Abstract
Frogs are characterized by a unique morphology associated with their saltatory lifestyle. Although variation in the form and function of the pelvic girdle and associated appendicular system related to specialized locomotor modes such as swimming or burrowing has been documented, the forelimbs have typically been viewed as relatively unspecialized. Yet, previous authors have noted versatility in forelimb function among arboreal frogs associated with feeding. Here we study the morphology and function of the forelimb and hand during locomotion in two species of arboreal frogs (Litoria caerulea and Phyllomedusa bicolor). Our data show a complex arrangement of the distal forelimb and hand musculature with some notable differences between species. Analyses of high-speed video and video fluoroscopy recordings show that forelimbs are used in alternating fashion in a diagonal sequence footfall pattern and that the position of the hand is adjusted when walking on substrates of different diameters. Electromyographic recordings show that the flexors of the hand are active during substrate contact, suggesting the use of gripping to generate a stabilizing torque. Measurements of grasping forces in vivo and during stimulation experiments show that both species, are capable of executing a so-called power grip but also indicates marked differences between species, in the magnitude of forces generated. Stimulation experiments showed an increased control of digit flexion in the more specialized of the two species, allowing it to execute a precision grip paralleled only by that seen in primates.
Keywords: arboreal frogs, grasping, muscle morphology
Introduction
Frogs are characterized by a specialized morphology including a shortened trunk and tail, elongated ilia, and elongated hind limbs, all traits thought to be associated with their saltatory mode of life (Gans & Parsons, 1966; Lutz & Rome, 1994; Shubin & Jenkins, 1995). This morphology was already present in the earliest fossils assigned to the Anura (Shubin & Jenkins, 1995; Jenkins & Shubin, 1998). Despite this common body plan, diverse lifestyles have evolved among frogs including specialist aquatic, fossorial and arboreal species characterized by unique modes of locomotion (Duellman & Trueb, 1986; Frost et al. 2006). Pipid frogs, for example, are highly specialized aquatic frogs characterized by a sliding pelvis thought to enhance their swimming capacity (Videler & Jorna, 1985). Pseudis and Lysapsus, aquatic hylids frogs, have ilio-sacral specializations related to their floating behaviour at the water surface (Manzano & Barg, 2005). Many burrowers, by contrast, show specializations of the pelvic girdle and hind-limbs thought to improve their burrowing ability (Emerson, 1976).
In contrast to the hindlimbs, the forelimbs are generally considered to be conserved among frogs. Their main function is thought to be associated with providing body support during sitting or walking, and/or the absorption of impact forces during landing (Nauwelaerts & Aerts, 2006). Frog forelimbs are typically short as the hind limbs are the principal limb pair generating propulsion. Moreover, while at rest most of the body weight is also displaced towards the hind limbs in frogs. However, distinct sexual dimorphism in forelimb length has been noted and is thought to be related to the ability of males to hold on to females during amplexus (Emerson, 1991). The pectoral girdle is also relatively unspecialized, although two structurally different types have been noted (Havelková & Roček, 2006).
One major exception to the relative lack of specialization among frog forelimbs is found in arboreal frogs. Arboreal frogs often have relatively long forelimbs that are capable of considerable dexterity during feeding (Gray et al. 1997). For example, although the use of the hands during feeding is not unusual among frogs, many arboreal frogs use their hands to manipulate food and even bring food to the mouth using complex rotations at the wrist. Moreover, these complex behaviours arose independently at least three times in arboreal frogs (Gray et al. 1997).
Consequently, the ability to execute these complex movements was interpreted as an exaptation of the specialization of the forelimbs for arboreal locomotion (Gray et al. 1997).
Indeed, it can be expected that for arboreal frogs to move across narrow substrates they not only need to move their arms independently from one another (in contrast to typical bilaterally simultaneous movements during landing or hopping), but will also need to be able to close the hand (i.e. execute a power grip sensuNapier 1956) to generate a balancing torque. Increased flexion capacity of the manus and increased mobility at the wrist seem to be important features as these allow closure of the hand around the substrate (Cartmill, 1985; Isler, 2005). Generating a balancing torque is probably crucial when moving on substrates equal to or narrower than the width of the body to counteract the moment of force induced by lateral displacements of the centre of mass during locomotion (Cartmill, 1985; Sargis, 2001; Schmitt & Lemelin, 2004). Indeed, the evolution of grasping is often thought to be associated with specialized arboreal habits in ancestral or early primates (Napier, 1967; Martin, 1990; Sargis, 2001; Bloch & Boyer, 2002).
Unfortunately, little is known about the morphology and function of the forelimbs in frogs with the exception of studies investigating the role thereof during landing (Nauwelaerts & Aerts, 2006), the morphology of the intercalary elements (Manzano et al. 2007), and the mechanism of attachment and detachment of the toe pads in arboreal frogs (Hanna & Barnes, 1991). Thus, we decided to examine the morphology and function of the forelimb during locomotion to understand better the origin of the increased mobility of the hand and wrist observed in arboreal frogs (Gray et al. 1997). We selected two species, one a more generalized arboreal frog, Litoria caerulea, and the other a representative of highly specialized arboreal frogs well known for their slow but precise limb movements (Phyllomedusa). Phyllomedusine frogs are particularly interesting to study as an unusual degree of dexterity was previously described (Blaylock et al. 1976), as these frogs use their hands and feet to distribute serous substances over their bodies. Specifically, we study the detailed anatomy of the forelimb and hand muscles, quantify how the forelimbs and hands are used while walking on a narrow substrate, investigate the muscle activity patterns during locomotion, quantify grasping performance, and explore potential for muscular control of the digits using stimulation experiments. Based on the known dexterity of Phyllomedusa we predicted anatomical differences that would also be reflected in grasping ability and movement patterns during locomotion in these frogs.
Materials and methods
Animals
Three adult Litoria caerulea (snout–vent length, SVL=69.7±2.2mm) and one adult Phyllomedusa bicolor (SVL=105.7mm) obtained through the pet trade were used in the experiments. Animals were kept in separate terraria with dense vegetation and were misted daily. Animals were fed ad libitum and were maintained in a climate-controlled room at 25°C. Before the experiments, all specimens were weighed and the dimensions of the body (SVL), head, forelimbs and hind-limbs were determined using digital calipers (Mitutoyo CD-30C and CD-15B; ±0.01mm). One adult preserved specimen of Phyllomedusa bicolor, three adult P. sauvagii and two adults L. caerulea were used for morphological analysis. Specimens of P. sauvagii are deposited in the herpetological collections of Fundacion Miguel Lillo – Tucumán, Argentina (FML04899, two specimens) and CICyTTP-CONICET-Entre Ríos, Argentina (DIAM 0337, one specimen). Specimens of L. caerulea and P. bicolor are deposited in the personal collection of A. Herrel, and one specimen of L. caerulea in CICyTTP-CONICET-Entre Ríos, Argentina (DIAM 0313).
High-speed video recordings
Animals were filmed in lateral view walking on a narrow dowel (17mm) using a Redlake MotionPro 500 camera set at 100 frames s−1. At least five walking sequences were recorded and analysed for each individual. Videos were reviewed in a Midas player (version 2.1.5; Xcitex Inc.) and contact times and durations were recorded. Qualitative descriptions of the placement of the hand onto the substrate were made based on these videos as well. Additionally, we recorded the number of times an animal lost balance while moving across the narrow dowel.
X-ray recordings and electromyography
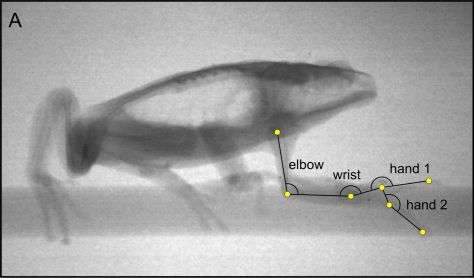
Before X-ray recordings were made, animals were anaesthetized using a buffered MS222 solution, and small metal markers were inserted subcutaneously at the proximal and distal ends of the humerus, at the proximal and distal ends of the radius, at the base of the carpals, at the base of the phalanges and at the last phalanx of digit II. Markers were implanted in the muscle tissue close to the bone using hypodermic needles and marker placement was checked on X-rays. Animals were filmed in lateral view while moving on a narrow dowel (17mm). X-rays were generated using a Philips optimus M200 X-ray generator and recorded using a Philips image intensifier with a Redlake MotionPro2000 camera attached. At least five trials were recorded for each animal. The following points were digitized using Didge (version 2.2.0.; A. Cullum) for the frame where the hand was in full contact with the substrate (mid stance) and the frame just before release of the substrate (toe-off) for all steps recorded in each sequence: the shoulder, the elbow, the wrist, the base of digits 3 and 4, the tip of digits 3 and 4, and the tip of the snout. Based on these points, the elbow, wrist and hand angles were calculated as well as the average velocity of movement (Fig. 1).
Fig. 1.
Image from a high-speed X-ray recording of Phyllomedusa bicolor walking on a narrow substrate. Indicated are the points digitized and the angles used to describe differences in forelimb movement during locomotion.
Bipolar Ni–Cr twisted hook electrodes were inserted percutaneously into the following muscles in P. bicolor, m. flexor proprius digiti II, m. palmaris profundus, m. flexor digitorum communis longus, m. biceps brachii, m. extensor digitorum communis longus, m. abductor indicis longus and the m. triceps brachii. For L. caerulea, electrodes were inserted in the same muscles with the exception of the m. palmaris profundus. Signals were amplified 10000 times using Gould Universal pre-amplifiers with notch filter and Honeywell Accudata 117DC amplifiers. Signals were recorded digitally on tape using a TEAC 145T DAT recorder. To allow synchronization between the X-ray video recordings and muscle activity patterns, a synchronization signal from the X-ray generator was recorded on tape. Data were transferred digitally to a PC using the TEAC QuickVu software, and the onset and duration of the muscular activity relative to substrate contact was quantified in Microsoft Excel.
Grasping force
Grasping forces were measured using a Kistler Squirrel force platform. A glass dowel was mounted on the force plate and animals were allowed to grasp the dowel with both hands. Next, animals were pulled off the dowel at constant speed at an angle of 45° to the horizontal. Three trials including at least three pull-offs each were recorded for every animal. Trials were analysed using the Kistler Bioware software and peak forces in the x, y and z direction as well as the resultant forces were extracted. The peak grip (resultant force, including friction generated by the adhesive pads) and grasp (vertical component only) forces were recorded for each individual and species means were calculated.
Kinematic analyses
All variables were log10 transformed before analyses, and normality and homoscedasticity were tested with Shapiro–Wilks and Levene's tests, respectively (Sokal & Rolph, 1995). First, we tested for differences in the velocity of movement between species. As movement velocity was not significantly different between species (F1,0.96=1.21; P=0.48), we did not use velocity as a covariate in our analysis. Next, nested analyses of variance, with individual assigned as random factor and nested within species, were used to test for differences in kinematics between species and contact time. Non-significant interaction effects were removed from the analysis.
Stimulation experiment
Bipolar Ni–Cr twisted electrodes were inserted in the following muscles in P. bicolor: the m. flexor digitorum communis longus, the m. flexor carpi radialis, the m. epitrochleocubitalis, the m. flexor proprius digiti II, the m. lumbricalis longus digit IV, and the m. palmaris profundus. In L. caerulea, electrodes were inserted into the same muscles with the exception of the m. epitrochleocubitalis. Stimulations were performed on one P. bicolor and two L. caerulea. Animals were brought under deep anaesthesia using ketamine (225mgkg−1 body mass) and the muscles of the right forelimb were exposed. Electrodes were inserted in the middle of the respective muscle bellies and connected to a stimulator (Grass S48). The stimulation circuit was charge balanced by a coupling capacitor and bleed resistor (Loeb & Gans, 1986) to avoid muscle damage and undue fatigue.
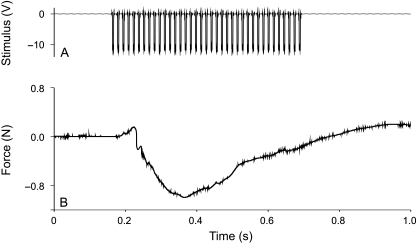
Muscles were stimulated at 12V with a pulse train of 500ms at 70Hz, and 3-ms pulse duration. Stimulation voltage was gradually increased from 5V upwards until no further increase in wrist flexion could be observed. Animals were positioned on their back on a custom-made platform and the lower arm was immobilized to allow visualization of movements at the wrist and hand. Animals were filmed in combined ventral and lateral view using a mirror positioned at an angle of 45° to the horizontal at the level of the arm. Muscles were stimulated one by one and movements were recorded. Next, combined stimulations were performed to understand the consequences of co-activation of the different muscles. Finally, the hand of the animal was positioned around two custom-made semicircular plates attached to a Kistler force transducer (type 9207, ±5N) and portable charge amplifier (type 5995). All muscles were stimulated at once, and both the stimulus and the corresponding grasping forces were recorded digitally on tape using a TEAC DAT recorder (Fig. 2). Three to five trials were performed for each individual and the maximal medially directed force per individual was retained and a species average was calculated. Forces were multiplied by two to allow for a comparison with the forces exerted using both hands in the in vivo trials using the force plate. At the end of the experiments, animals were killed via an overdose of ketamine (400mgkg−1 body mass).
Fig. 2.
Representative traces of a stimulation experiment in Phyllomedusa bicolor. Depicted are the 12-V stimulus train (A) and resulting grasping force measured using a force transducer (B).
All experiments were approved by the animal ethics committee at the University of Antwerp.
Results
Morphology
In the following descriptions of the muscles we follow the terminology of Gaupp (1896) unless otherwise noted, and for bones we follow Fabrezi (1992) and Fabrezi & Alberch (1996). Below, we describe those muscles specifically relevant to hand flexion in addition to those used during electromyographic and stimulation experiments.
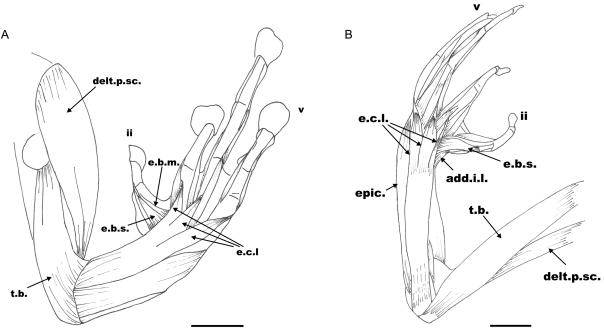
Extensor digitorum communis longus (e.c.l. Fig. 3A,B): This is a superficial, long, broad muscle that covers the dorsal surface of the radio-ulna. It originates on the distal condyle of the humerus and inserts on digits III, IV and V. Distally it divides into three branches, the lateral one inserting on the lateral base of metacarpus V by a short tendon, the central branch inserting on the middle of metacarpus IV by a tendon and the medial one inserting on the medial base of digit III by a short tendon. No differences related to this muscle were observed between the three species.
Fig. 3.
Dorsal view of the hand showing the extensor musculature: (A) Litoria caerulea, right hand. (B) Phyllomedusa sauvagii, left hand. Abbreviations: e.c.l., m. extensor communis longus; e.b.s., m. extensor brevis superficialis; e.b.m., m. extensor brevis medius; delt.p.sc., m. deltoideus pars scapularis; t.b., m. triceps brachii; add.i.l., m. adductor indicis longus; epic., m. epicondylo-cubitalis. Scale bars=5mm.
Extensor indicis brevis superficialis (e.b.s. Fig. 3A,B): This is one of the three branches of the m. extensor brevis superficialis that, in L. caerulea, originates on the ulnar side of the distal epicondyle of the radio-ulna and extends obliquely onto the dorsal face of the carpals. It inserts on the dorsum of metacarpal II and continues with a tendinous fascia to the metacarpal–phalangeal joint. In P. sauvagii it originates on the dorsum of the radiale and extends over almost the entire dorsal surface of digit II. Is a triangular and broad muscle, larger than in L. caerulea, which inserts on the metacarpal–phalangeal joint by a tendon. It is partially covered by the long and triangular m. abductor indicis longus that inserts on the dorsal face of the first phalanx by means of a wide and broad tendon.
Deltoideus (delt. Figs 3A,B and 4B): In L. caerulea this is a broad and long muscle that covers the entire ventro-lateral surface of the humerus. It has three branches that join on the proximal condyle of the humerus: a pars episternalis arising from the base of the omosternum; a pars clavicularis arising from the proximal extreme of the epicoracoid cartilage; and a pars scapularis (delt.p.sc. Fig. 3A,B) arising from the proximal scapulo-clavicular joint. It inserts on the distal extreme of the humerus. In P. sauvagii the muscle covers the deltoid crest and inserts on the ventro-lateral face of the proximal half of the humerus.
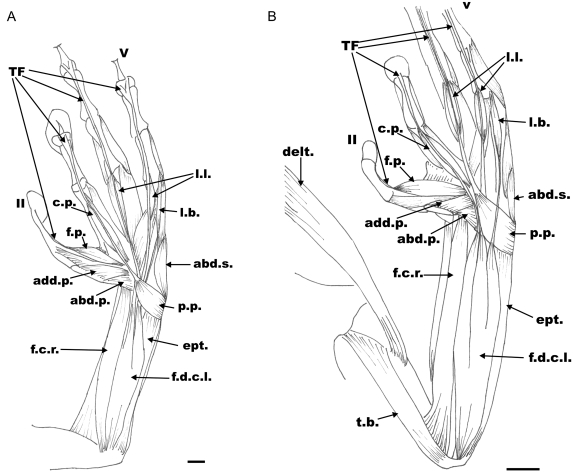
Fig. 4.
Ventral view of the hand showing the flexor musculature. (A) Litoria caerulea, left hand. Scale bar=1mm. (B) Phyllomedusa sauvagii, left hand. Scale bar=5mm. Abbreviations: f.d.c.l., m. flexor digitorum communis longus; ept., m. epitrochleocubitalis; p.p., m. palmaris profundus; abd.s., abductor secundus digiti V; l.b., m. lumbricalis brevis; l.l., m. lumbricalis longus; c.p., caput profundus digiti III; f.p., m. flexor indicis superficialis proprius digiti II; f.c.r., m. flexor carpis radialis; T.F., main flexor tendons; delt., m. deltoideus; t.b., m. triceps brachii.
Triceps brachii (anconeus sensuGaupp, 1896) (t.b. Figs 3A,B and 4B): In L. caerulea and P. sauvagii, this is a broad and bulky muscle that covers the entire ventro-lateral and dorso-lateral surfaces of the humerus. It has three branches: a ventral branch originating on the ventro-lateral base of the proximal condyle of the humerus and continuing to give rise to the elbow aponuerosis; a dorsal branch arising from the dorso-lateral base of the proximal condyle of the humerus and merging with the elbow aponeurosis; and a lateral branch arising by a short and broad tendon, from the proximal and posterior border of the scapula. It extends on the lateral surface of the humerus, covering part of the other two branches.
Flexor digitorum communis longus (sensuEcker, 1889) (f.d.c.l. Fig. 4A,B): In P. bicolor, this is a bulky and superficially positioned muscle located at the centre of the antebrachium. The muscle arises by a wide and short tendon from the aponeurosis covering the elbow. Distally the muscle splits into three branches, the medial, central and lateral branches, each one continuing with a strong and superficial tendon that insert on the last phalanx of digits III, IV and V. The tendon of origin of the m. lumbricalis brevis V arises from the tendon of the lateral branch of the m. flexor digitorum communis longus. At the level of the manus the three flexor tendons are joined by a tendinous fascia that arises from the m. palmaris profundus and the m. flexor digitorum communis longus. In L. caerulea and P. sauvagii the medial branch gives origin to the fifth tendon, the central branch to the fourth tendon, and the lateral one merges distally with a short fascia that provides the origin for the third tendon and the tendon of origin of m. lumbricalis brevis V.
Palmaris profundus (p.p. Fig. 4A,B): In P. bicolor and L. caerulea this is a short, rectangular and superficial muscle that runs transversely on the ventral face of the manus. It originates from the latero-distal edge of the ulnar side of the radio-ulna and joins the superficial tendons III, IV and V by a tendinous fascia. In Litoria, the muscle covers the tendon of the m. lumbricalis brevis V and is joined to it by connective tissue. In P. sauvagii this is a broad muscle that inserts on the superficial tendon IV and is joined to the other tendons by a small fascia. In this species it is, however, not related to the tendon of the m. lumbricalis brevis V.
Flexor capi radialis (f.c.r. Fig. 4A,B): This is a bulky, subtriangular, and superficial muscle located on the radial side of the antebrachium, covering the m. flexor antebrachii caput superior. It arises from the distal half of the humerus and inserts fleshy on the medial side of the radiale, and by a tendon on element Y. No differences related to this muscle have been observed among the three species studied.
Epitrochleocubitalis (ept. Fig. 4A,B): In P. bicolor this is a bulky and wide muscle that originates from the distal head of the humerus and inserts fleshy along the ventral face of the ulnar side of the radio-ulna, and by a short and broad tendon on the transverse crest of the distal carpal 5-4-3. In L. caerulea the distal tendon inserts on the ulnar side of distal condyle of the radio-ulna and in P. sauvagii it inserts on the ulnar side of the distal condyle of the radio-ulna and at the base of the ulnare.
Adductor pollicis (add.p. Fig. 4A,B): A short, wide, subtriangular muscle that arises from the medial border of the distal carpal 5-4-3 by a short tendon. It inserts along the medial border of the prepollex elements. No differences related to this muscle have been found between the three species analysed.
Abductor pollicis (abd.p. Fig. 4A,B): This has two branches that arise from the medial border of the ulnare. Both branches are broad and triangular and insert at the base of the prepollex close each other. In L. caerulea the origin of both branches is tendinous.
Flexor indicis superficialis proprius II (f.p. Fig. 4A,B): A broad muscle that covers the ventral face of metacarpus II, originated fleshy on medial border of the distal carpal 5-4-3 and inserts by TS II, at the base of the last phalanx. It is located superficially between digits II and III. No differences related to this muscle have been found between the three species analysed.
Tendo superficialis (superficial tendon) and caput profundum III (TF and c.p. Fig. 4A,B): This is a complex system formed by the superficial tendon of digit III and the muscle caput profundum that joint together at the level of the distal half of metacarpal III. The muscle arises fleshy from the transversal crest of distal carpals 5-4-3, close to the tendon of the m. lumbricalis brevis III. It is a bulky and superficial muscle located close to the m. lumbricalis brevis III, which inserts on the superficial tendon III. The superficial tendon III arises from the m. flexor digitorum communis longus and joins the m. caput profundum on it distal half, inserting at the base of the last phalanx. No differences related to this muscle–tendon complex have been found between the three species analysed.
Lumbricalis longus IV (l.l. Fig. 4A,B): This is a complex muscle with two sets of short branches, two medial and two external branches. The medial branches are thin and short, and originate on the superficial tendon IV at the level of the proximal half of metacarpal IV by means of two short tendons parallel to the superficial tendon. Both branches extend in parallel along the distal half of the metacarpus and distally join the distal extremity of metacarpal IV. The external branches originate with the internal ones on the superficial tendon IV by the same tendons. They run in parallel between the superficial tendon and the medial branch, continuing forward by means of two long tendons. These tendons run in parallel to the superficial tendon and insert on the distal third of the subterminal (penultimate) phalanx. The external branches insert on both sides of the distal extreme of metacarpal IV. In L. caerulea the muscle is single but continues forward via two tendons similar to the medial branch described above.
Movement patterns
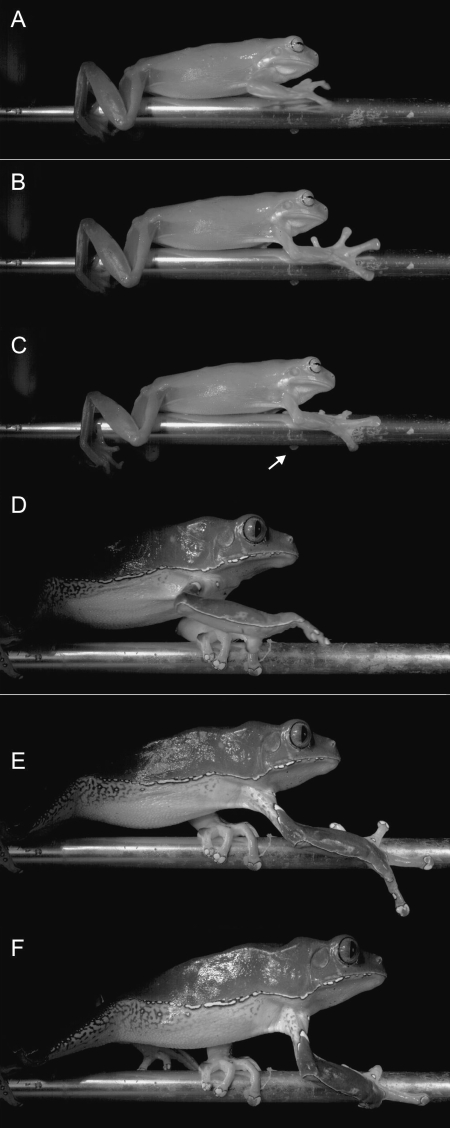
Our analysis of the high-speed video recordings indicates that the overall forelimb movement pattern is very similar in the two species (Fig. 5). During the swing phase the digits are flexed and digit 2 is adducted while the elbow is flexed and the humerus protracted. Close to substrate contact the elbow and wrist are extended, and the fingers extended and spread fully. During substrate contact, the fingers are flexed around the dowel and the wrist and elbow are flexed during stance. One notable difference that can be observed between species is the degree to which they can close the hand around the dowel. Whereas in P. bicolor closure is typically complete, in L. caerulea, the terminal phalanx of the third or fourth digits of the contralateral hand is not flexed and remains visible in lateral view (Fig. 5).
Fig. 5.
Selected images from high-speed video recordings (100frames per second) of walking on a narrow substrate in Litoria caerulea (A–C) and Phyllomedusa bicolor (D–F). Note the flexion of the hand and adduction of digit 2 during the swing phase (A, D) and extension and abduction of the digits right before substrate contact (B, E) in both species. During substrate contact, however, P. bicolor is able to close its fingers more completely and actively flexes the last phalanx of each digit; L. caerulea, by contrast, cannot fully flex the last phalanges (arrow) when grasping the substrate.
One other striking difference between the two species was that whereas P. bicolor, despite its larger size, never lost balance or stumbled when walking across the narrowest substrate, L. caerulea does. In slightly over half of the trials (53.85%) L. caerulea lost balance or stumbled when walking across the same substrate. Our analysis of the step parameters indicates that this may be due to the longer contact time observed in P. bicolor (1.19±0.46s) compared with L. caerulea (0.68±0.41s). Limb movements in general are about twice as slow in P. bicolor as in L. caerulea moving across the same substrate, as indicated by the swing phase duration (0.53±0.12 vs. 0.29±0.21s).
An analysis of the elbow angle showed no significant species (F1,0.93=0.1; P=0.81), contact time (F1,85=0.81; P=0.37) or interaction effects (F1,84=3.93; P=0.05). Wrist angle, by contrast, showed significant interaction effects (F1,84=11.43; P=0.001). Differences in wrist angle during the different contact phases were also significant (F1,84=10.54; P=0.002) with angles during mid-stance being greater (i.e. wrist more extended than during toe-off). Species were different in wrist angle only during toe-off (F1,46=37.54; P<0.001), with L. caerulea having greater angles and thus a more extended wrist than P. bicolor. Wrist angle was, however, not significantly different during mid-stance (F1,39=0.84; P=0.37). Hand angle 1 was not different between species (F1,0.68=0.64; P=0.62), or contact phase (F1,85=1.04; P=0.31) and also showed no significant interaction effects (F1,84=0.87; P=0.36). Hand angle 2 showed significant interaction (F1,84=4.10; P=0.04) and contact phase (F1,84=3.98; P=0.049) effects, with angles being smaller (i.e. the toe being more adducted) during toe-off. Species were different only during mid-stance (F1,39=11.86; P=0.001), with P. bicolor displaying greater angles than L. caerulea, but not during toe-off (F1,46=0.99; P=0.33).
Muscle activity patterns
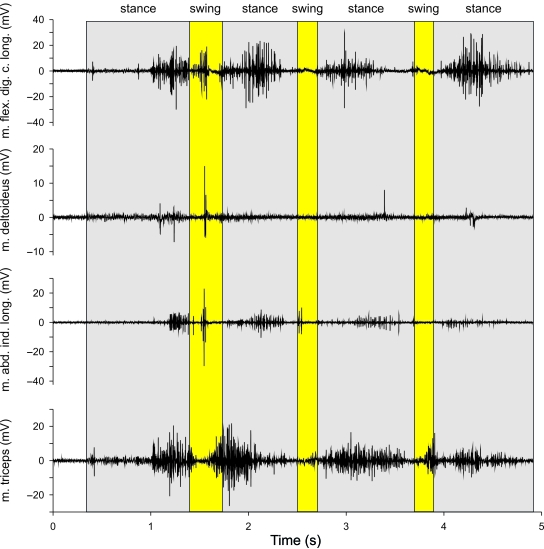
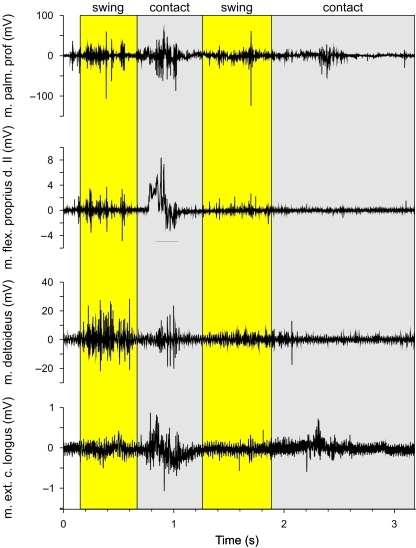
Our electromyographic recordings show that the flexors of the hand are active during substrate contact in both L. caerulea (m. flexor digitorum communis longus; Fig. 6) and P. bicolor (combined activity of m. flexor digitorum communis longus, m. palmaris profundus and m. flexor indicis superficialis proprius II; Fig. 7). The onset of activity of the m. flexor digitorum communis longus was 50ms after the onset of contact on average, and remained active for an average of 500ms in L. caerulea. In P. bicolor, the m. palmaris profundus was active on average for 400ms following initial substrate contact. Interestingly, the muscle also showed distinct activity during swing, coinciding with a flexion of the fingers (Fig. 5). Unfortunately, the electromyographic signal of the m. flexor digitorum communis longus in P. bicolor was too noisy to quantify its activity. The m. flexor i. s. proprius II (m. flexor indicis superficialis proprius II) was active for 200ms on average during stance and during the entire swing phase, causing adduction of digit 2 (Fig. 5). These data suggest that in both species the hand is actively flexed after being positioned onto the substrate. Two to 300ms before the onset of the swing phase, the flexor muscles cease their activity to allow extension of the hand in preparation for the swing phase in both species. This is corroborated by the late onset of the m. abductor indicis longus during late stance and early swing in L. caerulea (Fig. 7).
Fig. 6.
Representative electromyographic traces of selected forelimb muscles in Litoria caerulea. Grey bars represent the ipsilateral contact phase; yellow bars represent the swing phase. Note how the flexor becomes active slightly after substrate contact, suggesting that the hand is first put down and subsequently flexed. Also note how the triceps (elbow extensor) is active during the contact phase but may also show activity during the swing phase as seen in the last step.
Fig. 7.
Representative electromyographic traces of selected forelimb muscles in Phyllomedusa bicolor. Grey bars represent the ipsilateral contact phase; yellow bars represent the swing phase. Note the activity of the m. palmaris profundus, important in flexing the hand and adducting the fingers during the contact phase. The m. deltoideus and the m. flexor i. s. proprius, on the other hand, show the greatest activity during the swing phase, suggesting flexion at the elbow.
Activities of other muscles studied were more variable. In general, the m. triceps brachii was active during stance in L. caerulea, although occasionally a distinct activity burst was present during the swing phase (Fig. 6). Similarly, the wrist extensor (m. extensor digitorum communis longus) in P. bicolor showed a pronounced activity burst of variable duration during stance. The m. deltoideus in P. bicolor showed a pronounced activity during the swing phase but invariably showed a second activity burst during stance. In L. caerulea the activity of the m. deltoideus was variable, but again showed activity during both stance and swing phases.
Grasping forces
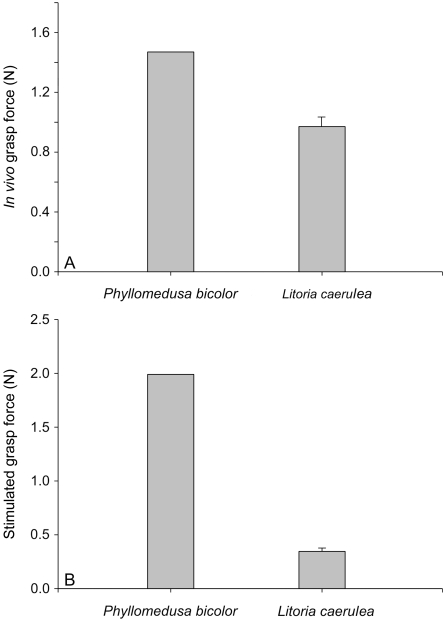
Whereas L. caerulea was able to generate 1.32±0.10 N of grasping force on average, the one P. bicolor for which measurements were obtained was able to generate 2.41 N of force (Fig. 8). This difference was significant (F1,2=47.82; P=0.02) but should be interpreted with some caution given that only a single individual of P. bicolor was measured. Maximal grasping forces obtained through stimulation of the forearm and hand flexors (Fig. 8) were somewhat lower for both P. bicolor (1.99N) and L. caerulea (0.79±0.30).
Fig. 8.
(A) Graph illustrating in vivo grasp forces in Phyllomedusa bicolor and Litoria caerulea. Note how forces are lower in L. caerulea than in Phyllomedusa bicolor.(B) Graph illustrating the maximal grasping forces obtained by electrical stimulation of the hand flexors. Both in vivo and stimulation data indicate that P. bicolor can generate higher grasp forces than L. caerulea. Bars represent average maximal grasp forces+one standard deviation.
Stimulation experiment
M. flexor digitorum communis longus: In L. caerulea, stimulation of the m. flexor digitorum communis longus causes flexion of the wrist to about 90° relative to the horizontal. Additionally, stimulation of this muscle causes flexion of the digits at all the different phalangeal joints. Stimulation of the same muscle in P. bicolor causes a similar degree of flexion at the wrist but resulted in flexion of the digits at the metacarpo-phalangeal joints only.
M. flexor carpi radialis: Stimulation of the m. flexor carpi radialis causes flexion of the wrist and a rotation of the hand towards the side of digit 2 (endorotation) in both species studied.
M. epitrochleocubitalis: The action of this muscle was investigated in P. bicolor only. Stimulation of the m. epitrochleocubitalis causes a rotation at the wrist towards the side of digit 5 (exorotation). When the lower arm is not stabilized relative to the substrate, stimulation of this muscle causes elbow flexion to an angle of about 90°. Little or no flexion of the wrist is observed upon stimulation of this muscle.
M. palmaris profundus: Stimulation of this muscle in L. caerulea causes an adduction of digit 5 and a slight but marked exorotation of the hand. In P. bicolor, however, stimulation of the m. palmaris profundus causes a displacement of the tendon of the m. flexor digitorum communis longus 2–3mm towards the side of digit 5.
M. lumbricalis longus digiti IV: Stimulation of the m. lumbricalis longus digiti IV causes complete flexion of digit 4 in both species.
M. flexor indicis superficialis proprius II: Stimulation of the m. flexor i.s. proprius II causes flexion of digit 2 in both species. In P. bicolor, a pronounced adduction of digit 2 is also observed upon stimulation of the muscle.
Combined stimulations: A combined stimulation of the m. flexor digitorum communis longus, m. palmaris profundus, m. lumbricalis of digit 4 and m. flexor i. s. proprius II of digit 2 results in a flexion of the wrist and closure of the hand in both species. Interestingly, stimulation of the m. lumbricalis of digit 4 and the m. flexor i. s. proprius II of digit 2 in P. bicolor results in a precision grip between digits 2 and 4. In L. caerulea the same stimulation results in flexion of digits 2 and 4 but the digits do not touch. A combined stimulation of the m. flexor digitorum communis longus and the m. palmaris profundus in P. bicolor resulted in a marked increase in the flexion at the wrist compared with a stimulation of the m. flexor digitorum communis longus by itself.
Discussion
Morphology of the forearm and hand
When comparing the anatomy of the forearm and hand of the species examined here with that observed for more generalized frogs (Gaupp, 1896; Burton, 1998), there appear to be some muscular characters related to the ability to climb, for example the elongation of the mm. extensores breves profundi and the presence of the mm. extensores breves distalis (Burton, 1998), and the intercalary element forming a complex system that appears to have evolved early in the history of frogs (Manzano et al. 2007). In some scansorial frogs, such as Eleutherodactylus, and in arboreal frogs such as most of the Hylids, Centrolenids, Rhacophorids and Hyperolids, a direct connection between the m. palmaris longus and the lateral tendo superficialis implies a reduction of the palmar aponeurosis that covers the hand musculature. This aponeurosis, which arises from the palmaris longus in most frogs (and even most vertebrates), gives origin to the superficial tendons of each digit. The species that we analysed have no aponeurosis on the palmar surface, and consequently the main flexor tendons arise directly from a muscle we consider to be the m. flexor digitorum communis longus. The main flexor tendons also show a close relationship with the m. palmaris profundus that joins these tendons by connective tissue and in Phyllomedusa species even attaches onto superficial tendon IV. In Litoria and Phyllomedusa species the m. lumbricalis brevis V originates with the superficial tendon III on the lateral branch of the flexor digitorum communis longus and only in Litoria is there a connection between this muscle and the m. palmaris profundus.
The hand musculature of the species of Litoria and Phyllomedusa examined here is very similar. There are, however, some peculiarities in Phyllomedusa: a general elongation and increase in the size of the muscles, the presence of strong and long tendons (like those of the m. extensor brevis or the m. adductor indicis longus); and the presence of elongated and naked bony areas (i.e. not covered by muscle; Manzano & Lavilla, 1995). The independence of the main flexor tendons from each other (resulting in the ability of each digit to flex independently), and the presence of muscles with accessory branches (resulting in additional insertion sites; Manzano & Lavilla, 1995) are some of the features unique to Phyllomedusa and may be related to their increased dexterity.
Indeed, our analysis of the use of the forelimbs during locomotion on a narrow substrate suggests that both species actively adjust the position of the hands and include a grasping type of support. On the narrow dowel, both species use a diagonal sequence gait typical of primates and other arboreal mammals when walking on narrow substrates (Jenkins, 1974; Sargis, 2001; Schmitt & Lemelin, 2004). Interestingly, even though both species appear to use a similar type of power grip when holding on to a narrow substrate, despite its larger body size and longer limbs Phyllomedusa appears much more stable and secure when moving across narrow substrates. Detailed observations based on the high-speed recordings, as well as the analysis of the X-ray data, suggest that this is because Phyllomedusa is able to generate a greater abduction of digit V and consequently is able to achieve a more complete (i.e. covering more of the substrate) and more secure grip on the substrate. Phyllomedusa bicolor also showed a greater flexion at the wrist, allowing it to maintain its grasp on the substrate for a longer time than L. caerulea.
That both species actively grasp the substrate is indicated by the results of our electromyographic analysis. In L. caerulea, the flexor digitorum communis longus shows activity during the stance phase, ending before the end of stance and coinciding with contact of the contralateral limb on the substrate. Thus, these data suggest an active flexion of the hand during stance. Although the quality of the data for this muscle in P. bicolor is not great, they do suggest a similar pattern of activity. Corroborating this pattern is the activity of the m. palmaris profundus, which, as shown by the stimulation experiment, increases the moment arm of the m. flexor digitorum communis longus and thus actively assists hand and wrist flexion. In addition, the activity of the flexor i. s. proprius II of digit 2 during stance corroborates this idea. Consequently, both species actively create a grasping posture of the hand during stance which is maintained until contra-lateral hand contact. Although to our knowledge no comparative data are available on the activity of hand flexor muscles during grasping associated with locomotion on narrow substrates, Tuttle & Basmajian (1974) do describe distinct activity in the superficial and deep m. flexor digitorum in gorillas while grasping objects such as food or toys, suggesting that these muscles may be important during grasping in general.
Our in vivo measurements of grasping force and the results of the stimulation experiment suggest that both species of frog are able to exert considerable centripetally directed force, and can thus indeed use this power grip to generate a counter-torque on the substrate to help stabilize their body. The ability of these animals to flex the hand into a power grip posture thus appears to be closely associated with the locomotion on these narrow substrates. Phyllomedusa, which is more specialized in its habitat use, moves more securely on narrow substrates, and is capable of generating higher grasping forces. Although these data suggest that the evolution of a high-performance power grip has gone hand in hand with the occupation of complex arboreal substrates with supports of narrow diameter, this hypothesis needs to be tested in a broad comparative framework. Arboreality has arisen many times independently in frogs and the group thus presents an ideal system to study the potential co-evolution of grasping and locomotion on narrow substrates.
One unexpected outcome of our stimulation experiments is that Phyllomedusa is mechanically capable of executing what is called a precision grip, known only from higher primates and so characteristic of human manipulative skills (Napier, 1956; Landsmeer, 1962; Marzke et al. 1992). A precision grip involves the adduction of the thumb towards the digits such that the palmar surfaces of the thumb and digit touch each other. The combined stimulation of the m. lumbricalis of digit 4 and the flexor i. s. proprius of digit 2 produced exactly such a precision grip. Interestingly, P. sauvagii was observed using this type of grip during locomotion on very narrow branches as well as during wiping behavior (Blaylock et al. 1976). This suggests that a precision grip may be used during locomotion on very narrow substrates and/or in the manipulation of small food items (Gray et al. 1997) in phyllomedusine frogs in general. When moving on very narrow substrates, a typical power grip would result in the digits of the fingers overlapping and thus potentially hindering the creation of a secure grip. Adduction of the first finger (digit 2 in this case) towards digit 3 combined with flexion of the remaining digits may (the way humans hold a stick or pen when pointing at an object), however, allow a secure grip on very narrow substrates. Vertical climbing on narrow substrates probably also benefits from a modified grip. To keep the centre of mass close to the substrate, and thus allow an efficient climbing style, the hand cannot be closed around the substrate in a typical power grip (with flexed thumb), but rather involves adduction of a straight thumb towards the palmar side of the other digits (Isler, 2005). Clearly these hypotheses need to be tested by observing locomotion of these animals on very narrow substrates of different orientations. Moreover, the potential use of the hand to manipulate small food objects, although common in arboreal frogs (Gray et al. 1997), remains to be investigated in this species.
In summary, we suggest that arboreal frogs may be a model system to understand the ecological context of the evolution of grasping. Despite long-standing interest in the evolution of human grasping and object manipulation skills, a true understanding of the origin of this functional capacity has been lacking due to the lack of independent origins of the behaviour among mammals. Frogs, despite their distant phylogenetic affinity, may thus provide us with a window to understand the evolution of human grasping abilities.
Acknowledgments
We would like to thank Vicky Schaerlaeken for help with experiments and data collection; and Bieke Vanhooydonck and Vicky Schaerlaeken for measuring animals and sending data to Argentina which allowed us to finish the paper in a timely fashion. This research was supported by a collaborative project between the FWO-Flanders and SECyT-Argentina, PIP CONICET 6347, and PI-UADER.
References
- Blaylock L, Ruibal R, Platt-Aloia K. Skin structure and wiping behavior of Phyllomedusinae frogs. Copeia. 1976;2:283–295. [Google Scholar]
- Bloch JI, Boyer DM. Grasping primate origins. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- Burton TC. Are the distal extensor muscles of the fingers of anuran an adaptation to arboreality? J Herpetol. 1998;32:611–617. [Google Scholar]
- Cartmill M. Climbing. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge, MA: Belknap Press; 1985. pp. 73–88. [Google Scholar]
- Duellman WE, Trueb L. Biology of Amphibians. New York: McGraw-Hill; 1986. [Google Scholar]
- Ecker A. The Anatomy of the Frog. Oxford: Clarendon Press; 1889. [Google Scholar]
- Emerson SB. Burrowing in frogs. J Morphol. 1976;149:437–457. doi: 10.1002/jmor.1051490402. [DOI] [PubMed] [Google Scholar]
- Emerson SB. A biomechanical perspective on the use of forelimb length as a measure of sexual selection in frogs. J Evol Biol. 1991;4:671–678. [Google Scholar]
- Fabrezi M. El carpo de los anuros. Alytes. 1992;10:1–29. [Google Scholar]
- Fabrezi M, Alberch P. The carpal elements of anurans. Herpetologica. 1996;52:188–204. [Google Scholar]
- Frost DR, Grant T, Faivovich J, Bain RH, et al. The amphibian tree of life. Bull Am Mus Nat Hist. 2006;297:1–370. [Google Scholar]
- Gans C, Parsons T. On the origin of the jumping mechanism in frogs. Evolution. 1966;20:92–99. doi: 10.1111/j.1558-5646.1966.tb03345.x. [DOI] [PubMed] [Google Scholar]
- Gaupp E. Anatomie des Frosches. Braunschweig: Friedrich Vieweg und Sohn; 1896. [Google Scholar]
- Gray L, O’Reilly JC, Nishikawa KC. Evolution of forelimb movement patterns for prey manipulation in anurans. J Exp Zool. 1997;277:417–424. doi: 10.1002/(sici)1097-010x(19970415)277:6<417::aid-jez1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hanna G, Barnes WJP. Adhesion and detachment of the toe pads of tree frogs. J Exp Biol. 1991;155:103–125. [Google Scholar]
- Havelková P, Roček Z. Transformation of the pectoral girdle in the evolutionary origin in frogs: insights from the primitive anuran Discoglossus. J Anat. 2006;209:1–11. doi: 10.1111/j.1469-7580.2006.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- Jenkins FA. Tree shrew locomotion and the origins of primate arborealism. In: Jenkins FA, editor. Primate Locomotion. New York: Academic Press; 1974. pp. 85–115. [Google Scholar]
- Jenkins FA, Shubin HH. Prosalirus bitis and the anuran caudopelvic mechanism. J Vert Paleontol. 1998;18:495–510. [Google Scholar]
- Landsmeer JMF. Power grip and precision handling. Ann Rheum Dis. 1962;21:164–170. doi: 10.1136/ard.21.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago: The University of Chicago Press; 1986. [Google Scholar]
- Lutz GJ, Rome LC. Built for jumping: the design of the frog muscular system. Science. 1994;263:370–372. doi: 10.1126/science.8278808. [DOI] [PubMed] [Google Scholar]
- Manzano AS, Barg M. The iliosacral articulation in Pseudinae (Anura: Hylidae) Herpetologica. 2005;61:259–267. [Google Scholar]
- Manzano AS, Fabrezi M, Vences M. Intercalary elements, treefrogs, and the early differentiation of a complex system in the Neobatrachia. Anat Rec. 2007;290:1551–1567. doi: 10.1002/ar.20608. [DOI] [PubMed] [Google Scholar]
- Manzano AS, Lavilla EO. Notas sobre la miología apendicular de Phyllomedusa hypocondrialis (Anura, Hylidae) Alytes. 1995;12:169–174. [Google Scholar]
- Martin RD. Primate Origins and Evolution. Princeton, NJ: Princeton University Press; 1990. [Google Scholar]
- Marzke MW, Wullstein KL, Viegas SF. Evolution of the power (‘squeeze’) grip and its morphological correlates in hominids. Am J Phys Anthropol. 1992;89:283–298. doi: 10.1002/ajpa.1330890303. [DOI] [PubMed] [Google Scholar]
- Napier JR. The prehensile movements of the human hand. J Bone Joint Surg. 1956;38:902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- Napier JR. Evolutionary aspects of primate locomotion. Am J Phys Anthropol. 1967;27:333–342. doi: 10.1002/ajpa.1330270306. [DOI] [PubMed] [Google Scholar]
- Nauwelaerts S, Aerts P. Take-off and landing forces in jumping frogs. J Exp Biol. 2006;209:66–77. doi: 10.1242/jeb.01969. [DOI] [PubMed] [Google Scholar]
- Sargis EJ. The grasping behavior, locomotion and substrate use of the tree shrews Tupaia minor and T. tana(Mammalia, Scandentia) J Zool Lond. 2001;253:485–490. [Google Scholar]
- Schmitt D, Lemelin P. Locomotor mechanics of the slender loris (Loris tardigradus. J Hum Evol. 2004;47:85–94. doi: 10.1016/j.jhevol.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shubin NH, Jenkins FA. An early Jurassic jumping frog. Nature. 1995;377:49–52. [Google Scholar]
- Sokal RR, Rolph FJ. Biometry. 3rd edn. New York: W. H. Freeman and Company; 1995. [Google Scholar]
- Tuttle RH, Basmajian JV. Electromyography of forearm musculature in Gorilla and problems related to knuckle walking. In: Jenkins FA, editor. Primate Locomotion. New York: Academic Press; 1974. pp. 293–347. [Google Scholar]
- Videler JJ, Jorna JT. Functions of the sliding pelvis in Xenopus laevis. Copeia. 1985;1985:254–257. [Google Scholar]