Abstract
Human 2D:4D ratios (measures of the relative lengths of index and ring fingers) attract considerable research interest because they exhibit sexual dimorphism and are associated with various morphological, physiological and behavioural traits as well as sporting abilities and medical conditions. In an attempt to identify potential confounding factors in such studies, we have examined how relative and absolute digit lengths vary with gender and tested whether they are influenced by age, right–left asymmetry and hand preference. Participants between 4 and 60years of age were recruited from local educational sites. Hand photocopies and calliper measurement were used to obtain digit lengths. We employed linear regression analysis to examine the growth trajectories of individual digits, analyses of variance to isolate main and interaction effects of age, gender and hand preference, and paired t-tests to identify lateral asymmetries. Both digits exhibited biphasic growth with an early growth phase followed by a stable length phase. Digits in females attained their maximum length about 2.2years (dextral subjects) or 5.1years (sinistral subjects) earlier than those in males. Sexual dimorphism in 2D:4D ratios was apparent by 4years of age and age changes in ratios depended on gender, side and hand preference. Relative and absolute lengths displayed age, gender, hand-preference and age×gender interaction effects. Lengths tended to be greater in females in younger subjects and greater in males in older subjects. Ratios tended to be greater in sinistral subjects. In dextral subjects, significant lateral asymmetries in 2D lengths were seen at all ages but asymmetries in males and 4D lengths seemed to be age-dependent. We conclude that age, lateral asymmetry and hand preference are potential confounding factors and that future study designs should take account of these as well as other known confounders such as ethnicity, birth order, menstrual cycle phase and sexual preference.
Keywords: index finger, lateral asymmetry, linear growth, ring finger, sexual dimorphism
Introduction
The relative lengths of fingers on the human hand continue to attract considerable research interest. Whilst 10 possible digit ratios are available for analysis on each hand, difficulties in measuring the first digit (1D, thumb) reduce this to six and, of these, the 2D:4D (index:ring) digit ratio has been the most extensively studied. This particular ratio exhibits sexual dimorphism and left–right asymmetry and is associated with a surprising variety of morphological, physiological, sexual preference and behavioural traits as well as with ability in certain sports and with the risk of developing medical conditions such as autism, infertility and breast cancer (Manning & Bundred, 2000; Robinson & Manning, 2000; Williams et al. 2000; Manning et al. 2001, 2002; Brown et al. 2002a,b; McFadden & Shubel, 2002; Paul et al. 2006; Voracek et al. 2006; Mayhew et al. 2007; Robertson et al. 2008).
It is generally agreed that 2D:4D ratios tend to be greater in females (closer to 1.0) and that gender differences tend to be larger for the right hand than for the left (Manning et al. 1998; Williams et al. 2000; Brown et al. 2002a; McFadden & Shubel, 2002). This sexual dimorphism in 2D:4D ratios is apparent by 2years of age and appears to be established early in life, possibly by the 14th week of gestation (Garn et al. 1975; Manning et al. 1998; Malas et al. 2006). The differences may be linked to the prenatal production of testosterone and oestradiol and, in the case of testosterone, to interactions with the homeobox genes Hoxa and Hoxd, which control differentiation of the urogenital system and development of the digits (Kondo et al. 1997; Mortlock & Innis, 1997; Manning et al. 1998; Lutchmaya et al. 2004; Hönekopp et al. 2007). Low 2D:4D ratios appear to be associated with high levels of fetal testosterone relative to oestradiol, and high ratios with relatively high levels of fetal oestradiol (Lutchmaya et al. 2004). The inverse sensitivity to testosterone is indicated by variation in composition of the androgen receptor gene (Manning et al. 2002, 2003). Moreover, maternal smoking during pregnancy is linked to high fetal testosterone and is associated with low 2D:4D ratios in the right hands of male offspring (Rizwan et al. 2007).
Whilst there is evidence that 2D:4D ratios do not change between 9 and 40 weeks of gestation (Malas et al. 2006), the way in which 2D:4D ratios vary with postnatal age is less certain. Whilst some studies (Manning et al. 1998; Williams et al. 2003; McIntyre et al. 2005) have adduced evidence for a positive correlation between 2D:4D ratio and age, other studies, using different population samples, have found either negative correlations (Fink et al. 2004; Manning et al. 2004) or no effect of age (Manning et al. 1998; Robertson et al. 2008). The reasons for these discrepancies are unclear but might include differences in age ranges and ethnicity (Manning et al. 2004, 2007) and temporal differences in the growth trajectories of individual digits. It has also been suggested that age changes in 2D:4D ratios are greater in the left hand (Trivers et al. 2006) suggesting that handedness could be another confounder. Recently, we found that the stage of the menstrual cycle also affects 2D:4D ratios (Mayhew et al. 2007) so this is a further potential confounder of studies comparing pre- and post-pubertal females.
The present study has two main aims: (1) to examine how absolute and relative digit lengths vary over a wide range of postnatal ages, and (2) to test whether these variables are influenced by gender, laterality and handedness effects.
Materials and methods
Participants were recruited from five different centres (Schools in Nottinghamshire and Leicestershire and the University of Nottingham) and were school pupils (4–18years of age) or their teachers (24–60years of age) or medical undergraduates (18–23years of age). They were approved by the University of Nottingham Medical School Ethics Committee, the Director of Education of Nottinghamshire County Council and the Head Teachers and Boards of Governors of relevant Schools. A copy of the permission letter was sent out to parents of all children under 16years of age with a covering letter which explained the purpose of the study. Parents consented by means of a reply slip. Privately funded schools consented on behalf of their pupils but each individual over 16years of age decided whether or not they wished to participate based on both written and verbal explanations of the study followed by an opportunity to ask questions.
At the selected centres, the overwhelming majority (at least 75%) of individuals can be classified as White (Caucasian), 6–25% as non-Chinese Asian or Middle-Eastern (almost exclusively Indian or Pakistani), 3–8% as Black and 2% as Chinese. This ethnic distribution, with small proportions of Black and Chinese, is reassuring as recent analyses have shown these two groups tend to have lower 2D:4D ratios than other groups (Manning et al. 2007). The final set of participants was divided into eight different age groups for the purpose of statistical analysis. The ages, numbers of participants, numbers of males and females and numbers of right- and left-handed individuals in each group are summarized in Table 1.
Table 1.
Age, sample size, gender mix and handedness incidence in each study group
| Age group | Age (range),years | Sample size | Female/male (%) | Sinistral/dextral (%) |
|---|---|---|---|---|
| Group 1 | 5.59 (4–7) | 82 | 39/43 (47.6, 52.4) | 8/74 (9.8, 90.2) |
| Group 2 | 7.60 (7–9) | 20 | 12/8 (60.0, 40.0) | 2/18 (10.0, 90.0) |
| Group 3 | 9.74 (9–11) | 103 | 43/60 (41.7, 58.3) | 14/89 (13.6, 86.4) |
| Group 4 | 11.19 (11–12) | 21 | 9/12 (42.9, 57.1) | 3/18 (14.3, 85.7) |
| Group 5 | 15.39 (15–17) | 56 | 34/22 (60.7, 39.3) | 4/52 (7.1, 92.9) |
| Group 6 | 17.00 (16–18) | 25 | 16/9 (64.0, 36.0) | 3/22 (12.0, 88.0) |
| Group 7 | 20.27 (18–23) | 48 | 25/23 (52.1, 47.9) | 7/41 (14.6, 85.4) |
| Group 8 | 42.32 (24–60) | 53 | 25/38 (47.2, 52.8) | 6/47 (11.3, 88.7) |
| Total | 15.57 (4–60) | 408 | 203/205 (49.8, 50.2) | 47/361 (11.5, 88.5) |
Hand photocopying
This was used to obtain length measurements of digits 2D and 4D (Manning et al. 2001; Peters et al. 2002; Rahman & Wilson, 2003; Mayhew et al. 2007). Although it has been suggested that photocopies can distort lateral asymmetries (Manning et al. 2006), further studies are required to test whether or not such biases are significant in other contexts. At least for the present context, relative biases should be equal and comparisons between age groups, genders and sides should retain their comparative worth. Moreover, photocopying is known to provide measurements which correlate well with those obtained directly from the hands with callipers (Robinson & Manning, 2000) or with calibrated finger tubes (Nicholls et al. 2008) and can provide lower 2D:4D ratios with smaller inter-observer variation (Peters et al. 2002; Manning et al. 2005). For internal consistency, photocopies were made according to a strict protocol designed on the basis of a pilot study, which compared the effect of different hand positions on digit length measurement. With this protocol, we have found that inter-individual variation in absolute and relative lengths is low (Mayhew et al. 2007). Coefficients of variation (CV=standard deviation/group mean) for absolute digit length accounted for 6–7% of group means and CV values were lower again (<4%) for 2D:4D ratios.
The photocopier contrast was adjusted to a setting which produced a clear hand image, where both the proximal crease and finger tip of digits 2D and 4D were visible and distinct from the background. Where necessary, participants were asked to remove rings or jewellery that would compromise measurement. Also, we attempted to eliminate other potential confounders such as damage to bone through disease or trauma. For younger children, a safety stool was used to reach the photocopier.
The palmar surfaces of both hands were placed on the photocopier with the three middle digits straight and adducted and first and fifth digits slightly splayed (Peters et al. 2002). Arms were kept straight, extended through the elbow, with the third digit in line with the midpoint of the radius and ulna, avoiding abduction or adduction at the wrist. Visual checks were made that the three middle digits of each hand did not overlap and were all within the photocopy area. Then the photocopier lid was closed (to avoid unwanted stray light) and, after photocopying, the image was observed to ensure all of the above conditions had been satisfied. If not, the process was repeated.
Participants remained anonymous and the only other information taken was their age, gender and dominant hand. This information was recorded on the reverse of the photocopy to conceal it from the measurer. Individuals were classed as dextral (right-handed) or sinistral (left-handed) on the basis of volunteered information about hand preference or empirical evidence of preference from observing them writing on a piece of paper.
Outcome measures
The primary measures were the lengths of digits 2D and 4D on the left and right hands of each individual. Measurements were taken from every photocopy using digital callipers accurate to 0.01mm. The measuring errors introduced by use of callipers had a negligible impact on the observed CV between individuals (Mayhew et al. 2007). To eliminate inter-observer error, all digits were measured by one observer. The lengths of digits 2D and 4D were measured from the midpoint of each proximal crease to the finger tip. The secondary measure, 2D:4D ratio, was obtained by simply dividing 2D length by 4D length. This ratio was preferred over the alternative measures of 2D–4D difference and distal tip extent because it offers greater precision and, for a given sample size, is better suited to detecting subtle differences between groups (Buck et al. 2003; Manning et al. 2005; Mayhew et al. 2007).
Study comparisons and statistical analyses
One study aim was to compare growth patterns in digits 2D and 4D across study groups. In addition, we tested the null hypotheses that age, gender and handedness had no effects on absolute or relative digit lengths.
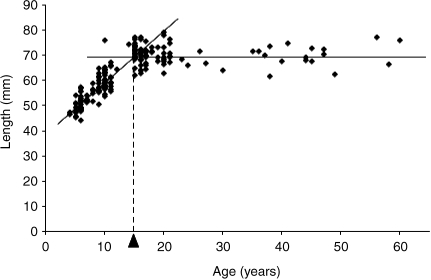
To analyse the growth in length of each digit, age (A inyears of age) was taken as the independent variable and digit length (L in mm) as the dependent variable. These were subjected to linear regression analysis (Sokal & Rohlf, 1981) as preliminary plots had indicated biphasic linear growth (e.g. see Fig. 1). To this end, each group pattern was subjected to two regression analyses, the first covering the period 4–12years of age and the second 15–60years of age Each regression equation was solved as L=S.A+I, where S represents the slope of the line and I is the intercept on the L axis. Each value of S and I was calculated together with its standard error of the mean (SEM). By solving the equations for a common value of L, it was possible to predict the inflection point where both lines intersected. In practice, this point represented the age at which the maximum digit length was achieved and beyond which it was constant. Regression equations for age and 2D:4D ratios covered the entire age range as slopes were not significantly different from zero in early or late phases.
Fig. 1.
Example of the relationship between digit length (mm) and age (years). Each point represents digit 2D length in the right hand of a dextral female. As with other digits and other sub-groups, the growth pattern seems to be biphasic with a linear increase between 4 and 12years of age followed by a plateau between 15 and 60years of age. For this reason, all growth relationships were examined in two phases by linear regression analysis (fitted lines). The age at which digit length switches from one phase to the other is referred to as the inflection point (dotted line and inverted arrowhead).
To examine the main effects of age, gender and handedness on outcome measures, a three-way analysis of variance (anova) was undertaken. This analysis generates first-order (age×gender, age ×handedness, gender ×handedness) and second-order (age ×gender ×handedness) interaction terms (Sokal & Rohlf, 1981). Because of inequalities in sample sizes between dextral and sinistral individuals, and to avoid confusion between hand-preference and left–right asymmetry effects, we also undertook separate two-way anova for these groups with age and gender as the main effects and age ×gender as the interaction term. Also, because left–right asymmetries were properly analysed as paired data sets, lateral asymmetries were analysed by means of a paired Student t-test.
In terms of inferential statistics, each null hypothesis was rejected at the probability level of P<0.05. All calculations and tests were performed with Unistat v5.5 software (Unistat Ltd, London, UK).
Results
Findings are summarized in Tables2–6 and Fig. 2. CV values were calculated for the absolute 2D and 4D lengths of the right and left hands for each age and gender group. The CV for each digit was relatively small in each age group: 4–8% (2D, right hand), 2–8% (4D, right hand), 2–8% (2D, left hand) and 1–8% (4D, left hand). Corresponding ranges for 2D:4D ratios were even smaller: 1–6% for both right and left hands.
Table 2.
Linear regression analyses of digital growth (mm) with age (years) for dextral individuals from 4–12years of age and 15–60years of age. The inflection point represents the age at which linear growth attains its maximum and then plateaus
| Side, digit | Period | Females Slope (SEM) | Females Intercept (SEM) | Inflection, years of age | Males Slope (SEM) | Males Intercept (SEM) | Inflection, years of age |
|---|---|---|---|---|---|---|---|
| Right, 2D | 4–12years of age | 2.161 (0.174) | 38.50 (1.450) | 14.76 | 2.205 (0.135) | 37.60 (1.152) | 16.76 |
| Right, 2D | 15–60years of age | –0.008 (0.037) | 70.53 (0.942) | 0.055 (0.040) | 73.64 (1.183) | ||
| Right, 4D | 4–12years of age | 2.174 (0.166) | 40.63 (1.390) | 14.39 | 2.248 (0.146) | 40.08 (1.242) | 16.80 |
| Right, 4D | 15–60years of age | –0.010 (0.036) | 72.06 (0.914) | 0.091 (0.044) | 76.31 (1.297) | ||
| Left, 2D | 4–12years of age | 2.125 (0.179) | 38.50 (1.495) | 14.68 | 2.172 (0.136) | 37.78 (1.162) | 16.87 |
| Left, 2D | 15–60years of age | 0.002 (0.035) | 69.67 (0.900) | 0.067 (0.041) | 73.29 (1.205) | ||
| Left, 4D | 4–12years of age | 1.977 (0.174) | 42.22 (1.450) | 14.85 | 2.151 (0.142) | 40.87 (1.210) | 17.13 |
| Left, 4D | 15–60years of age | –0.008 (0.036) | 71.68 (0.915) | 0.074 (0.043) | 76.44 (1.287) |
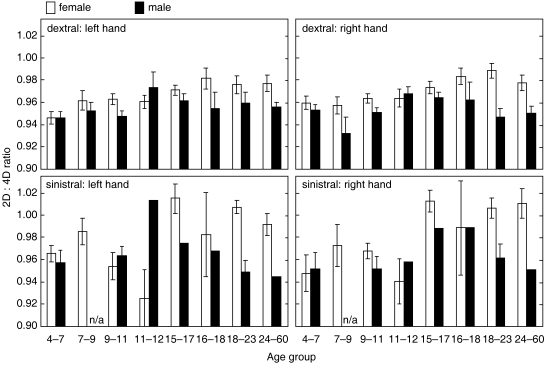
Fig. 2.
2D:4D ratios in female (open bars) and male (closed bars) subjects as a function of age group (see text). Values are plotted for both the left and right hands of right-handed (dextral) and left-handed (sinistral) subjects. Values are group means (SEM) except where group size is <3, in which case only the mean or individual values, or data not available (n/a), are indicated.
Growth patterns
The growth characteristics of digits 2D and 4D obtained by linear regression analysis, and grouped according to gender and hand preference, are provided in Table 2 (dextrals) and Table 3 (sinistrals). All digits grew in the same biphasic pattern and the slopes of the regression lines in the later phase were not significantly different from 0. Slopes in the early phase did not differ significantly between digits or between genders in either left- or right-handed individuals. However, intercepts (corresponding to digit lengths at phase onset) were larger in female digits in the first phase and longer in male digits in the second phase. These findings indicate that female digits begin with a length advantage over males at 4years of age but that the second growth phase begins with male digits having a length advantage over females. As the following shows, this gender difference is probably related to the delayed inflection point in males compared to females.
Table 3.
Linear regression analyses of digital growth (mm) with age (years) for sinistral individuals from 4–12years of age and 15–60years of age. The inflection point represents the age at which linear growth attains its maximum and then plateaus
| Side, digit | Period | Females Slope (SEM) | Females Intercept (SEM) | Inflection, years of age | Males Slope (SEM) | Males Intercept (SEM) | Inflection, years of age |
|---|---|---|---|---|---|---|---|
| Right, 2D | 4–12years of age | 2.416 (0.527) | 38.12 (4.559) | 14.43 | 2.167 (0.278) | 36.95 (2.380) | 18.70 |
| Right, 2D | 15–60years of age | –0.018 (0.080) | 73.25 (2.512) | –0.279 (0.416) | 82.68 (8.719) | ||
| Right, 4D | 4–12years of age | 2.540 (0.497) | 39.62 (4.295) | 13.07 | 2.255 (0.295) | 39.00 (2.521) | 18.00 |
| Right, 4D | 15–60years of age | –0.035 (0.085) | 73.28 (2.654) | –0.047 (0.411) | 80.42 (8.606) | ||
| Left, 2D | 4–12years of age | 2.282 (0.526) | 39.13 (4.549) | 14.77 | 2.122 (0.318) | 37.42 (2.726) | 18.51 |
| Left, 2D | 15–60years of age | –0.007 (0.076) | 72.93 (2.364) | –0.151 (0.353) | 79.50 (7.386) | ||
| Left, 4D | 4–12years of age | 2.781 (0.461) | 37.60 (3.986) | 12.51 | 1.960 (0.287) | 40.76 (2.456) | 19.99 |
| Left, 4D | 15–60years of age | 0.025 (0.082) | 72.09 (2.563) | 0.029 (0.347) | 79.34 (7.274) |
The regression equations for each digit allowed estimation of the mean age at which the inflection point was attained. For example, digit 2D in the right hands of dextral females grew in the initial period (4–12years of age) according to the equation L=2.161A+38.50 and, in the second period, by the equation L=–0.008A+70.53. For the same value of L, it follows that 2.169A equals 32.03, or A (the inflection point) is approximately 14.8years (see Fig. 1). Findings from such paired equations suggest that the digits of females tend to attain their maximum length at an earlier age than those in males. Moreover, this difference appears to be exaggerated in sinistrals (Table 3: females 13.7years of age, males 18.8years of age) compared to dextrals (Table 2: 14.7 and 16.9years of age respectively).
In dextral (right and left hands) and sinistral females (right hand only), regression equations for 2D:4D ratios demonstrated slopes which were significantly different from zero (data not shown). In sinistral females (left hand) and all males (dextral or sinistral, right or left hands), ratios did not change with age over the period 4–60years of age However, in all sub-groups, intercepts were significantly different from 1, indicating that inequalities in digit length ratios are established at an early age.
Effects of age, gender and handedness
The effects of age, gender and handedness on absolute and relative digit lengths were examined initially by three-way anova. This indicated that there were significant main effects of age and gender, together with a significant first-order interaction between age and gender, for 2D and 4D lengths in both right and left hands (P<0.001 in all cases). Digit lengths increased with age and tended to be longer in males but did not differ with hand preference.
In the right hand, there were significant age (P<0.01), gender (P<0.001) and hand preference (P<0.05) effects on the 2D:4D ratio and a significant age×gender interaction (P<0.05). Ratios tended to be higher in left-handed individuals. In the left hand, there were also significant age (P<0.001), gender (P<0.001) and hand preference (P<0.05) effects on the 2D:4D ratio but no significant age×gender interaction effect. Again, 2D:4D ratios were higher in left-handers.
To avoid the complications of unequal sample sizes and interpreting second-order interaction terms, age and gender effects and interactions were explored separately in dextrals and sinistrals by two-way anova.
Dextral individuals
In the case of dextral individuals (Table 4), there were significant age and gender effects, and a significant age× gender interaction effect, on digits 2D and 4D in either hand. The 2D:4D ratios were also subject to age and gender effects but without interaction.
Table 4.
Results of two-way anova applied to digit lengths (mm) and 2D:4D ratios in dextral individuals. Values are group means (CV%)
| Right, 2D | Right, 4D | Right, 2D:4D | Left, 2D | Left, 4D | Left, 2D:4D | |
|---|---|---|---|---|---|---|
| Group 1, female | 50.7 (6.6%) | 52.8 (6.4%) | 0.960 (3.5%) | 50.4 (6.7%) | 53.3 (6.8%) | 0.946 (3.7%) |
| Group 1, male | 50.0 (7.8%) | 52.5 (7.8%) | 0.953 (3.4%) | 50.0 (7.4%) | 52.8 (7.0%) | 0.947 (3.6%) |
| Group 2, female | 53.9 (5.1%) | 56.3 (4.9%) | 0.957 (2.5%) | 54.4 (5.8%) | 56.6 (5.5%) | 0.962 (2.8%) |
| Group 2, male | 53.5 (6.6%) | 57.5 (8.1%) | 0.932 (4.4%) | 54.0 (7.0%) | 56.8 (7.8%) | 0.952 (2.3%) |
| Group 3, female | 60.4 (7.0%) | 62.7 (6.2%) | 0.964 (2.7%) | 59.9 (7.4%) | 62.2 (6.2%) | 0.963 (3.0%) |
| Group 3, male | 59.1 (5.8%) | 62.2 (5.7%) | 0.951 (3.3%) | 58.8 (5.9%) | 62.0 (5.7%) | 0.948 (3.3%) |
| Group 4, female | 61.0 (6.5%) | 63.3 (5.8%) | 0.964 (2.2%) | 60.5 (7.1%) | 62.9 (6.1%) | 0.961 (1.7%) |
| Group 4, male | 61.9 (3.8%) | 63.9 (3.4%) | 0.968 (2.0%) | 62.3 (4.6%) | 64.1 (4.2%) | 0.974 (4.7%) |
| Group 5, female | 70.8 (5.6%) | 72.8 (5.3%) | 0.973 (3.2%) | 70.2 (5.3%) | 72.3 (5.4%) | 0.971 (2.6%) |
| Group 5, male | 75.5 (7.3%) | 78.4 (6.8%) | 0.964 (2.6%) | 75.4 (7.3%) | 78.5 (6.8%) | 0.961 (3.2%) |
| Group 6, female | 69.5 (4.5%) | 70.7 (4.5%) | 0.983 (2.9%) | 69.2 (4.9%) | 70.5 (4.3%) | 0.981 (3.6%) |
| Group 6, male | 74.8 (7.3%) | 77.8 (5.1%) | 0.962 (4.7%) | 74.8 (7.0%) | 78.3 (5.3%) | 0.955 (4.1%) |
| Group 7, female | 70.9 (5.6%) | 71.7 (5.0%) | 0.989 (3.1%) | 69.9 (5.3%) | 71.7 (5.3%) | 0.976 (3.7%) |
| Group 7, male | 73.6 (5.6%) | 77.9 (7.0%) | 0.947 (3.7%) | 73.5 (6.2%) | 76.7 (7.4%) | 0.960 (4.5%) |
| Group 8, female | 69.6 (6.1%) | 71.3 (5.9%) | 0.978 (3.3%) | 69.2 (6.0%) | 70.8 (5.7%) | 0.977 (3.4%) |
| Group 8, male | 76.0 (5.3%) | 80.0 (6.6%) | 0.951 (3.1%) | 76.1 (5.4%) | 79.6 (5.8%) | 0.956 (2.7%) |
| Effects | A, G,A×G | A, G, A×G | A, G | A, G, A×G | A, G, A×G | A, G |
A, significant age effect; G, significant gender effect; A×G, significant age×gender interaction effect.
In the right (dominant) hand, digit 2D increased in length with age (variance ratio, F=272.06; degrees of freedom, df=7,345; P<0.001). Length also varied with gender (F=16.42; df=1,345; P<0.001), with values tending to be greater in males. However, there was a significant age×gender interaction (F=7.01; df=7,345; P<0.001), with 2D length being greater in females at early ages (4–11years) but increasingly greater in males thereafter. This pattern was repeated in digit 4D on the right hand and in digits 2D and 4D on the left (non-dominant) hand.
In the right hand, the 2D:4D ratio tended to increase with age (F=2.35; P<0.05; see Fig. 2) and to be smaller in males (F=24.96; P<0.001; Fig. 2). No interaction term was detected. In the left hand, the effects of age and gender were similar to those in the right hand (Fig. 2).
Sinistral individuals
In contrast to dextrals, sinistral individuals (Table 5) displayed significant age effects on both digits and in both hands but we did not detect any gender effects and age×gender interactions were confined to digit 4D on each hand. The 2D:4D ratio did vary with age but there was a significant age×gender interaction in the left hand. There were no significant gender effects.
Table 5.
Results of two-way anova applied to digit lengths (mm) and 2D:4D ratios in sinistral individuals. Values are group means (CV%)
| Right, 2D | Right, 4D | Right, 2D:4D | Left, 2D | Left, 4D | Left, 2D:4D | |
|---|---|---|---|---|---|---|
| Group 1, female | 50.7 (6.1%) | 53.5 (3.2%) | 0.948 (3.0%) | 51.2 (6.2%) | 53.0 (5.1%) | 0.966 (1.3%) |
| Group 1, male | 48.9 (5.7%) | 51.4 (7.6%) | 0.952 (3.4%) | 49.3 (6.4%) | 51.5 (6.0%) | 0.957 (2.6%) |
| Group 2, female | 57.3 (7.6%) | 58.9 (4.9%) | 0.973 (2.7%) | 57.4 (2.0%) | 58.3 (3.7%) | 0.986 (1.7%) |
| Group 2, male | dna | dna | dna | dna | dna | dna |
| Group 3, female | 60.4 (7.2%) | 62.4 (7.2%) | 0.968 (1.4%) | 59.9 (7.6%) | 62.7 (5.5%) | 0.954 (2.6%) |
| Group 3, male | 57.3 (4.9%) | 60.2 (4.2%) | 0.952 (3.5%) | 57.2 (5.5%) | 59.3 (4.6%) | 0.964 (2.9%) |
| Group 4, female | 66.6 (5.7%) | 70.7 (2.7%) | 0.941 (3.0%) | 66.2 (8.3%) | 71.5 (4.4%) | 0.925 (3.9%) |
| Group 4, male | 61.8 (dna) | 64.5 (dna) | 0.958 (dna) | 62.7 (dna) | 61.9 (dna) | 1.013 (dna) |
| Group 5, female | 70.6 (7.7%) | 69.7 (7.3%) | 1.013 (1.7%) | 70.5 (6.1%) | 69.5 (6.5%) | 1.015 (2.3%) |
| Group 5, male | 82.4 (dna) | 83.4 (dna) | 0.988 (dna) | 79.3 (dna) | 81.4 (dna) | 0.975 (dna) |
| Group 6, female | 77.4 (4.4%) | 78.3 (1.7%) | 0.989 (6.1%) | 77.2 (6.1%) | 78.5 (0.7%) | 0.983 (5.4%) |
| Group 6, male | 74.6 (dna) | 75.4 (dna) | 0.989 (dna) | 75.4 (dna) | 78.0 (dna) | 0.967 (dna) |
| Group 7, female | 73.1 (5.9%) | 72.6 (5.5%) | 1.007 (1.8%) | 72.7 (6.0%) | 72.2 (5.3%) | 1.007 (1.2%) |
| Group 7, male | 76.3 (6.3%) | 79.4 (5.8%) | 0.961 (2.4%) | 76.0 (6.3%) | 80.1 (6.1%) | 0.949 (1.9%) |
| Group 8, female | 71.9 (4.9%) | 71.2 (5.8%) | 1.010 (3.0%) | 72.3 (4.3%) | 73.0 (5.4%) | 0.992 (2.2%) |
| Group 8, male | 75.8 (dna) | 79.7 (dna) | 0.951 (dna) | 75.7 (dna) | 80.2 (dna) | 0.944 (dna) |
| Effects | A | A, A×G | A | A | A, A×G | A×G |
Dna, data not available; A, significant age effect; G, significant gender effect; A×G, significant age×gender interaction effect.
In the right (non-dominant) hand, the length of digit 2D increased with age (F=41.31; df=7,32; P<0.001). Length also varied with age for digit 4D (F=37.64; P<0.001) for which there was also a significant interaction effect (F=4.13; df=6,32; P<0.01), with values tending to be greater in females at early ages (4–12years) but greater in males at later ages. These age and interaction effects were similar for corresponding digits on the left (dominant) hand.
In the right hand, the 2D:4D ratio increased with age (F=2.43; P<0.05; Fig. 2) but no gender or interaction terms were found. In the left hand, there no main effects of age or gender but there was a significant interaction effect (F=3.54; P<0.01) in which the normal pattern of higher ratios in females seemed to be perturbed during the period between 9–12years of age (Fig. 2).
Lateral asymmetries
Table 6 provides the results of paired t-tests applied to different age groups to test for left–right asymmetry involving digits 2D and 4D. Because of inadequate sample sizes for sinistral individuals, findings are limited to dextrals. Age groups were conflated to increase sample sizes and the groups roughly represented pre-adolescents (4–11years of age), adolescents+young adults (11–18years of age) and adults (18–60years of age).
Table 6.
Results of paired t-tests applied to right minus left digit lengths (mm) and 2D:4D ratios in dextral individuals. Values are group mean differences (SEM)
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Groups | 2D R-L | 4D R-L | 2D:4D R-L | 2D R-L | 4D R-L | 2D:4D R-L |
| Groups 1–3 | 0.27 (0.14)* | –0.03 (0.14) | 0.006 (0.003) | 0.15 (0.13) | 0.03 (0.15) | 0.002 (0.003) |
| Groups 4–6 | 0.54 (0.19)* | 0.41 (0.19)* | 0.002 (0.003) | –0.07 (0.24) | –0.23 (0.19) | 0.001 (0.004) |
| Groups 7–8 | 0.73 (0.22)* | 0.24 (0.19) | 0.007 (0.004) | –0.01 (0.23) | 0.68 (0.21)* | –0.008 (0.004)* |
| All (1–8) | 0.46 (0.10)* | 0.16 (0.10) | 0.005 (0.002)* | 0.06 (0.10) | 0.14 (0.11) | –0.001 (0.002) |
Significant lateral asymmetry.
In dextral females, regardless of age grouping, there were significant lateral differences in 2D length, with digits tending to be longer in the right hand. For all dextral females (n = 179), the right 2D was 0.46 (0.10)mm longer than the left (paired t=4.54; df=178; P<0.001). We detected no corresponding lateral asymmetry when all sinistral females were examined.
Although there was a significant asymmetry in 4D lengths in the group of 11–18years of age females, dextral females as a whole showed no significant 4D asymmetry. Also, no lateral asymmetry was detected when all sinistral females were examined.
We found no significant lateral asymmetries in 2D:4D ratios in the individual age groups. However, 2D:4D ratios overall showed lateral asymmetry with values tending to be larger in the right hand. We detected no comparable asymmetry when sinistral females were analysed collectively.
In contrast to females, dextral males showed no evidence of asymmetry in 2D lengths. We detected evidence of significant asymmetry in 4D lengths in the 18–60years of age group but, for dextral males as a whole, there was no evidence of 4D asymmetry. Again, no lateral asymmetry was detected for all sinistral males.
The 2D:4D ratios overall showed no evidence of lateral asymmetry in dextral or sinistral males, although values in the left hand of dextral males exceeded those in the right in the 18–60years of age group.
Taken together, the lateral asymmetry findings indicate a gender difference, with females showing lateral asymmetries in 2D length and 2D:4D ratio and an age-dependent asymmetry in 4D length around adolescence. In each case, values were greater in the right hand. Males showed no lateral asymmetries overall but did display a later age-dependent asymmetry in 4D length (values greater on the right) and in 2D:4D ratio (values greater on the left). Except for the age-dependent effects in males, these differences persisted when all individuals (regardless of age or hand preference) were analysed in each gender group (data not shown).
Discussion
Using subjects ranging in age from 4 to 60 years, we made calliper measurements on photocopier images of digits 2D and 4D to examine the growth trajectories of digit lengths and to test for the effects of gender, laterality and handedness. We found that, regardless of hand preference, both digits displayed biphasic growth with the inflection between phases occurring between 13 and 20years of age In the first phase, slopes of regression lines did not differ by digit, gender or laterality and slopes in the second phase did not differ significantly from zero. Compared to males, digits in females tended to reach their greatest length earlier and this was more exaggerated in left-handers compared to right-handers. Although 2D:4D ratios did not alter consistently with gender, laterality and hand preference over this age range, analyses of variance indicated significant age, gender and interaction effects on 2D and 4D lengths and the 2D:4D ratios tended to be greater in left-handed individuals. Moreover, there was evidence that ratios are perturbed in females around 9–12years of age. Finally, we found that 2D lengths in dextral females were longer in the right hand but that this lateral asymmetry was absent in males. Also, 4D lengths and 2D:4D ratios showed evidence of age-dependent lateral asymmetries at 11–18years of age (females) and 18–60years of age (males). Coefficients of variation between subjects within groups were low (1–8%), confirming earlier findings that relative and absolute digit lengths are tightly regulated.
Sexual dimorphism
These findings confirm the repeated previous observations that 2D:4D ratios exhibit sexual dimorphism in healthy human subjects, with lower ratios tending to be found in males (Williams et al. 2000; Buck et al. 2003). It is generally accepted that 2D:4D ratios are, on average, greater in females and that gender differences tend to be larger for the right hand than for the left (Manning et al. 1998; Williams et al. 2000; Brown et al. 2002a; McFadden & Shubel, 2002). The present findings suggest that the magnitude of these differences may be influenced by the effects of age, laterality and handedness.
It has been proposed (Manning et al. 1998) that sexual dimorphism in 2D:4D ratios reflects a negative correlation with testosterone levels in men and a positive correlation with oestrogen levels in women. Further, it has been suggested that concentrations of oestrogen and testosterone are retrospective markers of prenatal gonadal differentiation by members of the Hox gene family, which also influence development of the appendicular skeleton. This is based on the finding of significant gender differences across all age groups, suggesting that the 2D:4D ratio is established prenatally and remains fixed through childhood into adult life (Garn et al. 1975; Manning et al. 1998; Malas et al. 2006). Also, Brown et al. (2002a) found that women with congenital adrenal hyperplasia, who are known to be exposed to high levels of androgen prenatally, have masculinized 2D:4D ratios when compared to healthy controls. Our results are consistent with the notion that 2D:4D ratios are established early but, of course, provide no evidence that they are established prenatally.
Recently, we have found that human 2D:4D ratios vary postnatally during the menstrual cycle (Mayhew et al. 2007). It is conceivable that 2D:4D ratios in males and females might alter also postnatally, particularly in the peripubertal period when large changes occur in androgenic and oestrogenic hormone levels. The present study does not provide clear evidence for this and other studies have failed to demonstrate significant effects of adult sex hormone levels (Hönekopp et al. 2007). Linear regression analysis suggested that 2D and 4D lengths in females tended to attain their maxima before those in males (12–15 vs. 17–20years of age) and this is consistent with females entering puberty and attaining fertility earlier than males. However, regression analysis also demonstrated that 2D:4D ratios are influenced by laterality and handedness effects. Analysis of variance indicated that ratios tend to be higher in the left and right hands of sinistral individuals and to be perturbed in the left hands of females at 9–12years of age. 2D lengths were also longer in dextral females compared to sinistral females or to males. Laterality differences also varied by age and gender. All these attendant main and interaction effects indicate the advisability of catering for potential confounders in future studies on 2D:4D ratios. They also raise the possibility that, although determined prenatally, the 2D:4D ratio is only fully established once digital growth has finished.
It has been pointed out (Jackson, 2008) that, whilst the 2D:4D ratio is related to prenatal androgen exposure, digit length is related to post-puberty levels. Using proxy measures of cerebral hemisphere asymmetry (handedness), prenatal androgen exposure (2D:4D ratio) and post-pubertal exposure (digit length), it was found that dextrality was associated consistently with both phases of androgen exposure, whereas sinistrality was more variably associated with these phases.
There is much evidence to suggest that prenatal androgens and oestrogens play a key role in the development of the 2D:4D ratio. The involvement of oestrogen is strongly supported by the presence of oestrogen receptors in osteoclasts and osteoblasts (Buck et al. 2003) and evidence that oestrogens can modulate Hox gene expression (Taylor et al. 1999). With testosterone, evidence has been adduced that it is positively correlated with many traits including autism and mental rotation, which are also negatively correlated with the 2D:4D ratio (Manning et al. 2001; Manning & Taylor, 2001). Lutchmaya et al. (2004) found a significant negative association between 2D:4D ratios in the right hand at 2years of age and the fetal testosterone:oestrogen ratio measured after amniocentesis in the second trimester of pregnancy. It has also been reported recently that delayed menarche in female college students is associated with low 2D:4D ratios, the suggestion being that high androgen levels in early development are a common cause (Matchock, 2008). Nevertheless, it remains uncertain about exactly when the 2D:4D ratio is established by the balance of sex steroid hormones. Studies on human fetuses between 9 and 40 weeks of gestation have shown that digit lengths in utero increase with gestational age and that the digit ratio increased from the prenatal to the early postnatal period (Malas et al. 2006). However, digit lengths did not vary by gender or laterality and the ratio did not vary by gender or gestational age. A longitudinal study on infants between 1 and 12 months of age also found no gender differences in 2D:4D ratios (McIntyre et al. 2005).
Age effects on absolute and relative digit lengths
Previous studies of the effects of age on 2D:4D ratios have been contradictory, suggesting that the effects are weak or absent or have been investigated over too narrow an age range. Whilst some have demonstrated positive correlations between 2D:4D ratio and age (Manning et al. 1998; Williams et al. 2003), other studies have found no effect of age (Manning et al. 1998) or negative associations between age and 2D:4D ratio (Fink et al. 2004; Manning et al. 2004). Our findings indicate that the 2D:4D ratio does increase with age. Moreover, whilst low 2D:4D ratios (0.94–0.96) have been recorded in pre-school children and children 8–10years of age (Williams et al. 2003; Fink et al. 2004; Manning et al. 2004), higher ratios (0.98–1.00) have been found in adults (Manning et al. 1998; Williams et al. 2000) and this appears to support an increase in 2D:4D ratio with age. It also supports the idea (Williams et al. 2003) that lower 2D:4D ratios are found in both genders early in postnatal life but stabilize at a higher value in adults. Given these discrepancies, and the fact that our results are based on cross-sectional data, longitudinal studies covering a wide range of postnatal ages are required to establish the nature of temporal changes in 2D:4D ratios. This seems particularly desirable given the clearly biphasic patterns of change in absolute digit lengths. In a longitudinal study of absolute and relative finger lengths in the left hands of male and female subjects between 1 and 17years of age (McIntyre et al. 2005), serial radiographic images were used to measure the lengths of proximal, intermediate and distal phalanges. The growth trajectories of individual phalanges all displayed inflection points at around 13years of age, at which age the proximal phalanges in females were significantly longer than those in males. More recently, a 4-year longitudinal study on children between 7 and 17years of age found that 2D:4D ratios increased slightly with age, with the effect more marked in the left hand (Trivers et al. 2006). Though based on cross-sectional data, our present findings are consistent with results of these longitudinal studies.
Effects of hand preference on absolute and relative digit lengths
Our analyses indicated that 2D lengths in dextral females were longer in the right hand but that this lateral asymmetry was absent in males. A recent study in which digit lengths were measured by inserting fingers into a clear plastic tube with an attached measuring scale, found that the right fingers were longer than the left for dextrals but not for sinistrals (Nicholls et al. 2008). The present studies on sinistrals were based on small samples and further studies on larger samples are required to test whether or not the findings for sinistrals can be confirmed.
The significant effect of hand preference found in this study, with sinistral males and females having higher 2D:4D ratios than dextrals, has not been described by previous research. Indeed, Nicholls et al. (2008) reported lower ratios in sinistrals. Recent radiographic studies (Robertson et al. 2008) failed to detect differences in ratios for dominant, non-dominant or ambidextrous hands, although it has been suggested that radiographic studies reflect bones at the expense of soft tissues, which might also contribute to ratio differences measured directly or on photocopies (Vehmas et al. 2006). Grimshaw et al. (1995) demonstrated that girls exposed to higher levels of prenatal testosterone (lower 2D:4D ratios) were more strongly right-handed. However, the only research analysing the direct effect of hand preference on the 2D:4D ratio (Manning et al. 2000) showed that in right-handed Jamaican children, a low 2D:4D in the right hand of boys and girls was associated with a reduced rightward performance asymmetry. Overall these findings were also replicated by Fink et al. (2004). An association between low 2D:4D and increased likelihood of left-hand preference (due to reduced rightward performance) seems sensible given that a positive correlation exists between left-hand performance and mental rotation ability (males have lower 2D:4D ratios and score higher than females at such tasks, see Reio et al. 2004). Although males and females in the present study showed similar incidences of left-handedness, the handedness trait was not rigorously tested and we did not define a subset of ambidextrous subjects. It is reported that left-hand preference is a trait occurring more commonly in males than females (Manning et al. 2000) and there is a higher incidence of sinistrals among individuals with chronic adrenal hyperplasia (Kelso et al. 2000).
There are several reports of greater sex differences in the right hand than in the left, supporting a hypothesis that the right hand is more sensitive to fetal androgens (Manning et al. 1998; McFadden & Shubel, 2002). On the whole, the findings of this study replicated this, as sexual dimorphism was more pronounced in the right hand 2D:4D ratio. It has been proposed (Tanner et al. 1990) that this is due to sexually dimorphic traits expressing the most ‘male’ form on the right side of the body. This pattern is also seen in human toes (McFadden & Shubel, 2002).
In conclusion, it is evident that sexual dimorphism in the 2D:4D ratio is replicable in humans. Sexual dimorphism in the 2D:4D ratio appears to be more pronounced in the right hand than the left and this is supported by earlier work. To determine the age at which the 2D:4D ratio is established requires a longitudinal study controlling for any potential confounders. The present study confirms that lateral asymmetry and handedness are potential confounders of comparisons of relative and absolute digit lengths. Other studies have shown that ethnicity (Manning et al. 2004, 2007), birth order (Williams et al. 2000), menstrual cycle (Mayhew et al. 2007), sexual orientation (Brown et al. 2002a) and, at least at University level, academic discipline (Brosnan, 2006) should also be considered in future investigations. Finally, though a prenatal origin for the 2D:4D ratio (involving a combination of Hox genes and androgens) seems most likely, the influence of postnatal changes in hormone levels warrants further investigation.
Acknowledgments
We are extremely grateful to all who facilitated or participated in this study. In particular, we thank the staff, pupils and parents associated with Arnbrook Primary and Nursery, Carlton-Le-Willows, Heymanns and Oakham Schools. We also thank teachers Gemma Mayhew, Hazel Ebling and John Cheverton for their enthusiastic support and organizational skills.
References
- Brosnan MJ. Digit ratio and faculty membership: implications for the relationship between prenatal testosterone and academia. Br J Psychol. 2006;97:455–466. doi: 10.1348/000712605X85808. [DOI] [PubMed] [Google Scholar]
- Brown WM, Finn CJ, Cooke B, Breedlove SM. Differences in finger length ratios between self-identified ‘butch’ and ‘femme’ lesbians. Arch Sex Behav. 2002a;31:123–127. doi: 10.1023/a:1014091420590. [DOI] [PubMed] [Google Scholar]
- Brown WM, Hines MH, Fane BA, Breedlove SM. Masculinised finger length patterns in human males with congenital adrenal hyperplasia. Horm Behav. 2002b;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio-comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod. 2003;18:976–979. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- Fink B, Manning JT, Neave N, Tan U. Second to fourth digit ratio and hand skill in Austrian children. Biol Psychol. 2004;46:558–564. doi: 10.1016/j.biopsycho.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Garn SM, Burdi AR, Babler WJ, Stinson S. Early prenatal attainment of adult metacarpal-phalangeal rankings and proportions. Am J Phys Anthropol. 1975;43:327–332. doi: 10.1002/ajpa.1330430305. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Bryden MP, Finegan J-A. Relations between prenatal testosterone and cerebral lateralization in children. Neuropsychology. 1995;9:68–79. [Google Scholar]
- Hönekopp J, Bartholdt L, Beier L, Liebert A. Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: new data and a meta-analytic review. Psychoneuroendocrinology. 2007;32:313–321. doi: 10.1016/j.psyneuen.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Jackson C. Prediction of hemispheric asymmetry as measured by handedness from digit length and 2D:4D digit ratio. Laterality. 2008;13:34–50. doi: 10.1080/13576500701692507. [DOI] [PubMed] [Google Scholar]
- Kelso WM, Nicholls ME, Warne GL, Zacharin M. Cerebral lateralization and cognitive functioning in patients with congenital adrenal hyperplasia. Neuropsychology. 2000;14:370–378. doi: 10.1037//0894-4105.14.3.370. [DOI] [PubMed] [Google Scholar]
- Kondo T, Zakany J, Innis JW, Duboule D. Of fingers, toes and penises. Nature. 1997;390:29. doi: 10.1038/36234. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Malas MA, Dogan S, Evcil EH, Desdicioglu K. Fetal development of the hand, digits and digit ratio (2D:4D) Early Hum Dev. 2006;82:469–475. doi: 10.1016/j.earlhumdev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Manning JT, Bundred PE. The ratio of 2nd to 4th digit length: a new predictor of disease predisposition? Med Hypotheses. 2000;54:855–857. doi: 10.1054/mehy.1999.1150. [DOI] [PubMed] [Google Scholar]
- Manning JT, Taylor RP. Second to fourth digit ratio and male ability in sport: implications for sexual selection in humans. Evol Hum Behav. 2001;22:61–69. doi: 10.1016/s1090-5138(00)00063-5. [DOI] [PubMed] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Manning JT, Trivers RL, Thornhill R, Singh D. 2nd to 4th digit ratio and left lateralised preference in Jamaican children. Laterality. 2000;5:121–132. [PubMed] [Google Scholar]
- Manning JT, Baron-Cohen S, Wheelwright S, Saunders G. The 2nd to 4th digit ratio and autism. Dev Med Child Neurol. 2001;43:160–164. [PubMed] [Google Scholar]
- Manning JT, Bundred PE, Flanagan BF. The ratio of 2nd to 4th digit length: a proxy for transactivation of the androgen receptor gene? Med Hypotheses. 2002;59:334–336. doi: 10.1016/s0306-9877(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Manning JT, Bundred PE, Newton DJ, Flanagan BF. The 2nd to 4th digit ratio and variation in the androgen receptor gene. Evol Hum Behav. 2003;24:399–405. [Google Scholar]
- Manning JT, Stewart A, Bundred PE, Trivers RL. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Hum Dev. 2004;80:161–168. doi: 10.1016/j.earlhumdev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B, Neave N, Caswell N. Photocopies yield lower digit ratios (2D:4D) than direct finger measurements. Arch Sex Behav. 2005;34:329–333. doi: 10.1007/s10508-005-3121-y. [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B, Neave N, Szwed A. The second to fourth digit ratio and asymmetry. Ann Hum Biol. 2006;33:480–492. doi: 10.1080/03014460600802551. [DOI] [PubMed] [Google Scholar]
- Manning JT, Churchill AJG, Peters M. The effects of sex, ethnicity, and sexual orientation on self-measured digit ratio (2D:4D) Arch Sex Behav. 2007;36:223–233. doi: 10.1007/s10508-007-9171-6. [DOI] [PubMed] [Google Scholar]
- Matchock RL. Low digit ratio (2D:4D) is associated with delayed menarche. Am J Hum Biol. 2008;20:487–489. doi: 10.1002/ajhb.20763. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gillam L, McDonald R, Ebling FJP. Human 2D (index) and 4D (ring) digit lengths: their variation and relationships during the menstrual cycle. J Anat. 2007;211:630–638. doi: 10.1111/j.1469-7580.2007.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Shubel E. Relative lengths of fingers and toes in human males and females. Horm Behav. 2002;42:492–500. doi: 10.1006/hbeh.2002.1833. [DOI] [PubMed] [Google Scholar]
- McIntyre MH, Ellison PT, Lieberman DE, Demerath E, Towne B. The development of sex differences in digital formula from infancy in the Fels longitudinal study. Proc Biol Soc. 2005;272:1473–1479. doi: 10.1098/rspb.2005.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortlock DP, Innis JW. Mutation of Hoxa 13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Orr CA, Yates MJ, Loftus AM. A new means of measuring index/ring finger (2D:4D) ratio and its association with gender and hand preference. Laterality. 2008;13:71–91. doi: 10.1080/13576500701751287. [DOI] [PubMed] [Google Scholar]
- Paul SN, Kato BS, Hunkin JL, Vivekanandan S, Spector TD. The big finger – the second to fourth digit ratio is a predictor of sporting ability in women. Br J Sports Med. 2006;40:981–983. doi: 10.1136/bjsm.2006.027193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Mackenzie K, Bryden P. Finger length and distal finger extent patterns in humans. Am J Phys Anthropol. 2002;117:209–217. doi: 10.1002/ajpa.10029. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Wilson GD. Sexual orientation and the 2nd to 4th finger length ratio: evidence for organising effects of sex hormones or developmental instability? Psychoneuroendocrinology. 2003;28:288–303. doi: 10.1016/s0306-4530(02)00022-7. [DOI] [PubMed] [Google Scholar]
- Reio TG, Czarnolewski M, Eliot J. Handedness and spatial ability: differential patterns of relationships. Laterality. 2004;9:339–358. doi: 10.1080/13576500342000220. [DOI] [PubMed] [Google Scholar]
- Rizwan S, Manning JT, Brabin BJ. Maternal smoking during pregnancy and possible effects of in utero testosterone: evidence from the 2D:4D finger length ratio. Early Human Dev. 2007;83:87–90. doi: 10.1016/j.earlhumdev.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Robertson J, Zhang W, Liu JJ, Muir KR, Maciewicz RA, Dohert M. Radiographic assessment of the index to ring finger ratio (2D:4D) in adults. J Anat. 2008;212:42–48. doi: 10.1111/j.1469-7580.2007.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SJ, Manning JT. The ratio of 2nd to 4th digit length and male homosexuality. Evol Hum Behav. 2000;21:333–345. doi: 10.1016/s1090-5138(00)00052-0. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. The Principles and Practice of Statistics in Biological Research. 2nd edn. San Francisco: WH Freeman and Company; 1981. [Google Scholar]
- Tanner JM. Foetus into Man: Physical Growth from Conception to Maturity. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84:1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- Trivers R, Manning J, Jacobson A. A longitudinal study of digit ratio (2D:4D) and other finger ratios in Jamaican children. Horm Behav. 2006;49:150–156. doi: 10.1016/j.yhbeh.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Vehmas T, Solovieva S, Leino-Arjas P. Radiographic 2D:4D index in females: no relation to anthropometric, behavioural, nutritional, health-related, occupational or fertility variables. J Negat Results Biomed. 2006;5:12. doi: 10.1186/1477-5751-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voracek M, Reimer B, Ertl C, Dressler SG. Digit ratio (2D:4D), lateral preferences, and performance in fencing. Percept Mot Skills. 2006;103:427–446. doi: 10.2466/pms.103.2.427-446. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Greenhalgh KD, Manning JT. Second to fourth finger ratio and possible precursors of developmental psychopathy in preschool children. Early Hum Dev. 2003;72:57–65. doi: 10.1016/s0378-3782(03)00012-4. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Pepitone ME, Christensen SE, et al. Finger length ratios and sexual orientation. Nature. 2000;404:455–456. doi: 10.1038/35006555. [DOI] [PubMed] [Google Scholar]