamine in anhydrous DMF (30 min) (note: presently, an Fmoc-PEGO-OH has become commercially available from Novabiochem).
HPLC Analysis, S.E. Chromatography and Tryptophan Assay
The purity of final products was analyzed using Waters high-performance liquid chromatography (HPLC) apparatus and with a Vydac C18 reverse phase column (diameter × length: 4.6 mm × 75 mm, pore size: 3 μm). Buffer A was water with 0.1% TFA and buffer B was acetonitrile with 0.1% TFA. Peptides were analyzed using a linear gradient of buffer B under various gradient conditions at a flow rate of 1 ml/min and the separations monitored at 220 and 280 nm. Purification of compounds was achieved using a Waters 600 HPLC apparatus equipped with a Vydac C18 reverse phase column (22 mm × 250 mm, 5 μm) with similar buffers but with a gradient from 17% to 57% B in 40 min and 3 ml/min flow rate. The separations were monitored at 230 and 280 nm. Size exclusion chromatography was performed on a borosilicate glass column (2.6 mm × 250 mm, Sigma, St. Louis, MO) filled with medium sized Sephadex G-25 or G-10. The compounds were eluted with an isocratic flow of 1.0 M aqueous acetic acid.
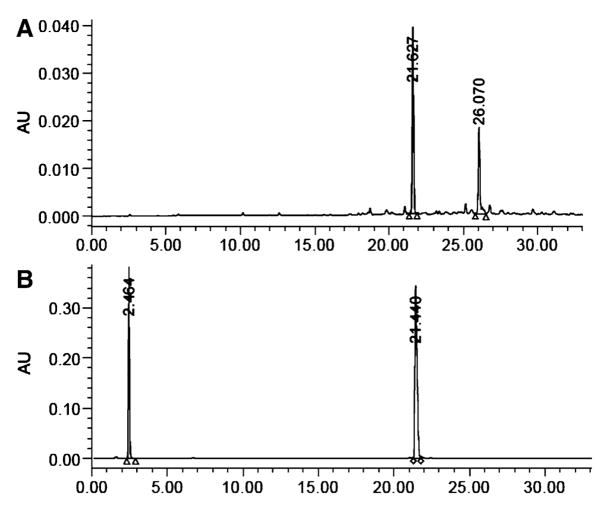
The peptide concentrations were determined by monitoring absorbance of peptides against 0.5 mM solutions of D-Tryptophan (or L-Tryptophan) in DMSO at 280 nm. The peptides were initially dissolved in DMSO at approximately 1–5 mM concentration. Co-injections of peptide and D-Trp were made on analytical HPLC and multiple measurements were recorded for different volumes of D-Trp and compound injected into the HPLC column (see Fig. 3). The peptide concentration was then calculated from area under the peaks using the formula given here.
Fig. 3.
(a) HPLC profile of crude compound 6 at 280 nm; (b) Peptide concentration determination of compound 6 using 0.5 mM D-Tryptophan as standard (with tR of 2.4 min in this figure) co-injected in analytical HPLC and monitored at 280 nm
where ε280 of compound was calculated for two tryptophans (ε280 = 5,500) in the heterobivalent construct and normalized to one tryptophan. There is no other chromophore in the peptide that absorbs significantly at 280 nm. The final concentration of peptide was deduced from at least three independent measurements.
Mass Spectrometry
Mass spectra of positive ions were recorded either with a single stage reflectron MALDI-TOF mass spectrometer (Bruker Rexlex-III, α-cyanocinnamic acid as a matrix) in reflectron mode or with a low resolution ESI mass spectrometer (Finnigan, Thermoquest LCQ ion trap instrument) and/or using high resolution Fourier transform mass spectrometer (FT-MS). For internal calibration, an appropriate mixture of standard peptides was used with an average resolution of 8,000–9,000.
Results and Discussion
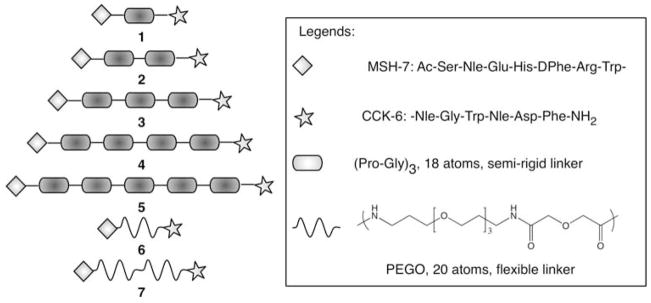
Several design and synthetic parameters such as binding affinity of ligands, spatial placement of ligands on the linker, type of linkers, pre-formed or on-resin construction of PEGO linker, on-resin reaction progress monitoring, recouplings and N-capping, etc were considered to optimize the yield and purity of final products. The synthetic scheme was designed to be simple and modular and was based on parallel solid-phase synthesis. Additionally, the strategy allows future incorporation of imaging and therapeutic tags such as fluorescent and lanthanide labels, radioisotopes, toxins and other latent functional groups (e.g., azides). Based upon the modeling of GPCR dimers, it was estimated that the distance between the two binding sites would be roughly 25–40 Å, depending upon the orientation of the binding sites. Therefore, the heterobivalent constructs were designed to have the linker lengths that meet this ligand spacing. Since the CCK peptide needs a free C-terminal amide for optimal binding (Fourmy et al. 2002), the design of these heterobivalent constructs necessitated placement of CCK ligand on the C-terminus and an α-MSH ligand at the N-terminus of the linker. Truncated ligands were chosen that possessed moderate binding affinities as we had previously observed a pronounced binding enhancement with homobivalent constructs when weaker monovalent ligands were used (Monguchi et al. 2005; Vagner et al. 2006). The binding affinities of monovalent MSH-7 [i.e., Ac-MSH(7)-NH2] and CCK-6 [i.e., Ac-CCK(6)-NH2] ligands, in terms of IC50, were 39 ± 4 nM and 26 ± 4 nM, respectively. Finally, most of these heterobivalent ligands had high molecular mass on the order of small proteins, requiring a combination of purification steps such as size-exclusion chromatography and preparative HPLC. Figure 1 lists the library of heterobivalent constructs 1–7 consisting of MSH-7 and CCK-6 ligands connected in a head-to-tail fashion by either a flexible polyethylene glycol based PEGO linker or by a semi-rigid poly(Pro-Gly) linker.
Fig. 1.
List of heterobivalent constructs composed of melanocortin and cholecystokinin ligands (MSH-7 and CCK-6, respectively). The heterobivalent ligands were either constructed from the semi-rigid poly(Pro-Gly) linker (compound 1–5) or from the flexible PEGO linker (compound 6–7). The legend shows the peptide sequences of MSH-7 and CCK-6 ligands and the structure of the PEGO linker (20 atoms)
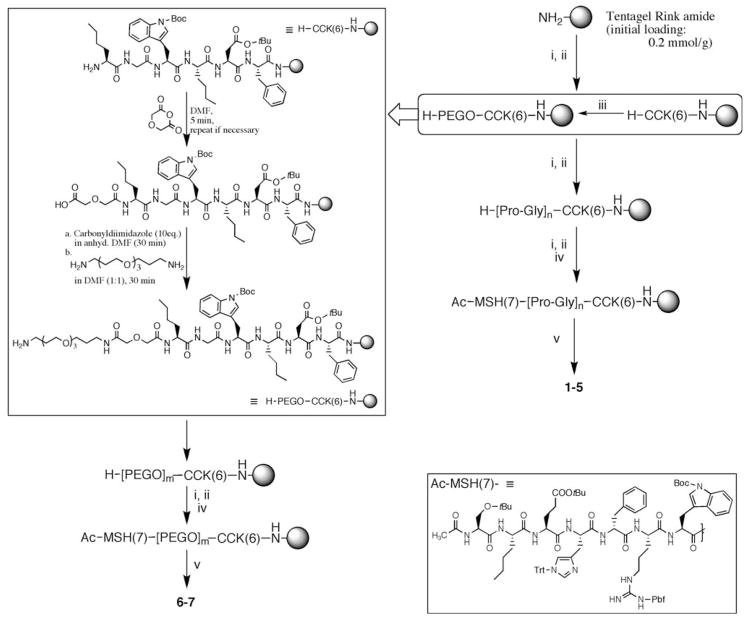
The compounds were assembled using the Nα-Fmoc/tBu strategy of solid-phase synthesis. To avoid deletions, a double coupling approach and N-capping were performed at each step. Starting with a Tentagel Rink amide polystyrene resin (initial loading 0.2 mmol/g), the hexapeptide CCK-6 ligand was constructed as shown in Fig. 2. The reaction progress was qualitatively and non-destructively monitored (on-resin) by bromophenol blue test. Kaiser test was performed for more accurate verification at the end of each coupling step and before the next amino acid addition. It was observed that coupling of the first two amino acids on resin was critical to avoid deletions.
Fig. 2.
Synthetic route for heterobivalent ligands. The inset on left, top shows addition of PEGO sequence. The inset on bottom, right shows the structure of protected Ac-MSH-7 ligand on resin. The reagents for each step are as follows—(i) Fmoc-amino acid-OH (3 eq), Cl-HOBt (3 eq), diisopropylcarbodiimide (6 eq); (ii) 20% Piperidine/DMF; (iii) PEGO linker assembly; refer text for details. (iv) 50% Ac2O in Pyridine; (v) TFA (91%), H2O (3%), Thioanisole (3%), Triisopropylsilane (3%) m is 1 or 2; n is 3, 6, 9, 12, 15
Following CCK-6 ligand construction, the resin was split in 7:3 portions for PEGO and Pro-Gly linker construction. The larger portion of the resin was coupled with proline and glycine residues to assemble poly(Pro-Gly) linkers for compounds 1–5 (Fig. 2). Polyproline helix Type II (PPII), such as present in collagen, provide high pitch/residue ratio for designing a linker. Although optimal for spacing, the PPII helix is fairly rigid and a rigid linker may not provide enough flexibility to the connected ligands to bind simultaneously due to entropic reasons (Vagner et al. 2006). Additionally, the polyproline sequence is harder to synthesize on resin beyond 10 residues length without encountering inefficient coupling rates. Therefore, glycine was introduced at alternate positions that not only provided the adequate flexibility to the linker backbone, but also made the linker readily amenable to synthesis. In our experience, we were able to build a 36 residue long Pro-Gly linker with good yields (Vagner et al. 2008). Further, we found the linker to be quite hydrophilic as products were readily soluble in water.
The PEGO spacers were coupled to the smaller portion of the resin 7:3 split. PEGO is a highly flexible linker composed of a 20-atom long chain of polyethylene glycol units and a short glycolic acid spacer (Vagner et al. 2004). In modeling studies, we found that this linker could extend up to ca. 18 Å. The inset on top left in Fig. 2 describes the protocol for assembling PEGO on resin. The free Nα-termini of H-CCK-6-Resin were reacted with diglycolic anhydride to give a free carboxylate. The carboxylate groups were then activated with carbonyldiimidazole (CDI) in anhydrous DMF followed by nucleophilic condensation with PEG-diamine. High concentration of diamine and vigorous stirring were required to avoid cyclative dimerization of peptide on the resin. Further, the final purity of the PEGO coupling protocol was best when couplings were kept short (30 min each for last two steps). The protocol allows ready incorporation of shorter or longer, alkyl or pegylated chains.
Following addition of linkers (Pro-Gly or PEGO), the free amine terminals of all resins were coupled with Fmoc-Trp(tBoc)-OH and the peptide synthesis continued to complete the MSH-7 sequence. After final residue coupling, the Nα-termini were deprotected and then acetylated with an excess of 50% Ac2O in pyridine for 5 min. The final peptides were then cleaved with TFA cocktail for 4 hours. When Boc-protected tryptophan byproduct was observed (the product appeared as a peak prior to the product peak in RP-HPLC), the final crude product was treated with 1 M aqueous AcOH for 3 h. The crude compounds were purified by Gel-Filtration and RP-HPLC and lyophilized to yield white amorphous product. The compounds were then characterized by ESI-MS and/or MALDI-TOF and/or FT-ICR mass spectrometry (see Fig. 3 and Table 1). The purified ligands were dissolved in DMSO and the concentration was established by co-injection of sample and 0.5 mM solution of D-Trp in DMSO (as standard) in analytical HPLC and measuring the absorbance (area under peak) of compound against D-Trp at 280 nm (Fig. 3b).
Table 1.
HPLC & MS data of Ac-MSH(7)-NH2, Ac-CCK(6)-NH2 and heterobivalent ligands 1–7
| Compound | Mass calculated | Mass found | tR (purity %) | K′ |
|---|---|---|---|---|
| Ac-MSH(7)-NH2 | 1015.5035 (M + 1)1+ | 1015.4881a (M + 1)1+ | 17.0d (96) | 9.2 |
| Ac-CCK(6)-NH2 | 791.4014 (M + 1)1+ | 791.1a (M + 1)1+ | 15.4e | 7.7 |
| 1 (C107H145N27O25) | 1105.0452 (M + 2)2+ | 1105.2a (M + 2)2+ | 20.3f (99) | 11.9 |
| 2 (C128H175N33O31) | 1336.1566 (M + 2)2+ | 1336.3a (M + 2)2+ | 19.2f (100) | 11.3 |
| 3 (C149H205N39O37) | 1045.1786 (M + 3)3+ | 1045.1891b (M + 3)3+ | 18.3f (98) | 10.8 |
| 4 (C170H235N45O43) | 1199.2529 (M + 3)3+ | 1199.2559b (M + 3)3+ | 18.0f (99) | 10.6 |
| 5 (C191H265N51O49) | 4059.9942* (M + 1)1+ | 4060.5121c (M + 1)1+ | 17.7f (87) | 10.4 |
| 6 (C100H141N23O25) | 1033.0235 (M + 2)2+ | 1032.9a (M + 2)2+ | 21.5f (100) | 12.7 |
| 7 (C114H167N25O31) | 1192.1129 (M + 2)2+ | 1192.0a (M + 2)2+ | 21.2f (100) | 12.5 |
HPLC eluents: phase A (0.1% TFA in water); phase B (Acetonitrile); tR is the retention time of compound peak in HPLC; (purity of final product in percentage is given in parenthesis); K′ is retention time of compound peak/retention time of solvent peak
ESI-MS;
FT-ICR;
MALDI-MS;
10–40% B gradient in 30 min;
10–90% B gradient in 30 min;
20–60% B gradient in 50 min;
10–40% B gradient in 50 min
Mass of highest abundant isotope peak
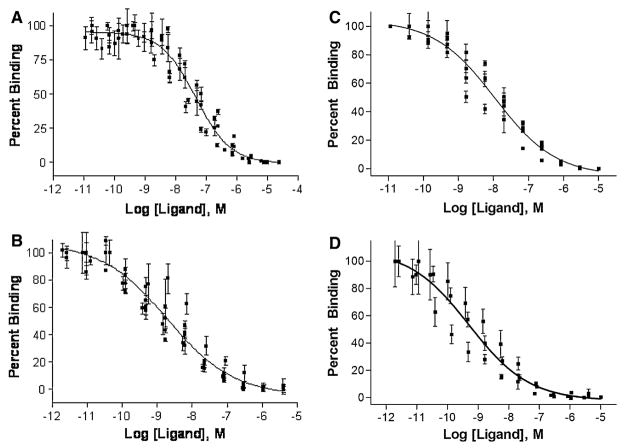
The heterobivalent constructs were evaluated for binding in Hek293 cells lines stably expressing only one of the complementary receptors (Hek293/hMC4R and Hek293/CCK-2R), which we termed as ‘mono-expressors’, compared to cells that expressed both receptors (Hek293/hMC4R/CCK-2R), which we termed as ‘di-expressors’ (Xu et al., unpublished) [see Ref. (Vagner et al. 2008) for assay procedures]. Ligand binding was evaluated via a competitive binding assay using lanthanide-based time-resolved fluorescence (TRF) as previously described (Handl and Gillies 2005; Handl et al. 2004b, 2005). Figure 4 shows representative binding curves for compound 2 and 6 for binding to cells mono-expressing CCK-2R and to cells di-expressing both hMC4R and CCK-2R. The relative enhancement in binding affinity to di-expressing vis-à-vis mono-expressing cells was expressed as a ratio of their respective IC50 values. For binding to CCK-2R, compound 2 exhibited an IC50 of 46 nM (40–52) against mono-expressing cells, and an IC50 of 2.3 nM (1.8–2.9) for binding to di-expressing cells. Thus, the bivalent construct 2 exhibited nearly 20-fold enhancement in binding affinity when targeted to cells that expressed both receptors as compared to cells that expressed only one of these. Similarly, a 22-fold enhancement in binding affinity was observed for compound 6. The results suggest that the compounds do so by non-covalent simultaneous cross-linking of heterologous cell-surface receptors via an apparent co-operativity effect. Notably, this enhancement was observed at the CCK-2R and little enhancement was observed for the hMC4R. We attribute this phenomenon to abundance (or expression ratio) of two receptors, where the binding enhancement is detectable only on the lower abundant receptor. This hypothesis was validated by switching the ratio of the two receptors and using a different co-expression system and the details have been discussed elsewhere (Vagner et al. 2008; Josan et al., unpublished). Nevertheless, enhancement at even one receptor yields an overall gain in affinity from multivalent constructs when compared to monovalent ligands, and this will yield increased binding to cells that express both complementary receptors.
Fig. 4.
Representative curves from the competitive binding assay of heterobivalent ligands evaluated for their monovalent and bivalent binding by competing them against Eu-labeled NDP-α-MSH and CCK-8 ligands. Single plot IC50 values were determined where data from all n measurements were pooled first and non-linear regression analysis performed. (a) Binding of Ligand 2 competed with 0.1 nM Eu-CCK8 in Hek293/CCK cells, with an IC50 of 46 nM (R2 = 0.90). (b) Binding of Ligand 2 competed with 0.1 nM Eu-CCK8 in Hek293/MC4R/CCK cells, with an IC50 of 2.3 nM (R2 = 0.89). (c) Binding of Ligand 6 competed with 0.1 nM Eu-CCK8 in Hek293/CCK cells, with an IC50 of 11 nM (R2 = 0.93). (d) Binding of Ligand 6 competed with 0.1 nM Eu-CCK8 in Hek293/MC4R/CCK cells, with an IC50 of 0.5 nM (R2 = 0.83)
Conclusion
The goal of this work was to develop a simple and practical, yet flexible and scalable approach to build heterobivalent ligands using solid-phase chemistry. In this report, we described a modular synthetic strategy for heterobivalent ligands where the first ligand was assembled on the resin, the appropriate linker built and then the second ligand assembled. Due to the modular nature, this strategy can be easily adapted to functionalize the multivalent constructs with fluorescent labels or other functional agents at any step in synthesis. Two linker types—a highly flexible PEGO and a semi-rigid Pro-Gly linker were used in the synthesis that displayed excellent compatibility with the solid-phase chemistry and provided a systematic tool to control hydrophilicity, length and rigidity of the linkage. The heterobivalent ligands were evaluated for cell-surface targeting by simultaneous binding of multiple (heterologous) cell-surface receptors and were shown to have higher affinity for cells expressing both complementary receptors compared to cells that expressed only one. It is our contention that a high degree of specificity can be achieved in the context of this heterobivalent affinity enhancement. We envision a novel targeting approach using combination of cell-surface receptors (receptor combination approach) and cross-linking them with heteromultivalent ligands. In the near future when a patient tissue expression profile could be available, it may become possible to tailor heteromultivalent ligands to a combination of receptors using these expressed patterns. Thus, a high degree of specificity in multivalent targeting coupled with the ability to identify unique receptor combinations will provide a novel and revolutionary platform technology with which to direct therapeutics to defined cell populations such as tumor tissue.
Acknowledgments
We thank Ms. Lucinda Begay and Mrs. Renata Patek for HPLC and technical assistance. This work was supported by grants R01 CA 123547 and RO1 CA097360 from the National Cancer Institute and Grant ABRC06-006 from the Arizona Biomedical Research Commission.
Abbreviations
- GPCR
G-protein coupled receptor
- hMC4R
Human melanocortin-4 receptor
- CCK-2R
Cholecystokinin-2 receptor
- α-MSH
α-Melanocortin stimulating hormone
- MSH-7
Ac-Ser-Nle-Glu-His-DPhe-Arg-Trp-
- CCK-6
-Nle-Gly-Trp-Nle-Asp-Phe-NH2
- PEGO
19-Amino-5-oxo-3,10,13,16-tetraoxa-6-azanonadecan-1-oic acid
- Fmoc
9-Fluorenylmethyloxycarbonyl
- Boc
Tert-butyloxycarbonyl
- HOBt
1-Hydroxybenzotriazole
- HOCt
6-Chloro-1-hydroxybenzotriazole
- HBTU
o-[1H-Benzotriazol-1-yl)(dimethylamino)methylene]uranium hexafluorophosphate N-oxide
- DIC
Diisopropylcarbodiimide
- DIEA
Diisopropylethylamine
- Pbf
2,2,4,6,7-Pentamethyldihydrobenzofuran-5-yl sulfonyl
- Trt
Triphenylmethyl (trityl)
- TFA
Trifluoroacetic acid
- MALDI-TOF
Matrix assisted laser desorption ionization-time of flight
- ESI-MS
Electrospray ionization-mass spectrometry
- FT-ICR
Fourier transform-ion cyclotron resonance
- Hek-293 cells
Human embryonic kidney cells
- TRF
Time-resolved fluorescence
References
- Caplan MR, Rosca EV. Targeting drugs to combinations of receptors: a modeling analysis of potential specificity. Ann Biomed Eng. 2005;33:1113–1124. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]
- Fourmy D, Escrieut C, Archer E, et al. Structure of cholecystokinin receptor binding sites and mechanism of activation/inactivation by agonists/antagonists. Pharmacol Toxicol. 2002;91:313–320. doi: 10.1034/j.1600-0773.2002.910608.x. [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Hruby VJ. Expression-driven reverse engineering of targeted imaging and therapeutic agents. Expert Opin Ther Targets. 2003;7:137–139. doi: 10.1517/14728222.7.2.137. [DOI] [PubMed] [Google Scholar]
- Handl H, Gillies RJ. Lanthanide-based luminescent assays for ligand-receptor interactions. Life Sci. 2005;77:361–371. doi: 10.1016/j.lfs.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Handl HL, Vagner J, Han HY, Mash E, Hruby VJ, Gillies RJ. Hitting multiple targets with multimeric ligands. Expert Opin Ther Targets. 2004a;8:565–586. doi: 10.1517/14728222.8.6.565. [DOI] [PubMed] [Google Scholar]
- Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Lanthanide-based time-resolved fluorescence of in cyto ligand-receptor interactions. Anal Biochem. 2004b;330:242–250. doi: 10.1016/j.ab.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Development of a lanthanide-based assay for detection of receptor-ligand interactions at the δ-opioid receptor. Anal Biochem. 2005;343:299–307. doi: 10.1016/j.ab.2005.05.040. [DOI] [PubMed] [Google Scholar]
- Handl HL, Sankaranarayanan R, Josan JS, et al. Synthesis and evaluation of bivalent NDP-α-MSH(7) peptide ligands for binding to the human melanocortin receptor 4 (hMC4R) Bioconjug Chem. 2007;18:1101–1109. doi: 10.1021/bc0603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick CL, Karush F. Antibody affinity-III. The role of multivalence. Immunochemistry. 1972;9:325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Krchnak V, Vagner J, Lebl M. Noninvasive continuous monitoring of solid-phase peptide synthesis by acid-base indicator. Int J Pept Protein Res. 1988;32:415–416. doi: 10.1111/j.1399-3011.1988.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Monguchi Y, Vagner J, Handl HL, et al. Design, synthesis, and validation of rigid linkers for bioactive peptides. Tet Lett. 2005;46:7589–7592. [Google Scholar]
- Sharma SD, Granberry ME, Jiang J, Leong SDL, Hadley ME, Hruby VJ. Multivalent melanotropic peptide and fluorescent macromolecular conjugates: new reagents for characterization of melanotropin receptors. Bioconjug Chem. 1994;5:591–601. doi: 10.1021/bc00030a015. [DOI] [PubMed] [Google Scholar]
- Sharma SD, Jiang J, Hadley ME, Bentley DL, Hruby VJ. Melanotropic peptide-conjugated beads for microscopic visualization and characterization of melanoma melanotropin receptors. Proc Nat Acad Sci USA. 1996;93:13715–13720. doi: 10.1073/pnas.93.24.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner J, Handl HL, Gillies RJ, Hruby VJ. Novel targeting strategy based on multimeric ligands for drug delivery and molecular imaging: homooligomers of α-MSH. Bioorg Med Chem Lett. 2004;2004:211–215. doi: 10.1016/j.bmcl.2003.09.079. [DOI] [PubMed] [Google Scholar]
- Vagner J, Handl HL, Monguchi Y, et al. Rigid linkers for bioactive peptides. Bioconjug Chem. 2006;17:1545–1550. doi: 10.1021/bc060154p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner J, Xu L, Handl HL, et al. Heterobivalent ligands crosslink multiple cell-surface receptors-the human melanocortin-4 and δ-opiod receptors. Angew Chem Int Ed. 2008;47:1685–1688. doi: 10.1002/anie.200702770. [DOI] [PMC free article] [PubMed] [Google Scholar]