Abstract
The microvascular architecture of developing lateral ventricle choroid plexus was investigated by corrosion casting and scanning electron microscopy in human fetuses aged 20 gestational weeks. The areas with different microvascular patterns corresponded to the particular parts of the mature plexus: anterior part, glomus, posterior part, the villous fringe and the free margin. In the posterior part, densely packed parallel arterioles and venules were surrounded by sheath-like capillary networks. Other areas contained compact capillary plexuses of the primary villi: the most prominent, protruding basket- and leaf-shaped plexuses were observed in the villous fringe, whilst less numerous and smaller plexuses occurred in the anterior part and glomus. The capillaries of the plexuses had a large diameter and sinusoidal dilations, and showed the presence of occasional short, blind sprouts indicative of angiogenesis. Short anastomoses between arterioles supplying the plexuses and venules draining them were only rarely observed. In the upper area of the choroid plexus, the superior choroidal vein was surrounded by a capillary network forming small, glomerular or rosette-shapes plexuses. The free margin of the choroid plexus was characterized by flat, multiple, arcade-like capillary loops. The general vascular architecture of the human choroid plexus at 20 gestational weeks seems to be similar to that of postnatal/mature plexus, still lacking, however, the complex vascular plexuses of the secondary villi.
Keywords: blood vessels, choroid plexus, corrosion casting, human fetus, SEM
Introduction
The choroid plexus, forming an interface between the blood and the cerebrospinal fluid (CSF) is now regarded as not merely a source of CSF, but as an organ greatly contributing to the maintenance of brain homeostasis by controlling the bi-directional transport of ions, nutrients, signaling molecules and growth factors as well as by participating in neuroimmune interactions (Strazielle & Ghersi-Egea, 2000). Moreover, it plays a significant role in brain development by providing the developing brain with a controlled microenvironment and by supplying morphogens and trophic factors (Dziegielewska et al. 2001).
Until the 1980s, the vascular and microvascular architecture of the human choroid plexus was investigated mostly in injected and cleared preparations (Pedget, 1948; Millen & Woollam, 1953; Hudson 1960; Maillot et al. 1976; Wolfram-Gabel et al. 1984, 1987a,b). The introduction of vascular corrosion casting technique in association with scanning electron microscopy (SEM) has offered highly improved resolution and attractive quasi three-dimensional images of the studied vascular networks (Lametschwandtner et al. 1990). It has been employed by many authors for detailed visualization of the complex vasculature of mammalian choroid plexus (Hodde 1979; Miodonski et al. 1979; Motti et al. 1986; Weiger et al. 1986). However, with the exception of the paper by Motti et al. (1986), this technique has not been used to examine human choroid plexus and there are no relevant data concerning the fetal period. This study presents for the first time the microvascular structure of the human fetal lateral ventricle choroid plexus revealed by SEM.
Materials and methods
Fetuses
The investigations were carried out on two fetuses (both male, aged 20 gestational weeks with the crown–rump length 190 and 193mm) belonging to the material collected for vascular corrosion cast studies in 1990–1994, in accordance with institutional requirements for the use of human material. The fetuses were obtained after spontaneous abortions from the Department of Obstetrics, Jagiellonian University Medical College, Krakow. The abortions were due to maternal disorders and the fetuses showed no developmental malformations and/or anomalies of large blood vessels upon macroscopic inspection.
Corrosion casting and scanning electron microscopy
After abortion, the thorax of each fetus was opened to expose the heart and large vessels. The heart apex was cut off and a cannula of appropriate diameter was inserted via the left ventricle into the aorta and fixed by ligation at the level of the ascending part. The vascular system of the fetus was subsequently perfused manually by a sequence of solutions with outflow occurring via the umbilical vessels and additionally incised posterior tibial veins.
The fetuses were first perfused with 300mL of prewarmed (37°C) heparinized (12.5IU mL−1) isotonic sodium chloride solution containing 3% Dextran, MW 70000, and 0.025% lidocaine (Lignocaine, Polfa). Perfusion fixation was then carried out with 200mL of 0.08% glutaraldehyde in 0.2M cacocylate buffer, pH 7.4, at 37ºC. Finally, 60mL of a mixture containing 8mL Mercox CL-2R (Vilene Comp, Tokyo, Japan) and 2mL methylmethacrylate (MMA; Fluka) containing 0.2g MA initiator per 10mL of the casting medium was injected. Following the injection, the fetuses were kept overnight in a water bath at 55°C to facilitate polymerization and tempering of resin (Miodonski et al. 1981).
After resin curing, the brain was exposed in saline bath and the meninges were carefully removed. Following a wash in distilled water, the specimens were macerated at 37°C in 10% potassium hydroxide for 5–7 days with alternating, gentle washes in hot tap water and distilled water. The resulting vascular casts were carefully and thoroughly cleaned in 2% formic acid, followed by washing in distilled water for a few days. Then, under a dissection microscope, the lumina of the lateral ventricles were opened from the dorsal, medial and lateral sides. In this way, choroid plexuses of both lateral ventricles were exposed in situ. The casts thus obtained, containing choroid plexuses as well as telencephalic, diencephalic and brain stem structures, were freeze-dried. Afterwards, they were mounted with colloidal silver and conductive bridges (Lametschwandtner et al. 1980) onto specially prepared specimen holders, enabling their tilting or rotation, coated with gold and examined in a Jeol JSM-35-CF scanning electron microscope at 20–25kV. After preliminary examination and documentation, the casts were microdissected under an operation microscope to remove the surrounding structures and to expose fully the choroid plexuses, again coated with gold, and used for further scanning electron microscopic observations.
Results
In the complete vascular cast, the choroid plexus appeared as a thin, demilunar fold with thalamus-attached margin and free margin. The lateral surface of the plexus showed areas with different microvascular patterns, corresponding to the particular parts of the mature plexus: anterior part, glomus, posterior part, the villous fringe and the free margin (Fig. 1). The main supplying and draining vessels, choroidal arteries (anterior and posterior) and veins (superior, medial and inferior), as well as their large ramifications, were mostly masked by the surrounding capillary networks; however, their location and course corresponded to that described for the choroid plexus of adult individuals (Millen & Woollam, 1953; Wolfram-Gabel et al. 1987b).
Fig. 1.
The total vascular cast of the right lateral ventricle plexus – lateral and slightly posterodorsal view. The plexus exhibits a demilunar form with its concave attached margin being in close apposition with the thalamus (TH). The particular parts of the plexus, distinguished on the basis of vascular patterns, are separated by dotted lines. The villous fringe (VF) runs along the lateral surface of the plexus just over and parallel to the attached margin. The anterior part of the plexus (AP), reaching the interventricular foramen region, is located on the right side of micrograph. The posterior part (PP), beginning in the vicinity of the inferior horn, is located in the left lower part of micrograph. The anterior and posterior parts flank the glomus area (GL). The free margin (FM) forms the convex border of the plexus, with the anterior part of contralateral plexus in the background. A superficial area of the glomus and the adjacent part of free margin were lost during preparation. Bar=1000µm.
The posterior part
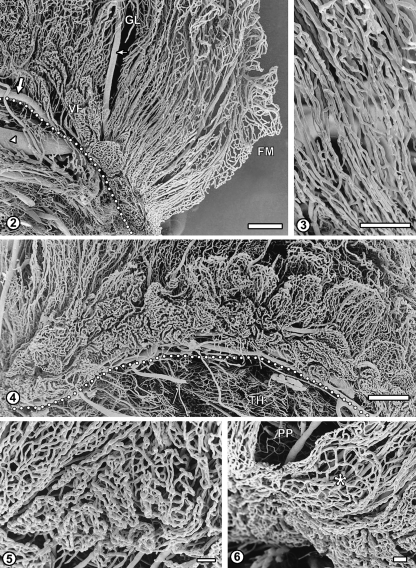
The posterior part, beginning in the vicinity of the inferior horn of the lateral ventricle, shows strikingly different microvascular architecture as compared to the other areas of the plexus. It contains an array of long, parallel and densely packed arterioles and venules (Figs 1, 2). These vessels are surrounded by dense, sheath-like capillary plexuses (Fig. 3). Posteriorly, the vessels branch into the capillary loops of the free margin, and superiorly, they blend with more irregular vascular network of the glomus.
Fig. 2.
The vascular pattern of the posterior part of the left ventricle plexus. This area is characterized by a dense array of straight, parallel blood vessels: arterioles, venules and capillaries which form tubular plexuses around bigger vessels. These vessels blend with irregular vascular network of the glomus (GL). In the narrow zone of villous fringe (VF) along the attached margin (dotted line), capillaries form relatively small, rounded or elongated baskets, which become bigger and more leaf-like as the fringe continues anteriorly. Flat multiple capillary arcades of the free margin (FM) are also seen. Large arrow: medial branch of anterior choroidal artery; small arrow: its branch; arrowhead: choroido-ventricular vein. Bar=1000µm.
Fig. 3 Sheath-like capillary networks surrounding parallel arterioles and venules in the posterior part of the choroid plexus. Bar=500µm.
Fig. 4 The C-shaped villous fringe of the right ventricle plexus composed of basket-like or leaf-like capillary plexuses of the choroid villi. The elongated villi emerge from behind and are extending dorsally over the basket-like structures. Dotted line indicates attached margin of the plexus. Anterior-posterior axis: right-to-left. TH, thalamus. Bar=1000µm.
Fig. 5 Higher magnification of the fringe posterior–anterior transition area (left-to-right). Small, nearly flat and very dense basket-like capillary networks located under the posterior part of the plexus become bigger, looser and more folded, being separated by narrow sulci. Bar=100µm.
Fig. 6 Fragment of the fringe under the posterior part of the plexus (PP): a flat, discoid capillary plexus (asterisk). Bar=100µm.
The villous fringe
In contrast to the smooth and regular microvascular pattern of the posterior part, the other areas of the choroid plexus are characterized by more or less developed irregular capillary plexuses, suggesting the presence of villous protrusions. The C-shaped villous fringe shows the presence of most prominent, protruding basket- and leaf-shaped capillary plexuses (Fig. 4).
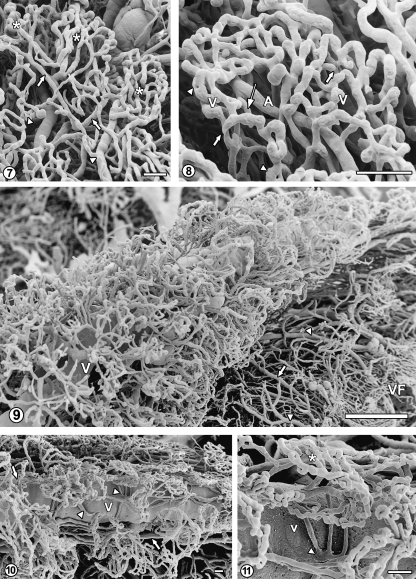
This area is the narrowest under the posterior part of the plexus, where small, sometimes elongated capillary baskets can be observed, and widens under the glomus and the anterior part, where capillary plexuses are larger, less dense and often form flat, leaf-like arrays (Fig. 5). Occasionally, single discoid plexuses can be observed in the posterior part of the fringe (Fig. 6). The leaf-like capillary networks are often composed of a few distinguishable plexuses (lobules) supplied and drained by separate arterioles and venules, respectively (Fig. 7). The capillaries, up to 20µm in diameter, show uneven contours, with nodular thickenings and constrictions as well as with occasional short, blind sprouts. Tight capillary loops with small ‘holes’ suggesting the intussusceptive growth, albeit rare, can also be observed. The capillary plexuses showed the presence of occasional short connections, approximately 15µm in diameter, between arterioles and venules (Fig. 8).
Fig. 7.
Villous fringe under the glomus. A leaf-like villous network composed of three separate capillary plexuses (lobules, asterisks). The arterioles (arrows) and slightly thicker venules (arrowheads) form short stalks for each plexus. Bar=100µm.
Fig. 8 Higher magnification of the villous capillary plexus (lobule) showing uneven capillary contours, blind sprouts (thick arrows) and tight loops with ‘holes’ (arrowheads). A, arteriole; V, venule (masked by capillaries). Thin arrow indicates connection between arteriole and venule. Bar=10µm.
Fig. 9 The anterior part of the right ventricle choroid plexus. The vascular bed of this part, constituting simultaneously a fragment of the free margin, is located around and along the course of the superior choroidal vein (V), only partially masked in the lower left corner of the micrograph. The bed tapers approaching the interventricular foramen region (upper part of the micrograph). Note arteriole (arrow) and venules (arrowheads) interconnecting the periphery of the bed with the villous fringe area (VF). Bar=1000µm.
Fig. 10 The imediate surroundings of the superior choroidal vein (V). Note small glomerular or rosette-like capillary plexuses. The arteries (arrows) supplying arteriolar branches and capillaries run parallel to the vein. Note also the presence of short collecting venules (arrowheads) directly emptying into the superior choroidal vein. Bar=100µm.
Fig. 11 Higher magnification presenting a rosette-like capillary plexus (asterisk) drained by three postcapillary venules discharging via a short common venular stem (arrowhead) directly into the superior choroidal vein (V). Bar=100µm.
The anterior part and the glomus
As compared to the villous fringe, the anterior part and the glomus are generally characterized by lower density of capillaries as well as lower number and size of distinct capillary plexuses (Fig. 1). Only in the upper area, located below the free margin, is the density of capillary network surrounding the superior choroidal vein higher (Fig. 9). The capillaries located in the vicinity of the vein, form small and dense, glomerular or rosette-shapes plexuses. This system is supplied by arterioles running parallel to the vein via short, radial branches and drained by venules which frequently empty directly into the vein (Figs 10, 11). Below this area, an array of nearly parallel arterioles and venules interconnects the capillary network located around the superior choroidal vein with the villous fringe (Fig. 9).
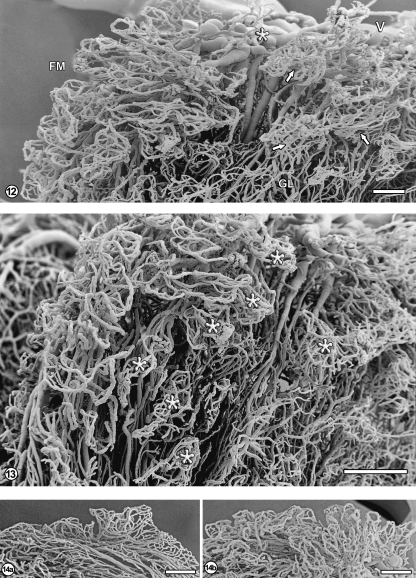
In the superior part of the glomus, relatively large veins formed in its deeper regions unite to form the superior choroidal vein (Fig. 12). The lateral surfaces of the glomus show the presence of irregular capillary plexuses, often possessing long vascular stalks composed of relatively straight arterioles and venules (Fig. 13). The venules empty into venous vessels which finally either gather into the superior choroidal vein or drain into the inferior choroidal vein. In the anterior part of the choroid plexus and especially in the glomus, some relatively large avascular areas were observed (Fig. 13).
Fig. 12.
Free margin (FM) with the underlying part of the glomus (GL). The veins emerging from the glomus converge (asterisk) into the superior choroidal vein (V). The capillary network of the free margin has mostly the form of multiple flat loops (see also Figs 1 and 2). The glomus in this area is composed of irregular villous networks, mostly with long vascular stalks and dense capillary plexuses forming ‘heads’ (arrows). Bar=1000µm.
Fig. 13 The glomus part viewed from the free margin edge. Note irregular villous plexuses (asterisks) with their vascular stalks, forming the underlying array of mostly parallel vessels and some nearly avascular spaces. Bar=1000µm.
Fig. 14 Capillary arcades of the free margin: one row of arcades over the posterior part (a) and several rows over the glomic part of the choroid plexus (b). Bar=1000µm.
The free margin
Anatomically, the free margin is in fact the outer margin of the anterior part, glomus and posterior part; however, it shows a common microvascular architecture. The margin is characterized by flat, multiple, arcade-like capillary loops (Figs 2, 9, 12, 13). The morphological indicators of angiogenesis – blind capillary sprouts or small ‘holes’– are not observed in this area. The free margin is the thinnest – just one row of arcades – over the posterior part (Fig. 14a) and thickens anteriorly, so that several rows of capillary loops constitute the margin over the glomus and the anterior part (Figs 9, 14b).
Discussion
The fetal period investigated in this study (20 gestational weeks) corresponds to so-called stage 3 of the choroid plexus development (17–28 gestational weeks; Catala, 1998). According to the authors of relevant publications, the choroid plexus at the 4th gestational month shows the presence of primary villi; true villi develop afterwards (Kappers, 1958; Shuangshoti & Netsky, 1966; Milhorat, 1976; Catala, 1998). The vascular patterns observed in our material remain in accordance with these observations: relatively simple capillary plexuses protruding in the areas of villous fringe, anterior part and the glomus seem to represent the vascular systems of the primary villi, whereas more complex, elongated and branched systems characteristic of the true villi are not yet observed.
On the other hand, the division of the choroid plexus into its parts described in adult individuals (Millen & Woollam, 1953) is already distinct in this relatively early developmental period: the anterior and posterior parts, the villous fringe, as well as the glomus can be easily distinguished in corrosion casts because of different vascular patterns. Not surprisingly it is the villous fringe area that shows the most prominent capillary plexuses of the primary villi. In the other regions, such plexuses are less numerous, smaller and more irregular.
It should be emphasized that our corrosion casts present the choroid plexus just before the onset of an intense angiogenesis leading to the formation of the complex, elongated vascular systems of the true villi. Indeed, particularly in the villous fringe region we observed the signs of angiogenesis in the form of either blind capillary sprouts or small ‘holes’ typical of intussusceptive capillary growth (Burri & Tarek, 1990). Interestingly, the latter feature was encountered only occasionally, most probably because during formation of true villi the capillary sprouting and elongation is the predominant form of angiogenesis, whereas the intussusceptive growth is more characteristic for 3-dimensional expansion of the preexisting capillary networks (Gorczyca et al. 1999).
Generally, the capillaries of the choroid plexus showed uneven contours with nodular dilations and constrictions, as well as a large diameter (up to 20µm). On one hand, both features have been reported in the description of the fully mature choroid plexus in man (Motti et al. 1986; (Strazielle & Ghersi-Egea, 2000) and in other mammalian species (Hodde, 1979; Miodonski et al. 1979; Weiger et al. 1986) – such a character of capillaries has been postulated to slow down blood flow locally and to facilitate blood–cerebrospinal fluid exchange; on the other hand, sinusoidal contours and large diameter seem to be common characteristics of the capillaries in various human fetal organs (Gorczyca et al. 1999). Interestingly, there is some evidence that during the first and second trimester of development such capillaries of the choroid plexus can be fragile, leading to microhemorrhagic events (Scott & Bergevin, 2005).
The only corrosion cast/SEM study of mature human choroid plexus was published by Motti et al. (1986) Generally, their observations concerning the character of vascular patterns agree with ours; however, there are also several substantial differences between their findings and the results of the present study. The leaf-like and glomerular capillary meshes in the villous areas, microglomeruli which we describe as rosette-shaped plexuses, large diameter of capillaries, as well as their sinusoidal dilations have been observed in both studies. On the other hand, Motti et al. (1986) distinguished two morphologically different regions in the vascular cast of the plexus: (1) the rostral area and free margin characterized by villous infoldings and (2) a central smooth segment. The structure of the latter: the network of thin capillaries with predominantly longitudinal arrangement (so-called garland capillaries), surrounding larger vessels, corresponds well to what has been observed in the present study in the posterior part of the plexus. This location discrepancy may result from the fact that Motti et al. (1986) presented sort of joint characteristics of rat, dog and human choroid plexuses and they did not follow the precise anatomical division of the human plexus into its parts presented by Millen & Woollam (1953). Moreover, they failed to obtain a complete corrosion cast of the glomus area.
Numerous short arteriovenous connections at the origin of the villous capillary networks, probably acting as thoroughfare channels that can regulate blood flow in the villi, were described in mature human choroid plexus investigated by both dye injection and corrosion casting methods (Millen & Woollam, 1953; Motti et al. 1986). In our material, however, such connections observed between arterioles and venules in the capillary plexuses of the primary villi were rare – they probably develop later in the fetal life, when secondary villi with their elaborate capillary networks are formed.
In the present study no manifestation was found of what has been described by Dziegielewska et al. (2001) as ‘a grossly distended tissue with a large central mass of amorphous material’, in their opinion a striking feature of the human fetal choroid plexus. This feature was, however, observed mostly at earlier developmental stages. We could not find in our material any remnants of ‘lingula’, a long process of the choroid plexus present in some animals (Hodde, 1979; Weiger et al. 1986) and reported to occur occasionally in fetal human plexus (Millen & Woollam, 1953), although never present in adults. The latter authors speculate that in humans, the glomus may be a homologue of lingula, but the issue remains controversial, as other authors, investigating a large material, could not find lingula in any human fetal choroid plexus (Pleeging & Kappers, 1962).
The glomus, characterized by irregular capillary plexuses, contained relatively large, avascular areas, not observed in mature human choroid plexus. As reported by Pleeging & Kappers (1962), during fetal development the glomus is a site of connective tissue accumulation which can be reflected by local scarcity of blood vessels in this region, although some of the avascular areas could represent spaces between deeper folds of the plexus.
In conclusion, the vascular architecture of the human choroid plexus at 20 gestational weeks seems to have achieved the general pattern of the postnatal/mature plexus, with just one important component still to be developed: the complex vascular plexuses of the secondary villi.
Acknowledgments
This study was supported by a grant 501/P/192/L/UJCM from the Jagiellonian University Medical College to A.J.M.
References
- Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec. 1990;228:35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- Catala M. Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: the ventricular system, meninges and choroid plexus. Arch Anat Cytol Pathol. 1998;46:153–169. [PubMed] [Google Scholar]
- Dziegielewska KM, Ek CJ, Habgood MD, Saunders NR. Development of the choroid plexus. Microsc Res Tech. 2001;52:5–20. doi: 10.1002/1097-0029(20010101)52:1<5::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gorczyca J, Litwin JA, Nowogrodzka-Zagórska M, Skawina A, Miodoński AJ. Architecture of blood vessels in human fetal gastric corpus: a corrosion casting study. Ann Anat. 1999;181:353–358. doi: 10.1016/S0940-9602(99)80127-7. [DOI] [PubMed] [Google Scholar]
- Hodde CK. The vascularization of the choroid plexus of the lateral ventricle of the rat. Beitr Elektronenmikrosk Direktabb Oberfl (BEDO) 1979;12:395–400. [Google Scholar]
- Hudson AJ. The development of the vascular pattern of the choroid plexus of the lateral ventricle. J Comp Neurol. 1960;115:171–186. doi: 10.1002/cne.901150206. [DOI] [PubMed] [Google Scholar]
- Kappers AJ. Structural and functional changes in the telencephalic choroid plexus during human ontogenesis. In: Wolstenholme GEW, O’Connor CM, editors. The Cerebrospinal Fluid. Boston: Little Brown; 1958. pp. 3–31. [Google Scholar]
- Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts – techniques and applications: updated review. Scanning Microsc. 1990;4:889–941. [PubMed] [Google Scholar]
- Lametschwandtner A, Miodonski A, Simonsberger P. On the prevention of specimen charging in scanning electron microscopy by attaching conductive bridges. Mikroskopie. 1980;36:270–273. [PubMed] [Google Scholar]
- Maillot C, Koritke JG, Laude M. The vascular patterns in choroid tela of the fourth ventricle in man. Arch Anat Histol Embryol. 1976;59:33–70. [PubMed] [Google Scholar]
- Milhorat TH. Structure and function of the choroid plexus and other sites of cerebrospinal fluid formation. Int Rev Cytol. 1976;47:225–288. doi: 10.1016/s0074-7696(08)60090-x. [DOI] [PubMed] [Google Scholar]
- Millen JW, Woollam DHM. Vascular patterns in the choroid plexus. J Anat. 1953;87:114–123. [PMC free article] [PubMed] [Google Scholar]
- Miodonski A, Kus J, Tyrankiewicz R. SEM blood vessel casts analysis. In: Allen DJ, Motta PM, Didio LJA, editors. Three-Dimensional Anatomy of Cells and Tissue Surfaces. Amsterdam: Elsevier; 1981. pp. 71–87. [Google Scholar]
- Miodonski A, Poborowska J, Friedhuber de Grubenthal A. SEM study of the choroid plexus of the lateral ventricle in the cat. Anat Embryol. 1979;155:407–416. doi: 10.1007/BF00317645. [DOI] [PubMed] [Google Scholar]
- Motti EDF, Imhof HG, Janzer RC, Marquardt K, Yasargil GM. The capillary bed in the choroid plexus of the lateral ventricles: a study of luminal casts. Scanning Electron Microsc. 1986;IV:1501–1513. [PubMed] [Google Scholar]
- Pedget DH. The development of the central arteries in the human embryo. Contrib Embryol. 1948;32:205–262. [Google Scholar]
- Pleeging JH, Kappers ÄJ. Comparative anatomy, embryological development and microscopical structure of the glomus choroideum. In: Jacob H, editor. Proceedings IV International Congress Neuropathol 1961. III. Stuttgart: Thieme; 1962. pp. 455–459. [Google Scholar]
- Scott DE, Bergevin M. Fine structural correlates of the choroid plexus of the lateral cerebral ventricle of the human fetal brain. Anat Rec. 2005;282A:8–12. doi: 10.1002/ar.a.20104. [DOI] [PubMed] [Google Scholar]
- Shuangshoti S, Netsky MG. Histogenesis of the choroid plexus in man. Am J Anat. 1966;118:283–316. doi: 10.1002/aja.1001180114. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF. Choroid plexus in central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59:561–574. doi: 10.1093/jnen/59.7.561. [DOI] [PubMed] [Google Scholar]
- Weiger T, Lametchwandtner A, Hodde CK, Adam H. The angioarchitecture of the choroid plexus of the lateral ventricle of the rabbit. A scanning electron microscopic study of vascular corrosion casts. Brain Res. 1986;378:285–296. doi: 10.1016/0006-8993(86)90931-5. [DOI] [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C, Koritke JG, Laude M. Vascularization of the tela choroidea of the 3rd ventricle in man. Arch Anat Histol Embryol. 1984;67:3–42. [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot C, Koritke JG, Laude M. The vascularization of the human tela choroidea of the lateral ventricle. Acta Anat (Basel) 1987b;128:301–321. [PubMed] [Google Scholar]
- Wolfram-Gabel R, Maillot CL, Koritke JG. The vascular pattern in the tela choroidea of the prosencephalon in man. J Neuroradiol. 1987a;14:10–26. [PubMed] [Google Scholar]