Abstract
Mandibular condylar cartilage is the best-studied mammalian secondary cartilage, differing from primary cartilage in that it originates from alkaline phosphatase-positive progenitor cells. We previously demonstrated that three transcription factors related to bone and cartilage formation, namely Runx2, Osterix and Sox9, are simultaneously expressed in the anlage of mandibular condylar cartilage (condylar anlage) at embryonic day (E)14. In this study, expression of these transcription factors was investigated in the anlagen of mandibular bone (mandibular anlagen) from E11.0 to 14.0. Runx2 mRNA was first expressed in the mandibular anlage at E11.5. Osterix mRNA was first expressed at E12.0, and showed a different expression pattern from that of Runx2 from E12.5 to E14.0, confirming that Osterix acts downstream of Runx2. Sox9 mRNA was expressed in Meckel's cartilage and its anlagen throughout the experimental period, but not clearly in the mandibular anlagen until E13.0. At E13.5, the condylar anlage was morphologically identified at the posterior end of the mandibular anlage, and enhanced Sox9 mRNA expression was detected here. At this stage, Runx2 and Osterix mRNA were simultaneously detected in the condylar anlage. These results indicate that the Sox9 mRNA-expressing condylar anlage is derived from Runx2/Osterix mRNA-expressing mandibular anlage, and that upregulation of Sox9 in this region acts as a trigger for subsequent condylar cartilage formation.
Keywords: Meckel's cartilage, secondary cartilage, Sox5, transcription factor
Introduction
Secondary cartilage has several definitions, and detailed information relevant to this topic has been well reviewed (Beresford, 1981; Vinnka, 1982). One acceptable definition of secondary cartilage is that it arises from the periostea of membrane bone after (secondary to) bone formation.
Avian secondary cartilage, including the quadratojugal, has been extensively studied and characterized (Hall, 2005), and the mandibular condylar cartilage is the best-studied mammalian secondary cartilage. Secondary cartilage differs from primary cartilage in various ways, one of which is that condylar cartilage is derived from alkaline phosphatase (ALP)-positive progenitor cells of the periosteum continuous to the preliminarily formed ossifying mandible (Shibata et al. 1996, 1997; Miyake et al. 1997; Fukada et al. 1999; Fukuoka et al. 2007). These observations indicate the close relationship of condylar cartilage to the developing mandibular bone, although some differences exist among species (Hall, 2005). Progenitor cells of this cartilage (namely skeletoblasts, see Silbermann et al. 1987) rapidly differentiate into hypertrophic chondrocytes (Shibata et al. 1997; Fukada et al. 1999; Fukuoka et al. 2007), after which the temporomandibular joint, consisting of condylar cartilage, an articular disc, and upper and lower joint cavities, can be morphologically identified until E18.5 (Shibukawa et al. 2007). In addition, the periostea/perichondria of the dentary have different characteristics to the perichondria of the limb bud (primary) cartilage. The limb bud cartilage forms mesenchymal cell condensations before the perichondria are present, and the periostea replaces the perichondria adjacent to hypertrophic chondrocytes. Meanwhile, the periostea of the dentary are neural crest in origin, and do not form via chondrogenesis, but have bipotentiality that can differentiate into both bone and secondary cartilage (Fang & Hall, 1996, 1997).
In the last few years, several transcription factors essentially involved in bone and/or cartilage formation have been identified. Sox9 (SRY-box containing gene 9) is an essential factor for chondrocyte differentiation; it is expressed in the chondrogenic region (Wright et al. 1995; Ng et al. 1997; Zhao et al. 1997; Bi et al. 1999), directly regulates cartilage-specific genes (Bell et al. 1997; Lefebvre et al. 1997; Xie et al. 1999; Sekiya et al. 2000), and induces ectopic cartilage formation when mis-expressed (Bell et al. 1997; Healy et al. 1999). In addition, Sox9 interacts with Sox5 (L-Sox5) and Sox6 (Lefebvre et al. 1998; Smits et al. 2001). Using the Cre/loxP recombination system, Akiyama et al. (2002) demonstrated that Sox9 plays essential roles in the successive steps from undifferentiated mesenchymal cells to proliferating chondrocytes, and is required for Sox5 and Sox6 expression. Using Sox9-Cre;R26R mice, Akiyama et al. (2005) further demonstrated that osteo-chondroprogenitor cells are derived from Sox9-expressing precursors during mouse embryogenesis.
Runx2 (runt-related transcription factor 2) is an essential transcription factor for bone formation and Runx2 gene knockout mice completely lack bone tissue (Komori et al. 1997; Otto et al. 1997; Inada et al. 1999). Runx2 also regulates hypertrophic chondrocyte differentiation (Inada et al. 1999; Kim et al. 1999; Enomoto et al. 2000; Takeda et al. 2001; Ueta et al. 2001). Yoshida et al. (2004) demonstrated that Runx2 and Runx3 are essential for chondrocyte maturation, and that Runx2 regulates limb growth through the induction of Indian hedgehog (Ihh).
Further, Nakashima et al. (2002) reported that the gene knockout of a novel zinc-finger-containing transcription factor called Osterix causes a complete lack of bone formation, demonstrating that Osterix is another essential factor for osteoblast differentiation. Previous studies of these transcription factors have mainly focused on primary cartilage or long bone formation, but rarely on secondary cartilage formation.
Buxton et al. (2003) first reported that in avian secondary cartilage (quadratojugal), Runx2-expressing preosteoblasts exit from the cell cycle and rapidly differentiate into hypertrophic chondrocytes; a process that correlates with the upregulation of Sox9. We reported that mandibular condylar cartilage is completely absent in Runx2-deficient mice (Shibata et al. 2004), and bone morphogenetic protein 2 (BMP2) can rescue secondary cartilage formation in these mice, although BMP2 cannot rescue chondrocyte hypertrophy (Fukuoka et al. 2007).
Furthermore, we described that mRNAs for Runx2, Osterix and Sox9 are expressed in the condylar anlage, consisting of preosteoblasts/skeletoblasts, at E14.0, and that reduced expression of Osterix in combination with Sox9-Sox5 expression is important for the onset of condylar cartilage formation at E15.0 (Shibata et al. 2006). As described above, Sox9 is strongly expressed in the chondrogenic region, and condylar cartilage is derived from progenitor cells in the periosteum continuous to the preliminarily formed ossifying mandible. These findings led us to hypothesize that a strong Sox9-positive area may appear in the anlagen of mandibular bone at earlier stages of embryogenesis. The main purpose of this study was to confirm this hypothesis by standard in situ hybridization analysis.
Nakashima et al. (2002) argued that Osterix acts downstream of Runx2 in the process of osteoblast differentiation, and Akiyama et al. (2005) reported that Osterix mRNA is temporally expressed later than Runx2 during limb bud development. However, no in situ hybridization studies have been done relevant to the expression pattern of both transcription factors in mandibular bone formation. Furthermore, using the Wnt-1 Cre/loxP recombination system, Mori-Akiyama et al. (2003) reported that Sox9 is essential for cartilage derived from neural crest cells, including Meckel's cartilage. However, these findings have not been confirmed in the formation of Meckel's cartilage by in situ hybridization. Thus, another purpose of this study was to confirm the expression pattern of these transcription factors in the formation of Meckel's cartilage and mandibular bone using standard in situ hybridization.
Materials and methods
All animals were housed in facilities approved by the Health Sciences University of Hokkaido. Our animal-use protocol conformed to the ‘Institutional Administrator's Manual for Laboratory Animal Care and Use’ (NIH publication No. 88–2959) and was reviewed and approved by the Screening Committee for Animal Research of the Health Sciences University of Hokkaido.
Tissue preparation
A total of 10 pregnant ICR mice, of E11.0–14.0 (08:00 hours on the day of the vaginal plug was designated as E0), were used for this study. At each time point, the pregnant mice were killed by cervical dislocation under ether anesthesia, after which each fetal mouse was killed by cervical dislocation. The heads were then removed and immersed in 4% paraformaldehyde (0.1m phosphate buffer, pH 7.4) for 1day at 4°C. The specimens were embedded in paraffin using standard procedures. Sections (5µm) were cut in the horizontal or coronal (perpendicular to the sagittal plane and parallel to the long axis of condylar process) plane and stained with 0.1% Toluidine blue (0.1m phosphate buffer, pH 7.4) for histological observation. ALP activity was detected using the Azo-dye method as previously described (Shibata et al. 1997; Fukuoka et al. 2007).
In situ hybridization
Digoxigenin-labeled RNA probes for aggrecan and bone sialoprotein (BSP) from our previous studies were used (Fukada et al. 1999; Shibata et al. 2002, 2003). 35S-UTP-labeled RNA probes for Runx2, Osterix and Sox9, kindly donated by Dr. Kazuhisa Nakashima (Molecular Pharmacology, Department of Regulation of Internal Environment and Reproduction, Graduate School, Tokyo Medical and Dental University) and a probe for Sox5 synthesized by reverse transcriptase-polymerase chain reaction were used as in our previous in situ hybridization study (Shibata et al. 2006). In situ hybridization using the digoxigenin-labeled probes and the Nucleic Acid Detection Kit (Roche Diagnostics, Mannheim, Germany) was performed as previously described (Fukada et al. 1999; Shibata et al. 2002, 2003). When using 35S-UTP labeled probes, sections were dipped in emulsion (NTB, Kodak, Rochester, NY, USA) after hybridization and RNAase treatment, then exposed for 1 week at 4°C for autoradiography. Sections were observed after counterstaining with nuclear fast red or hematoxylin. Sense probes were used as negative controls.
Results
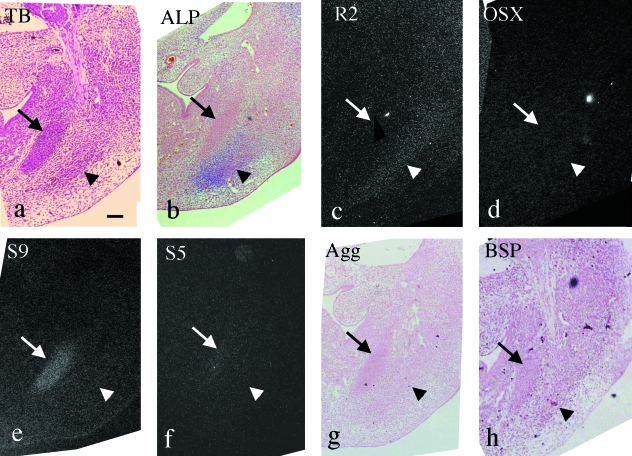
At E11.0, the mandibular nerve was formed, and a mesenchymal cell condensation of future Meckel's cartilage (termed Meckel's anlage) was faintly recognizable in the mandibular region (Fig. 1a). ALP activity was detected in the anterior part of the mandible and in the brain (Fig. 1b). Runx2 and Osterix mRNA were not expressed (Fig. 1c,d), but Sox9 and Sox5mRNA were expressed in Meckel's anlage (Fig. 1e,f). However, aggrecan and BSP mRNA were not expressed (Fig. 1g,h).
Fig. 1.
Mandible in the horizontal plane at E11.0. (a,c–f) Serial sections and (b,g,h) other sections cut in a similar plane. Toluidine blue staining (a), ALP staining (b) and in situ hybridization using 35S-UTP labeled probes of dark fields (c–f) and digoxigenin-labeled probes (g,h). (a) Meckel's anlage is faintly recognizable in the mandibular region (arrow). MN, mandibular nerve (b) ALP activity is detected in the anterior part of mandible and in the brain (arrows). (c–h) Runx2 (c), Osterix (d), aggrecan (g) and BSP (h) mRNA are not expressed, but Sox9 (e) and Sox5 (f) mRNA are expressed in the mandibular anlage (arrows in e and f). Width of view=100 µm.
At E11.5, Meckel's anlage was clearly recognizable and future mandibular bone (termed the mandibular anlage) was also identified (Fig. 2a). ALP activity was detected in the mandibular anlage (Fig. 2b). Runx2 mRNA was slightly expressed in the mandibular anlage (Fig. 2c) but Osterix was not (Fig. 2d). Sox9 mRNA was clearly expressed in Meckel's anlage but not in the mandibular anlage (Fig. 2e). Sox5 mRNA was also expressed in Meckel's anlage (Fig. 2f). Aggrecan and BSP mRNA were not expressed in either Meckel's anlage or in the mandibular anlage (Fig. 2g,h).
Fig. 2.
Mandible in the horizontal plane at E11.5. (a,c–f) Serial sections and (b,g,h) other sections cut in a similar plane. Toluidine blue staining (a), ALP staining (b) and in situ hybridization using 35S-UTP labeled probes of dark fields (c–f) and digoxigenin-labeled probes (g,h). (a) Meckel's anlage (arrow) and the mandibular anlage (arrowhead) are recognizable as mesenchymal cell condensations. (b) ALP activity is detected in the mandibular anlage (arrowhead), but not in Meckel's anlage (arrow). (c–h) Runx2 mRNA is expressed in the mandibular anlage (arrowhead in c), but Osterix and other mRNAs are not (arrowheads in d–h). Sox9 and Sox5 mRNA are expressed in Meckel's anlage (arrows in e and f) but other mRNAs are not (arrows in c,d,g,h). Width of view=100 µm.
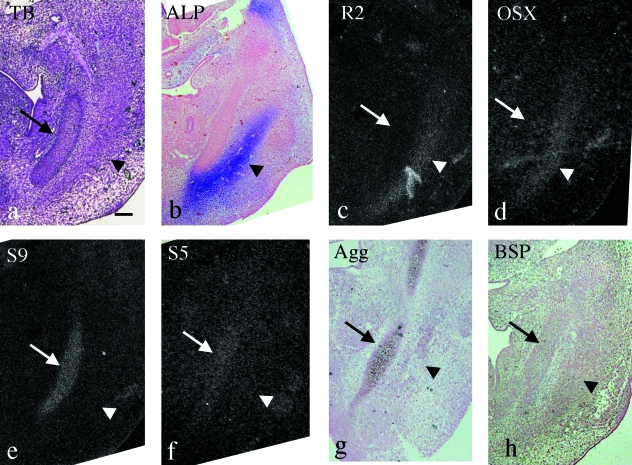
At E12.0, Meckel's cartilage was clearly formed and the mandibular anlage had expanded antero-posteriorly (Fig. 3a). ALP activity was detected in the expanded mandibular anlage (Fig. 3b). Runx2 and Osterix mRNA were identically detected in the mandibular anlage (Fig. 3c,d). Sox9, Sox5 and aggrecan mRNA were expressed in Meckel's cartilage but not in the mandibular anlage (Fig. 3e,f,g). BSP mRNA was also not expressed in the mandibular anlage at this stage (Fig. 3h).
Fig. 3.
Mandible in the horizontal plane at E12.0. (a,c–f) Serial sections and (b,g,h) other sections cut in a similar plane. Toluidine blue staining (a), ALP staining (b) and in situ hybridization using 35S-UTP labeled probes of dark fields (c– f) and digoxigenin-labeled probes (g,h). (a) Meckel's cartilage has clearly formed (arrow) and the mandibular anlage is recognizable as a mesenchymal cell condensation lateral to Meckel's cartilage (arrowhead). (b) ALP activity is detected in the mandibular anlage (arrowhead). (c–h) Runx2 and Osterix mRNA are expressed in the mandibular anlage (arrowhead in c and d) but other mRNAs are not (arrowheads in e–h). Sox9, Sox5 and aggrecan mRNA are expressed in Meckel's cartilage (arrows in e–g) but Runx2, Osterix and BSP mRNA are not (arrows in c,d,h) Width of view=100 µm.
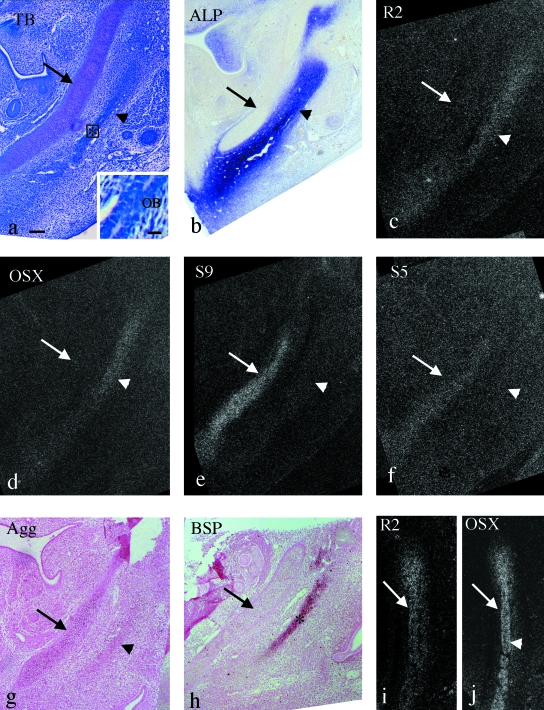
At E12.5, mandibular bone containing mature osteoblasts was clearly formed within the mandibular anlage lateral to Meckel's cartilage (Fig. 4a) and ALP activity was detected in the mandibular anlage (Fig. 4b). Runx2 and Osterix mRNA were expressed in the mandibular anlage, and Osterix tended to be expressed strongly in the center (Fig. 4c,d). Sox9, Sox5 and aggrecan mRNA were detected in Meckel's cartilage (Fig. 4e,f,g) and BSP mRNA was first detected in the mandibular bone area (Fig. 4h).
Fig. 4.
Mandible in the horizontal plane at E12.5 (a–h) and E13.0 (i,j). (a,c–f) Serial sections and (b,g,h) other sections cut in a similar plane. Toluidine blue staining (a), ALP staining (b) and in situ hybridization using 35S-UTP labeled probes of dark fields (c–f,i,j) and digoxigenin-labeled probes (g,h). (a) Meckel's cartilage (arrow) and the mandibular anlage (arrowhead) has expanded, and mandibular bone (*) has formed within it. Inset: enlargement of rectangular area. Mature osteoblasts (OB) are seen in the mandibular bone. (b) ALP activity is detected in the mandibular anlage (arrowhead) but not in Meckel's cartilage (arrow). (c–h) Runx2 and Osterix mRNA are expressed in the mandibular anlage, and Osterix tends to be expressed strongly in the center (arrowheads in c and d), but not in Meckel's cartilage (arrows in c and d). Sox9, Sox5 and aggrecan mRNA are expressed in Meckel's cartilage (arrows in e–g), but not in the mandibular anlage (arrowheads in e–g). BSP mRNA is expressed in the mandibular bone (* in h) within the mandibular anlage, but not in Meckel's cartilage (arrow in h). (i,j) Runx2 and Osterix mRNA are expressed in the mandibular anlage (arrows in i and j), and strong Osterix expression is clearly seen in the center (arrowhead in j). Width of view=100 µm and 20 µm in inset of a.
At E13.0, the difference of expression patterns of Runx2 and Osterix mRNA became distinctive, i.e. strong expression of Osterix mRNA in the center of the mandibular anlage was clearly recognized (Fig. 4i,j). However, the other mRNAs showed similar expression patterns to those seen at E12.5 (data not shown).
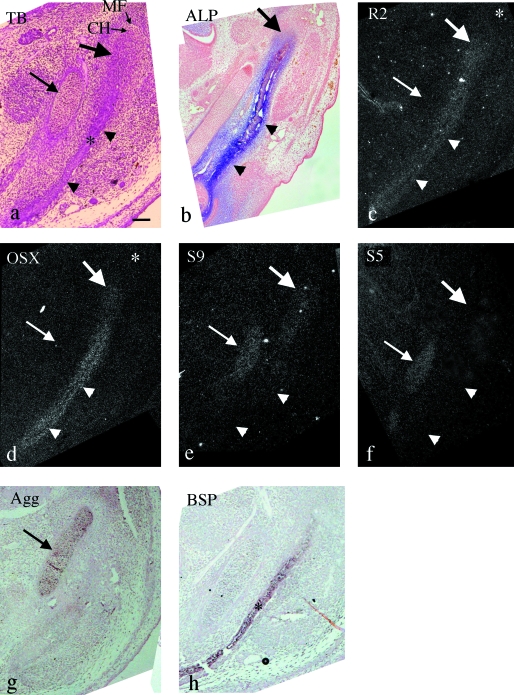
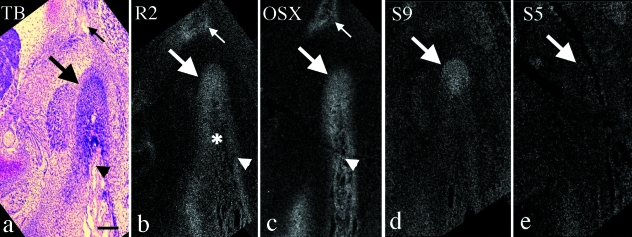
At E13.5, the mandibular anlage and mandibular bone within it had further expanded antero-posteriorly, and the outline of the future condylar head and mandibular fossa consisting of mesenchymal cell condensation of future temporal bone were morphologically recognizable at the posterior end of mandibular anlage. We therefore regarded this area as the condylar anlage (Fig. 5a). ALP activity was detected in the mandibular anlage and in the condylar anlage (Fig. 5b). Runx2 and Osterix mRNA showed similar expression patterns to those at E13.0 in the mandibular anlage, but enhanced expression of Osterix was not seen in the condylar anlage. Mesenchymal cell condensation of future temporal bone did not show clear mRNA expression of both molecules at this stage (Fig. 5c,d).
Fig. 5.
Mandible in horizontal plane at E13.5. (a,c–f) Serial sections and (b,g,h) other sections cut in a similar plane. Toluidine blue staining (a), ALP staining (b) and in situ hybridization using 35S-UTP labeled probes of dark fields (c–f) and digoxigenin-labeled probes (g,h). (a) The mandibular anlage (arrowheads) and mandibular bone (*) within it lateral to Meckel's cartilage (arrow) have expanded. The outline of the future condylar head (CH) and mandibular fossa (MF) consisting of mesenchymal cell condensation of future temporal bone are clearly recognized. Large arrow indicates position of condylar anlage. (b) ALP activity is detected in the mandibular anlage (arrowheads) and in the condylar anlage (large arrow). (c–h) Runx2 mRNA is evenly expressed in the mandibular anlage (arrowheads in c), and in the condylar anlage (large arrow in c) but not in Meckel's cartilage (arrow in c). Osterix mRNA is expressed strongly in the center of the mandibular anlage (arrowheads in d), moderately in the condylar cartilage (large arrow in d), but not in Meckel's cartilage (arrow in d). Note that Runx2 and Osterix mRNA are not clearly expressed in the mesenchymal cell condensation of future temporal bone (* in c and d). Sox9 mRNA is expressed in Meckel's cartilage (arrow in e) and is expressed in the condylar anlage (large arrow in e), but not in the anterior position of the mandibular anlage (arrowheads in e). Sox5 mRNA is expressed in Meckel's cartilage (arrow in f), but not in either the mandibular anlage (arrowheads in f) or the condylar anlage (large arrow in f). Aggrecan and BSP mRNA are expressed in Meckel's cartilage (arrow in g) and mandibular bone (* in h), respectively. Width of view=100 µm.
Sox9 mRNA was expressed in Meckel's cartilage and first detected in the condylar anlage but not in the anterior position of the mandibular anlage (Fig. 5e). Sox5 and aggrecan mRNA was expressed in Meckel's cartilage but not in either the condylar anlage or the mandibular anlage (Fig. 5f,g). BSP mRNA was detected in the center of the mandibular anlage corresponding to mandibular bone area (Fig. 5h).
At E14.0, mandibular bone had expanded and the condylar anlage was recognizable as a mesenchymal cell condensation at the posterior end of mandibular bone, as previously described (Shibata et al. 2006). The formation of temporal bone constituting mandibular fossa was also recognizable. Condylar cartilage had not formed by this stage (Fig. 6a). Runx2 mRNA was expressed in the condylar anlage and in the periphery of mandibular bone, but weakly in the center (Fig. 6b). In contrast, Osterix mRNA was expressed strongly in the center, but still moderately in the condylar anlage (Fig. 6c). Additionally, Runx2 and Osterix mRNA were clearly expressed in the temporal bone at this stage (see Fig. 6b,c).
Fig. 6.
Mandible in the coronal plane at E14.0. (a–e) Serial sections. Toluidine blue staining (a), and in situ hybridization using 35S-UTP labeled probes of dark fields (b–e). The condylar anlage is recognizable as a mesenchymal cell condensation (large arrow in a) at the posterior end of the expanded mandibular bone (arrowhead in a). The formation of temporal bone constituting mandibular fossa is recognizable (small arrow in a). Runx2 mRNA is expressed in the condylar anlage (large arrow in b) and in the periphery of the mandibular anlage (arrowhead in b), but shows lower expression in the center (* in b). Osterix mRNA is expressed strongly in the center (arrowhead in c), but still moderately in the condylar anlage (arrow in c). Note that Runx2 and Osterix mRNA are clearly expressed in the temporal bone (small arrows in b and c). Sox9 mRNA is expressed in the condylar anlage (arrow in d), but Sox5 mRNA is not expressed (arrow in e). Width of view=100 µm.
Sox9 mRNA was expressed in the condylar anlage (Fig. 6d), but Sox5 mRNA was not (Fig. 6e). Negative controls using sense probes did not show any positive reactions at any stage examined (data not shown).
Discussion
In the present study, ALP activity was diffusely detected in the mesenchymal cells at the anterior part of the mandible at E11.0, then detected in the mandibular anlage at E11.5, and then continuously detected in the mandibular anlagen including mandibular bone until E13.5. This distribution pattern is consistent with previous histochemical studies of ALP activity (Miyake et al. 1997). In addition, the ALP activity-positive areas were identical to the Runx2-positive areas from E11.5 to E13.5, indicating that ALP activity is utilized as a histological marker of mandibular bone formation in vivo.
Previous gene knockout studies have indicated that Runx2 and Osterix are essential for bone formation (Komori et al. 1997; Otto et al. 1997; Nakashima et al. 2002). Nakashima et al. (2002) reported that Osterix acts downstream of Runx2 in the lineage of osteoblast differentiation, i.e. Runx2 is mainly involved in the differentiation of mesenchymal cells into preosteoblasts, whereas Osterix acts on the differentiation of preosteoblasts into mature osteoblasts. In the present study, Runx2 mRNA was detected in the mandibular anlage at E11.5, whereas Osterix mRNA was first detected at E12.0. Thus, temporal expression of both molecules at the onset of mandibular bone formation is comparable to that in limb bud development indicated by a gene knockout study (Akiyama et al. 2005). However, the expression patterns of Runx2 and Osterix were different after BSP-expressing mature osteoblasts appeared in the center of the mandibular anlage at E12.5. Runx2 was evenly expressed in the mandibular anlagen, whereas Osterix was expressed strongly in the center. This different expression pattern became marked at E14. Therefore, the present findings provide the first support by in situ hybridization of mandibular bone formation of the hypothesis of Nakashima et al. (2002) based on the histological viewpoint.
Many previous standard in situ hybridization studies have demonstrated Sox9 expression just in mesenchymal tissues for the chondrogenic cell population of mesodermal origin (Wright et al. 1995; Ng et al. 1997; Zhao et al. 1997). Using a conditional gene knockout system, Akiyama et al. (2002, 2005) demonstrated that Sox9 plays essential roles in successive steps from undifferentiated mesenchymal cells to proliferating chondrocytes mesodermal in origin. In the present study, Sox9 mRNA was first detected in Meckel's anlage at E10.0 and was continuously detected in Meckel's cartilage until E13.5. This result indicates that Sox9 is also involved in cartilage formation neural crest in origin, and supports the results in a conditional gene knockout study performed by Mori-Akiyama et al. (2003).
In a study related to primary cartilage and subsequent endochondral bone formation, Akiyama et al. (2005) demonstrated that Runx2-expressing osteogenic cells are derived from Sox9-expressing progenitor cells during limb bud development. Meanwhile, Buxton et al. (2003) reported that chondrocytes of avian secondary cartilage form coincident with Sox9 upregulation from a precursor population expressing Runx2, and they insisted that this is a reversal of the normal sequence. In the present study, Sox9 mRNA was not clearly expressed in the mandibular anlagen until E13.0, but enhanced expression was seen in the condylar anlage at the posterior end of the mandibular anlage at E13.5. Furthermore, Runx2 and Osterix mRNA were simultaneously expressed in the condylar anlage at this stage, indicating that strong Sox9-expressing condylar anlage is derived from the Runx2/Osterix-expressing mandibular anlage. In addition, our previous study (Shibata et al. 2006) indicated that newly formed condylar cartilage appears within the condylar anlage at E15.0. Thus, upregulation of Sox9 in the condylar anlage acts as a trigger for subsequent condylar cartilage formation, and this result is comparable with that of Buxton et al. (2003). Therefore, the reversal expression pattern of Runx2 and Sox9 is possibly a unique feature of secondary cartilage formation.
Next, as described above, enhanced Osterix expression was seen in the center of the mandibular anlage, but not in the condylar anlage at E13.5 and 14.0. Our previous study (Shibata et al. 2006) indicated that downregulation of Osterix is apparently involved in the onset of condylar cartilage formation at E15.0. Meanwhile, mesenchymal cells in Runx2-deficient mice do not express Osterix but do still express Sox9 (Nakashima et al. 2002; Fukuoka et al. 2007), and condylar cartilage formation is rescued by BMP-2 in the absence of Runx2 (Fukuoka et al. 2007). In addition, downregulation of Runx2 is slight at the onset of condylar cartilage formation (Shibata et al. 2006). These results indicate that Osterix rather than Runx2 may have a direct interaction with Sox9 in condylar cartilage formation. A future study of transfection of Osterix/Runx2 into the condylar anlagen should clarify this issue.
Previous gene knockout studies indicate that interactions between Sox9 and Sox5 (L-Sox5) or Sox6 are important for cartilage formation (Lefebvre et al. 1998; Smits et al. 2001; Akiyama et al. 2002). A standard in situ hybridization study indicated that Sox9 and Sox5 mRNA are expressed in precartilaginous mesenchymal cell condensations during limb bud cartilage formation (Smits et al. 2001). In the present study, Sox5 mRNA was detected in Meckel's anlage at E11.0 before aggrecan mRNA expression. Thus, interactions between Sox9 and Sox5 are also adapted for primary cartilage formation of neural crest in origin. However, the present and previous results (Shibata et al. 2006) have shown that Sox5 mRNA is first expressed in the newly formed condylar cartilage. Chondrocytes in the mandibular condylar cartilage rapidly differentiate into hypertrophic chondrocytes (Shibata et al. 1997; Fukada et al. 1999; Fukuoka et al. 2007) and this rapid differentiation may account for the discrepancy relevant to Sox5 expression between condylar (secondary) cartilage and Meckel's (primary) cartilage.
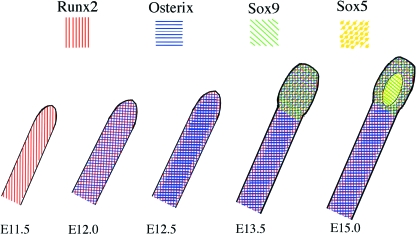
Our model of transcription factors active during mandibular condylar cartilage formation is summarized in Fig. 7.
Fig. 7.
Schematic representations of the expression pattern of transcription factors during mandibular bone and condylar cartilage formation, based on the present findings and previous reports of Akiyama et al. (2003, 2005), Buxton et al. (2003) and Shibata et al. (2006). Runx2 mRNA is first expressed in the mandibular anlage at E11.5*, and subsequently, Osterix mRNA expression overlaps Runx2 mRNA expression at E12.0. At E12.5, the mandibular bone containing mature osteoblasts is formed in the center of the mandibular anlage and enhanced Osterix mRNA expression is seen in the center. At E13.5, the condylar anlage is morphologically identified and enhanced Sox9 mRNA expression overlaps the Runx2/Osterix expression. Enhanced Osterix mRNA expression is seen in the center of the mandibular anlage, but not in the condylar anlage. Upregulation of Sox5 mRNA and downregulation of Osterix and Runx2** mRNA is seen at the onset of condylar cartilage formation at E15.0. *Sox9 mRNA may be expressed in the mandibular anlage (Akiyama et al. 2005), but only weakly. **Downregulation of Osterix mRNA is more predominant than that of Runx2 (Shibata et al. 2006).
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (No. 17591901) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Akiyama H, Chaboissier M-C, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kim J-E, Nakashima K, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DM, Leung KKH, Wheatley SC, et al. Sox9 directly regulates the type-II collagen gene. Nature Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Beresford WA. Chondroid Bone, Secondary Cartilage and Metaplasia. Baltimore: Urban & Schwarzenberg; 1981. [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nature Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Buxton PG, Hall B, Archer CW, Francis-West P. Secondary chondrocytes-derived Ihh stimulates proliferation of periosteal cells during chick development. Development. 2003;130:4729–4739. doi: 10.1242/dev.00610. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Enomoto-Iwamoto M, Iwamoto M, et al. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- Fang J, Hall BK. In vitro differentiation of the periosteal cells from a membrane bone, the quadratojugal of the embryonic chick. Dev Biol. 1996;180:701–712. doi: 10.1006/dbio.1996.0339. [DOI] [PubMed] [Google Scholar]
- Fang J, Hall BK. Chondrogenic cell differentiation from membrane bone periostea. Anat Embryol. 1997;196:349–362. doi: 10.1007/s004290050104. [DOI] [PubMed] [Google Scholar]
- Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T. In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J Anat. 1999;195:321–329. doi: 10.1046/j.1469-7580.1999.19530321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka H, Shibata S, Suda N, Yamashita Y, Komori T. Bone morphogenetic protein rescues lack of secondary cartilage in Runx2-deficient mice. J Anat. 2007;211:8–15. doi: 10.1111/j.1469-7580.2007.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. San Diego, London: Elsevier, Academic Press; 2005. Osteo- and chondroprogenitor cells. In; pp. 149–165. [Google Scholar]
- Healy C, Uwanogho D, Sharpe PT. Regulation and role of Sox9 in cartilage formation. Dev Dyn. 1999;215:69–78. doi: 10.1002/(SICI)1097-0177(199905)215:1<69::AID-DVDY8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Inada M, Yasui T, Nomura S, et al. Maturational disturbance of chondrocytes in cbfa1-deficient mice. Dev Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80:159–70. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, et al. Targeted disruption of cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. Sox9 is a potent activator of the chondrocyte-specific enhancer of the proα1(II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Cameron AM, Hall BK. Stage-specific expression patterns of alkaline phosphatase during development of the first arch skeleton in inbred C57BL/6 mouse embryos. J Anat. 1997;190:239–260. doi: 10.1046/j.1469-7580.1997.19020239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of chondrogenic cell linege in the cranial neural crest. Proc Natl Acad Sci USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Ng L-J, Wheatley S, Muscat GEO, et al. Sox9 binds DNA activates transcription, and coexpresses with Type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, et al. Sox9 enhances aggrecan gene promotor/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- Shibata S, Suzuki S, Tengan T, Ishii M, Kuroda T. A histological study of the developing condylar cartilage of the fetal mouse mandible using coronal sections. Arch Oral Biol. 1996;41:47–54. doi: 10.1016/0003-9969(95)00105-0. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Yamashita Y. Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. J Anat. 1997;191:561–570. doi: 10.1046/j.1469-7580.1997.19140561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y. In situ hybridization and immunohistochemistry of bone sialoprotein and secreted phosphoprotein 1 (osteopontin) in the developing mouse mandibular condylar cartilage compared with limb bud cartilage. J Anat. 2002;200:309–320. doi: 10.1046/j.1469-7580.2002.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Imai H, Abe T, Yamashita Y. In situ hybridization and immunohistochemistry of versican, aggrecan, and link protein and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003;203:425–432. doi: 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Suda N, Yoda S, et al. Runx2-deficient mice lack mandibular condylar cartilage and have deformed Meckel's cartilage. Anat Embryol. 2004;208:273–280. doi: 10.1007/s00429-004-0393-2. (Berl) [DOI] [PubMed] [Google Scholar]
- Shibata S, Suda N, Suzuki S, Fukuoka H, Yamashita An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–177. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, et al. Temporomandibular joint formation and condylar growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Reddi AH, Hand AR, Leapman RD, von der Mark K, Franzen A. Further characterization of the extracellular matrix in the mandibular condyle in neonatal mice. J Anat. 1987;151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, et al. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta C, Iwamoto M, Kanatani N, et al. Skeletal malformations caused by overexpression of Cbfa1or its dominant negative form in chondrocytes. J Cell Biol. 2001;153:87–99. doi: 10.1083/jcb.153.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinnka H. Secondary cartilages in the facial skeleton of the rat. Proc Finn Soc. 1982;78:1–137. [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nature Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Xie WF, Zhang X, Sakano S, Lefebvre V, Sandell LJ. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J Bone Miner Res. 1999;14:757–763. doi: 10.1359/jbmr.1999.14.5.757. [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Yamamoto H, Fujita T, et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Gene Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, de Crombrugghe B. Parallel expression of Sox9 and col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]